Abstract
The silver oxide nanoparticles (AgO2 ‐NPs) were synthesised using silver foil as a new precursor in wet chemical method. X‐ray diffraction analysis shows crystallographic structures of AgO2 ‐NPs with crystallite size of 35.54 nm well‐matched with standard cubic structure. Scanning electron microscopy analysis clearly shows the random distribution of spherical‐shaped nanoparticles. Energy dispersive X‐ray analysis confirmed the purity of the samples as it shows no impurity element. Fourier transforms infra‐red analysis confirmed the formation of AgO2 ‐NPs with the presence of Ag‐O‐Ag stretching bond. All the techniques also confirmed the loading of ceftriaxone drug on the surface of AgO2 ‐NPs. This study also described the effect of AgO2 ‐NPs having synergistic activity with β lactam antibiotic i.e. ceftriaxone against ESBL generating Escherichia coli (E. coli). Among isolated strains of E. coli, 60.0% were found to be ESBL producer. The synergistic activities of AgO2 ‐NPs with ceftriaxone suggest that these combinations are effective against MDR‐ESBL E. coli strains as evident by increase in zone sizes. The present study observed rise in MDR‐ESBL E. coli with polymorphism of blaCTXM and blaSHV causing UTI infections in Pakistani population. The antibiotic and AgO2 ‐NPs synergistic effect can be used as an efficient approach to combat uro‐pathogenic infections.
Inspec keywords: antibacterial activity, nanofabrication, nanomedicine, drugs, nanoparticles, microorganisms, crystallites, scanning electron microscopy, silver compounds, X‐ray diffraction, X‐ray chemical analysis, Fourier transform infrared spectra, organic compounds, genetics
Other keywords: synergistic evaluation, clinical strains, silver oxide nanoparticles, silver foil, wet chemical method, X‐ray diffraction analysis, crystallographic structures, standard cubic structure, spherical‐shaped nanoparticles, energy dispersive X‐ray analysis, ceftriaxone drug, synergistic activity, ESBL producer, scanning electron microscopy, Fourier transform infrared analysis, Escherichia coli, blaSHV gene positive ESBL, crystallite size, random distribution, β lactam antibiotics, MDR‐ESBL E. coli strains, polymorphism, blaCTXM, uro‐pathogenic infections, uro‐pathogenic E. coli, AgO2
1 Introduction
In the treatment of bacterial infections, use of cephalo sporins has led to the development of a novel class of broad‐spectrum enzymes called extended spectrum β ‐lactamases (ESBLs) [1]. Escherichia coli (E. coli) strains producing ESBL enzymes are considered as the well‐known reasons of infections in urinary tract of children [2]. K. pneumoniae producing ESBL was first described in 1983 [3] and consequently, E. coli producing ESBL enzymes was reported in 1987 [4]. Since then, reports on the production of ESBL‐producing microorganisms have been consistently expanding worldwide. ESBLs are encoded by mobile genes; the most frequently encountered ESBLs genes belong to the blaCTX‐M, blaSHV, and blaTEM. TEM (Temoniera), SHV (sulphydryl variable), and CTX‐M type are the most prominent variants [5]. The prevalence regarding antibiotic resistance among ESBL producing uro‐pathogenic E. coli continues to be increased inside both healthcare and local community settings and become a challenge to the healthcare professionals in terms of disease prevention, therapeutic development, and epidemiology [6]. Alternate antimicrobial agents are immensely needed to fight ESBL‐producing strains, nanoparticles in combination with existing antibiotics are an alternative technique to fight against infections caused by MDR bacteria [7]. Silver, due to its broad‐spectrum antimicrobial property, could represent a favourable option especially in its nanoform [8]. Silver oxide has been used to treat bacterial infections in the start of nineteenth century but when the era of antibiotics began, along with the discovery of penicillin [9]. Among the wide range of nanoparticle choices available, silver oxide nanoparticles due to its wide range of antibacterial properties constitute a very encouraging loom for the advancement of new antimicrobial agents [10]. Ag2 O nanoparticles exhibit antibacterial activity against both gram‐positive as well as gram‐negative bacteria but its efficiency is higher in case of gram‐negative bacteria. [10] It has been observed by Harshita Negi et al. during their research that by increasing the concentration of silver oxide nanoparticles, their inhibition zone increases. They exhibit higher efficiency due to the larger surface to volume ratio. Similarly, Xin Wang et al. [11] showed the efficiency of different shapes of Ag2 O nanoparticles. They found that antibacterial activity of Ag2 O nanoparticles against E. coli gram‐negative bacteria depends on the shape of nanoparticles. Silver oxide NPs have received rigorous attention in medicine, as they are able to act as antimicrobials, and elicit anti‐angiogenesis, ant‐inflammatory, and anti‐platelet activities [10, 12]. There are detailed reports regarding anti‐biofilm adequacy of silver nanoparticles against biofilm produced by expanded range β ‐lactamase isolates of E. coli [13]. Several studies about the synergistic action of nano‐materials in conjunction with antibiotics have been reported [14], nano‐silvers combined to polymixin B also confirmed synergistic consequences with regard to gram‐negative microorganisms [15].
The first objective of the present study is to synthesise AgO2 ‐NPs using a novel method. There are many reports on silver oxide synthesis [16, 17] but our methodology is unique in utilisation of Ag foil as precursor. The second objective is the isolation of uro‐pathogenic strains of E. coli producing ESBL enzymes and to analyse existence of blaCTX‐M gene, blaSHV gene, and blaTEM gene among E. coli strains showing phenotypic ESBL characteristic on antibiotic sensitivity plates. Furthermore to combat these ESBL‐producing uro‐pathogenic E. coli strains, AgO2 ‐NPs synergistic interaction with β ‐lactam antibiotic (ceftriaxone) was evaluated against ESBL‐producing E. coli strains. The prevalence of diseases caused by broad spectrum β ‐lactamases (ESBLs) is a worldwide problem. Urinary tract infection caused by E. coli s trains producing ESBL enzymes is known as a noteworthy hazard of childhood kidney diseases. Furthermore to combat these ESBL‐producing uro‐pathogenic E. coli strains silver nanoparticles synergistic interaction with β ‐lactam antibiotic ceftriaxone was evaluated against ESBL‐producing E. coli strains.
2 Experimental procedures
2.1 Materials and methods
Silver foil, sodium hydroxide (NaOH), nitric Acid (HNO3), antibiotics discs: Augmentin (20 µg amoxicillin + 10 µg clavulanic acid), Ceftazidime (30 µg), Ceftriaxone (30 µg), Aztreonam (ATM‐30 µg), and Cefotaxime (CTX‐30 µg) (Oxoid, UK). Culture media CLED agar, MacConkey agar, TSI agar, and Muller Hinton agar (MH) were purchased from (Oxoid, UK).
2.2 Synthesis of silver oxide nanoparticles
Silver foil was used as a precursor for synthesis of silver oxide nanoparticles by adopting wet chemical method. First of all, one molar aqueous solution of nitric acid (HNO3) was prepared and silver foil was dissolved into it with constant stirring (solution 1). Then, 40 mL of 0.5 M solution of sodium hydroxide (solution 2) was added into it drop‐wise until brownish precipitates are formed and allowed to stir for an hour. The solution was centrifuged for 12 min and washed with ethanol and distilled water. Then, it was sonicated for 5 to 10 min and dried. The schematic representation of the synthesis of silver oxide nanoparticles is shown in Fig. 1.
Fig. 1.
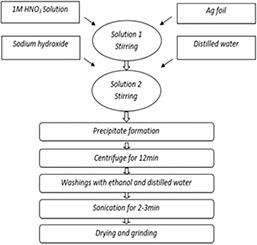
Schematic diagram of silver oxide nanoparticles (NPs)
2.3 Sample collection and bacterial strains identification
The urine samples of paediatric patients suffering from urinary tract infection from nephrology department of tertiary care hospital of Pakistan were brought into microbiology lab of International Islamic University and the collected urine samples were processed for the isolation of E. coli. The urine samples were cultured on the standard media including cytosine lactose electrolyte deficient (CLED) and MacConkey agar plates. After culturing, the plates were incubated at 37°C for 24 h. The colony showed fermentation of lactose on MacConkey agar and CLED media were refined and recognised by their morphology as round, rose‐pink to red states on MacConkey agar medium and yellow colonies on CLED agar. The bacterial isolates were identified by standard microbiological techniques as described in the Bergey's manual of systemic bacteriology, which involves colony characterisation, cell morphology, and biochemical tests i.e. biochemical reactions e.g. oxidase test, catalase, Indole and methyl red test, vogesproskaur reaction, urease, and citrate and H2 S production in triple sugar iron medium was examined [18].
2.4 Disc diffusion test for ESBL detection
Identification of ESBL‐producing E. coli was done using the Kirby–Bauer disc diffusion test on Muller–Hinton agar plates inoculated with the E. coli strains as recommended for standard disc diffusion susceptibility test CLSI (2012) [19]. Augmentin disc (20 µg amoxicillin + 10 µg clavulanic acid) were positioned at the middle of the plate, and discs containing ceftazidime (30 µg), ceftriaxone (30 µg), Aztreonam (ATM‐30 µg), and cefotaxime (CTX‐30 µg) were placed at a distance of 25 mm from Augmentin disc. After 24 h incubation at 37°C, a zone of inhibition around one or more cephalosporin discs was extended on the side nearest to the Augmentin disc. This synergy was designated as ESBL positive.
2.5 Antibiotics susceptibility profiling
The antibiotics susceptibility patterns of the ESBL‐producing E. coli were determined by disc diffusion method on MH plates as per CLSI rules 2012 [20]. Total 17 antibiotics discs of known concentration, i.e. Augmentin (30 µg), Cefipime (30 µg), nitrofurantion (300 µg), Cefixime (5 µg), ceftriaxone (30 µg), amikacin (30 µg), ciprofloxacin (5 µg), sulphanethoxazole SXT (25 µg), gentamicin CN (10 µg), piperacillin/tazobactam (110 µg), sulbuctum (105 µg), norfloxacin NOR (10 µg), impipenum (10 µg), ampicillin AMP (10 µg), and nalidixic acid NA (30 µg) were placed on the surface of these plates pre‐inoculated by ESBL‐producing E. coli. The plates were incubated overnight at 37°C for 24 h. Diameters of the zone of inhibition were measured in mm with the help of a ruler, and interpreted as per CLSI guidelines 2013 [19].
2.6 Genomic DNA extraction and genotyping
The genomic DNA of bacteria was extracted by phenol and chloroform routine extraction method [21]. The genomic DNA extraction was performed in strict understanding to the following steps: (i) quantitative determination of DNA by UV‐spectrophotometry using Nanodrop 2000c (Thermo Scientific, USA); (ii) PCR analysis of TEM, SHV, and CTX‐M genes in all isolated for the detection of ESBL resistance blaTEM, blaSHV, and blaCTX‐M genes, using primers provided in Table 1. The process of PCR was done in 96‐well thermal cycler (Thermo Electron Corporation, Mill passage, USA) utilising amplification protocol comprising of 30 cycles with an underlying initial denaturation at 94°C for 5 min and final extension at 72°C for 4 min. Each cycle comprised of denaturation at 94°C for 1 min, annealing for 1 min at 57°C for blaSHV, 55°C for blaCTX‐M, and at 50°C for blaTEM and final extension at 72°C for 1 min. The amplified PCR products were detected by electrophoresis using 2.0% of agarose gel to obtain better resolution of the amplified product which size was checked with standard molecular weight marker.
Table 1.
Sequence of the primers used for the genotyping of blaSHV, blaCTX‐M and blaTEM
| Gene | Primers | Sequence | Product size, bp |
|---|---|---|---|
| bla TEM | forward primer | ATG AGT ATT CAA CAT TTC CG | 858 |
| reverse primer | CCA ATG CTT AAT CAG TGA GG | — | |
| bla SHV | forward primer | ATG CGT TAT ATT CGC CTG TG | 859 |
| reverse primer | AGC GTT GCC AGT GCT CGA TC | — | |
| bla CTX‐M | forward primer | SCS ATG TGC AGY ACC AGT AA | 581 |
| reverse primer | ACC AGA AYV AGC GGB GC | — |
2.7 Evaluation of synergism of AgO2 ‐NPs with ceftriaxone
The tests were performed by using the disk diffusion (DD) strategy on Müller‐Hinton agar plates. In this examination, three kinds of disks were utilised (i) paper disc (10 mm) immersed with 10 µL of AgO2 ‐NPs; (ii) ceftriaxone discs of two different concentrations (10 µg/mL and 20 µg/mL and (iii) ceftriaxone discs saturated with 10 and 20 µL of AgO2 ‐NPs). Fresh cultures of ESBL E. coli with <12 h incubation were prepared at a concentration of 0.5 McFarland Scale29 (1.5 × 108 CFU/mL) and used as inoculums for swabbing on plates. After swabbing the prepared discs containing synergistic formulation were placed on inoculated plates and were incubated at 37°C for 24 h, the zones of inhibition were measured next day. The synergism was assessed by the equation {(C − B)/B } × 100, where, B = the zone of inhibition of the ceftriaxone alone and C = Ceftriaxone + AgO2 ‐NPs. This equation used to assess the expansion of the zone of inhibition around the bacteria caused by the antibiotic in combination with AgO2 ‐NPs.
2.8 Observation of phenotype changes of silver oxide nanoparticles and antibiotic‐treated cells
Culture of ESBL E. coli (A600, 0.05) at its exponential stage was grown in Luria broth and was treated with silver oxide nanoparticles and antibiotic formulation (10 µg/mL + 10 µg/mL) and incubated for 15 min at 37°C and slides were made in 1.0% agarose and observed under phase contrast microscope, to find their morphology, specific care was taken to reduce the sample exposure to UV light, image grabbing were performed basically as depicted by Edwards and Errington [22].
3 Results and discussion
Inappropriate and arbitrary utilisation of β ‐lactam drugs are the prominent reasons of emergence of resistant strains and their episodes. As per the current investigation, the predominance of β ‐lactamase genes in Pakistan is high. Therefore, with a specific end goal to control resistant strains, suitable strong measures, new antimicrobial formulations, and additional research to determine antibiotic resistance patterns in different regions are crucial. In addition, there is a pressing need to build up alternate antibacterial agents. The past decade has observed as a considerable rise in the global use of nano‐medicines as inventive tools to fight antimicrobial resistance dilemma. Silver oxide nanoparticles (AgO2 ‐NPs) are one of the notable antibacterial substances and showed up as reasonable possibility for use in combinations with traditional antibiotics [23]. Antibiotic resistance has evolved as a serious area under discussion against life threatening infections and remains a crucial rationale of morbidity and mortality. Infections in the urinary tract of the children are the well‐known microbial disease with an increasing incidence rate in girls [24]. E. coli distant as the main cause of UTI with prevalence ranging from 80 to 90% and are progressively revealed as causative agents of UTI diseases [4] and production ESBLs by uro‐pathogenic E. coli causes inactivation of large number of antibiotics and present major therapeutic dilemma [1]. Studies to recognise the microorganisms causing UTIs and determining the resistance prototype to antimicrobial agent's exhibit specifically geographical area prompts the choice of reasonable treatment possibilities. The current study investigated the antimicrobial susceptibility patterns of ESBL enzymes producing E. coli isolates separated from urine sample of paediatric patients experiencing infections in the urinary tract in tertiary‐care hospitals of Pakistan. We have prepared the AgO2 ‐NPs using Ag foil as cheaper precursor. These AgO2 ‐NPs are utilised in combination with antibiotic and their synergistic effect is studied. The current study includes120 uro‐pathogenic E. coli strains isolated from paediatric patients having UTI infections, the isolated strains of E. coli were screened for the production of ESBL enzymes by double‐disc synergy method and the existence of bla CTX‐M, bla SHV, and bla TEM genes were investigated by using PCR. High rate of resistance was observed against ceftriaxone (100%), followed by ciprofloxacin (94.0%) co‐trimoxazole (79.0%), whereas 71.0% of the isolates exhibited blaCTX‐M genes, 29.0% isolates were positive for blaSHV gene, 29.0% isolates harboured both blaCTX‐M and blaSHV genes, while blaTEM gene was not detected in any of the isolated strain. Rather than treatment with antimicrobials alone, we additionally analysed antibiotic/AgO2 ‐NPs combinations, our results revealed a positive synergism marked by significant increase in sizes zone of inhibition against MDR‐ESBL E. coli strains.
3.1 Characteristics of silver oxide nanoparticles
3.1.1 X‐ray diffraction spectroscopy
Silver oxide nanoparticles (AgO2 ‐NPs) have been synthesised successfully using a cheaper and easily available precursor i.e. silver foil. X‐ray diffraction (XRD) analyses of the samples were carried out using X‐ray diffractometer in which Cu kα of wavelength 1.54 Å radiations were used. Intensity of the diffracted beam was recorded as a function of position (2θ). Fig. 2 a presents the X‐ray diffraction pattern of the prepared sample. It gives the confirmation of AgO2 ‐NPs by comparing them with the standard crystal structures, crystallinity, and value of lattice parameters by using JCPDS card. It is evidently showed that all of the peaks of all the three samples well‐matched with the Bragg reflections of the standard cubic structure (Space group: Pn−3m, a = 4.7360, b = 4.7360, c = 4.7360, JCPDS: 012‐0793). The five sharp peaks at 32.88, 37.98, 55.28, 65.34, and 68.59 can be allotted to their characteristic (111), (200), (220), (311), and (222) indices, respectively. All the reflected peaks were matched with the Ag2 O phase having simple cube structure. In addition, it can be analysed that the product has a high purity as no characteristic peaks of other impurities are observed. The crystallite sizes of NPs were also calculated from Debye–Scherer's formula was 35.54 nm. Fig. 2 b depicts the X‐Ray diffraction pattern of ceftriaxone drug loaded Ag2 O material. The loaded sample shows all characteristic peaks of Ag2 O with change of intensity. There is no appearance of any peak related to drug loading due to less contents. Although there is change in intensity of (111) peak value which confirms the incorporation of dye molecules on the surface of Ag2 O.
Fig. 2.
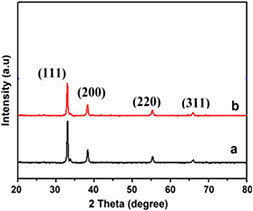
X‐ray diffraction analysis of various samples
(a) AgO2 ‐NPs and, (b) drug loaded AgO2 ‐NPs
3.1.2 Scanning electron microscopy
The morphology of prepared sample is shown in for pure (Figs. 3 A and B and ceftriaxone drug loaded samples Figs. 3 C and D). It is clear from the image that the particles were highly agglomerated in nature. The SEM pictures showed clearly, random distribution of nanoparticles of spherical shape which were grown in a very high density. The image revealed the average particle size of 100 nm. The sample after drug loading shows agglomeration of Ag nanoparticles with appearance of large‐sized crystal of drug material of ceftriaxone.
Fig. 3.
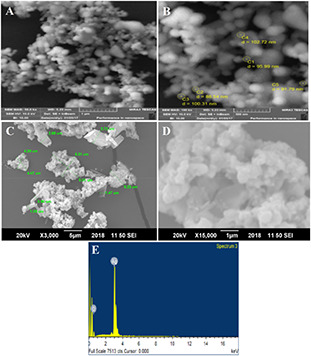
SEM images of AgO2 NPs and drug loaded AgO2 NPs at different magnifications and Energy dispersive X‐ray analysis of AgO2 ‐NPs
(A, B) SEM images of AgO2 NPs, (C, D) SEM images of drug loaded AgO2 NPs, (E) Energy dispersive X‐ray analysis of AgO2 ‐NPs
3.1.3 Energy dispersive X‐ray analysis
The energy dispersive X‐ray analysis of the prepared samples indicated the presence of oxygen and silver elemental composition. Fig. 3 E shows the EDS analysis of sample. It can be clearly seen that the sample just comprises on silver (wt.% 83.31) and oxygen (wt.% 16.69). It shows the purity of the sample as no other peak related with any impurity has been indicated by XRD analysis. Elemental analysis of sample AgO2 ‐NPs is given in Table 2.
Table 2.
Elemental analysis of sample AgO2 ‐NPs
| Elements | Weight, % | Atomic, % |
|---|---|---|
| O K | 16.69 | 57.47 |
| Ag L | 83.31 | 42.53 |
| Total | 100.00 | 100.00 |
3.1.4 Fourier transforms infra‐red
Fig. 4 shows FTIR spectra of pure AgO2 ‐NPs and ceftriaxone loaded AgO2 ‐NPs. It can be seen clearly from Fig. 4a that the sample of pure AgO2 ‐NPs shows a vibration band at 509 cm−1 which represents Ag‐O‐Ag stretching vibration in correspondence with literature. The ceftriaxone‐loaded AgO2 sample showed the characteristic peaks of ceftriaxone in addition to Ag‐O‐Ag stretching vibration. These bands are recorded at 2930 cm−1 for C‐H stretching, 1412 cm−1 of stretching C = N and 1640 cm−1 peak represents amide group C = O axial deformation.
Fig. 4.
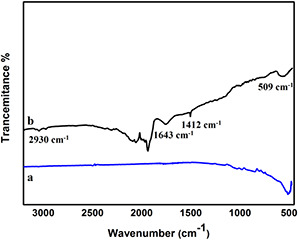
FTIR spectra of AgO2 ‐NPs and ceftriaxone‐loaded AgO2 ‐NPs
(a) AgO2 ‐NPs, (b) ceftriaxone‐loaded AgO2 ‐NPs
3.2 Morphological and biochemical characterisation of E. coli strains
On the basis of colonial morphology and gram staining, E. coli strains were recognised. On MacConkey agar, E. coli colonies were lactose fermenter. Gram staining showed that all of the strains of E. coli were gram negative and appeared as rod‐shaped. All of the isolated strains of E. coli were oxidase negative. In indole test, a red ring was appeared after adding Kavoc's reagent that was considered positive. Acidic reactions and gas formation were observed in TSI tubes inoculated.
3.2.1 Phenotypic detection of ESBL production
A total of 120 E. coli isolates were analysed by the double‐disc synergy method for the production of ESBL enzymes. Out of these 120 E. coli isolates, 72 isolates analysed evidenced ESBL‐producing activity. So the overall incidence rate of ESBL‐producing E. coli in our study was 60%. The double‐disc synergism is shown in Fig. 5.
Fig. 5.
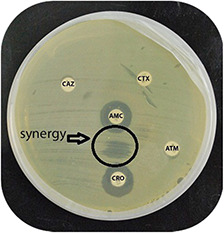
Phenotypic detection of ESBLs between Ceftriaxone (CRO) and Augmentin (AMC) disc
3.2.2 Antibiotic susceptibility profiling
Antibiotic susceptibility profiling of the ESBLs producing E. coli were determined by using disc‐diffusion method. All of the ESBL‐producing E. coli isolates (n = 72) were resistant to ceftazidime (CAZ 100%), ceftrixone (CRO 100%), cefixime (CFM 100%), ampicillin (AMP 100%), and nalidixic acid (NA 100%). All of the ESBLs isolates showed 100% susceptibility to imipenem, whereas 94% strains were susceptible to amikacin (AK), 81% to nitrofurantoin (F) 72% to cefoparazone, 62% piperacillin (TZP), 48% to Augmentin (AMC), 46% to Cefepime, 31% to Gentamicin (CN) 18% to Fosfomycin (FOS). Then, 13% to Co‐trimoxazole (SXT) and 10% to Norfloxacin (NOR) MDR characteristic are evident in Fig. 5. The resistance pattern exhibited by isolates against the test antibiotics is summarised in Figs. 5 and 6.
Fig. 6.
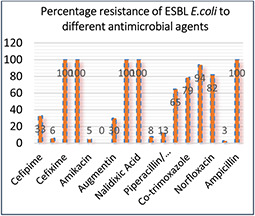
Antibiotic resistance profile of uro‐pathogenic ESBL E. coli strains
3.2.3 PCR analysis of blaTEM, blaCTX‐M and blaSHV genes
Among isolated strains, 21 isolates were positive for blaSHV genes and 51 showed prevalence of blaCTX‐M gene, whereas blaTEM gene was not detected in any of studied isolate. This information is presented in Figs. 7 and 8. The percentage distribution of blaCTX‐M, blaSHV, and blaTEM is summarised in Fig. 9.
Fig. 7.
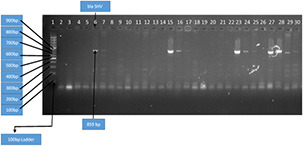
Amplification of blaCTX‐M gene by PCR with 100 bp DNA ladder Key. Lane 1 was 100 bp DNA ladder. Lane 2, 3, 4, 5,…, 31 was blaCTX‐M positive
Fig. 8.
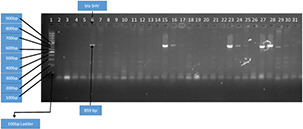
Amplification of blaSHV gene by PCR with 100 bp DNA ladder Key. Lane 1 was 100 bp DNA ladder. Lane 6, 7, 15, 16, 17, 23, 24, 25, 27, 28, 29 and 30 was blaSHV positive
Fig. 9.
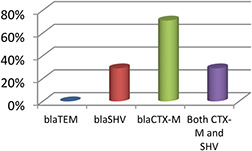
Percentage distribution of blaCTX‐M, blaSHV and blaTEM genes among ESBL E. coli
The prevalence of MDR‐ESBLs strains is growing resulting lack of usefulness of β ‐lactam class of antibiotics. In this study, 120 E. coli strains were screened from urine samples and among them 60% were ESBL‐producing strains proposing a higher predominance of these enzymes in paediatric patients having infection in urinary tract in patients. The researches in different regions have also informed increase ESBL enzymes prevalence in uro‐pathogens [25]. In this study, the prevalence of genes involved in MDR‐ESBL E. coli strains was studied as β ‐lactamase genes are resulting from TEM1, thus blaCTX‐M, blaSHV, and blaTEM genes were studied. Our data represent blaCTX‐M as the most prevalent genotype which is 71% followed by blaSHV 29% and presence of both blaCTX‐M‐blaSHV genes was found in 29% of the strains. The frequency of blaCTX‐M and blaSHV is in high agreement with the previously reported data [5, 26]. Masroor et al. reported 51% blaCTX‐M in uro‐pathogenic ESBL E. coli from tertiary care hospitals [27]. ESBLs belonging to the CTX‐M types are also most prevalent in Europe [28]. The first occurrence of a CTX‐M ESBL‐producing E. coli outbreak intervened mother to neonate transmission in an Irish neonatal intensive care unit become mentioned by O'Connor et al. 2017 [29]. In light of the above discoveries, it is far clear that the frequency of a wide variety of ESBL enzymes is growing.
3.2.4 Antibacterial effect of AgO2 ‐NPs in synergism with antibiotics
In our examination, we assessed the impact of AgO2 ‐NPs alone and in combination with the antimicrobial agent ceftriaxone (CRO). 10 µg/mL concentration of ceftriaxone produced a zone of clearance of 10 ± 1 mm; 10 µg/mL concentration of AgO2 ‐NPs alone demonstrated a restraint zone of 14.5 ± 1 mm, while combination of CRO + AgO2 ‐NPs produced a zone of inhibition of 26.4 ± 2 mm (Fig. 5). The relationship of CRO + AgO2 ‐NPs created a 30% expansion in the zone of inhibition when compared and the antibiotic alone (Table 3). In Fig. 10, we can observe the activity of this affiliation; the inhibition zones on the disc containing CRO + AgO2 ‐NPs in both the tested concentration are much larger than the disc with CRO alone and AgO2 ‐NPs alone. Rather than treatment with antimicrobials alone, we additionally analysed antibiotic/AgO2 ‐NPs combinations, our results revealed a positive synergism marked by significant increase in sizes zone of inhibition against MDR‐ESBL E. coli strains. In AgO2 ‐NPs and antibiotic‐treated cells, morphological changes were observed. Schreurs and Rosenberg [30] also reported changes in cell morphology in AgO2 ‐NPs‐treated bacterial cells because of increase in membrane permeability, which influence the cells capacity to appropriately manage the transport action through the plasma membrane for example, Ag weakens the uptake and release of phosphate ions in E. coli [30]. The functionalisation of AgO2 ‐NPs using antibiotics is not only a hopeful nano‐platform to combat bacterial resistance but may also trim down the dose and hence toxicity of the existing antibiotic against uro‐pathogenic strains [11, 12].
Table 3.
Antibacterial activity of antibacterial formulations against MDR‐ESBL E. coli
| Antibacterial formulation | Conc., µg/ml | Inhibition zone (mean ± SD), mm |
|---|---|---|
| ceftriaxone (CRO) | 10 | 12.0 |
| ceftriaxone (CRO) | 20 | 13.5 |
| (AgO2 ‐Nps) | 10 | 14.5 |
| (AgO2 ‐Nps) | 20 | 18.0 |
| (CRO + AgO2 ‐Nps) | 10 + 10 | 26.4 |
| (CRO + AgO2 ‐Nps) | 20 + 20 | 32.6 |
Fig. 10.

Synergism between synthesised AgO2 ‐NPs with ceftriaxone (CRO) against ESBL E. coli
3.2.5 Phenotypic changes in nanoparticle and antibiotic‐treated cells of E. coli
Cells of ESBL enzyme producing E. coli were treated with Ag nanoparticles synergistic formulation with antibiotic i.e. ceftriaxone. Morphological changes become noticeably clear upon treatment with antimicrobial formulation, phase contrast microscopic examination of treated E. coli cells showed cell lysis, while some giant and ghost cells were also evident in Fig. 11.
Fig. 11.
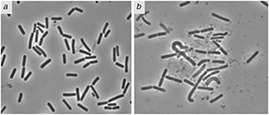
Effect of AgO2 nanoparticles with ceftriaxone on cellular morphology of E. coli
(a) control without treatment, (b) with treatment
4 Conclusion
AgO2 ‐NPs were synthesised using Ag foil as cheaper precursor. These AgO2 ‐NPs were utilised in combination with antibiotic. Their synergistic effect have been studied. Rather than treatment with antimicrobials alone, we additionally analysed antibiotic/AgO2 ‐NPs combinations. Antibiotics like ceftriaxone can be combined with AgO2 ‐NPs to achieve the treatment goals. Our results revealed a positive synergism marked by significant increase in sizes zone of inhibition against MDR‐ESBL E. coli strains. High rate of resistance was observed against ceftriaxone (100%). Treatments utilising antimicrobial nanoparticles seem to have promising additive or synergistic impacts on impending function of AgO2 ‐NPs to combat ESBL producing strains. The present study also concludes that there was a rise in ESBL‐producing uro‐pathogenic E. coli with blaSHV, blaCTX‐M, and both blaSHV and blaCTX‐M genetic polymorphism.
5 Acknowledgments
This work has been supported by International University Islamabad, Pakistan Institute of Engineering and Applied Sciences, Institute of Biomedical and Genetic Engineering Islamabad and Higher Education Commission of Pakistan.
6 References
- 1. Pitout J. D. D. Laupland K. B.: ‘Extended‐spectrum beta‐lactamase‐producing Entero‐bacteriaceae: an emerging public‐health concern’, Lancet Infect. Dis., 2008, 8, pp. 159 –166 [DOI] [PubMed] [Google Scholar]
- 2. Zorc J. J. Kiddoo D. A. Shaw K. N.: ‘Diagnosis and management of pediatric urinary tract infections’, Clin. Microbiol. Rev., 2005, 18, pp. 417 –422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knothe H. Shah P. Krcmery V. et al.: ‘Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiellapneumoniae and Serratiamarcescens ’, Infection, 1983, 11, pp. 315 –317 [DOI] [PubMed] [Google Scholar]
- 4. Bauernfeind A. Hörl G.: ‘Novel R‐factor borne beta‐lactamase of Escherichia coli confering resistance to cephalosporins’, Infection, 1987, 15, pp. 257 –259 [DOI] [PubMed] [Google Scholar]
- 5. Latifpour M. Gholipour A. Damavandi M.S.: ‘Prevalence of extended‐spectrum beta‐lactamase‐producing Klebsiellapneumoniae isolates in nosocomial and community‐acquired urinary tract infections’, Jundishapur J. Microbiol., 2016, 9, (3), p. e31179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perez F. Endimiani A. Hujer K.M. et al.: ‘The continuing challenge of ESBLs’, Curr. Opin. Pharmacol., 2007, 7, pp. 459 –469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoon K. Y. Byeon J. H. Park J.H. et al.: ‘Antimicrobial characteristics of silver aerosol nanoparticles against Bacillus subtilis ’, Science, 2008, 25, pp. 289 –293 [Google Scholar]
- 8. Durán N. Durán M. de Jesus M.B. et al.: ‘Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity’, Nanomedicine, 2016, 12, (3), pp. 789 –799 [DOI] [PubMed] [Google Scholar]
- 9. Boopathi S. Gopinath S. Boopathi T. et al.: ‘Characterization and antimicrobial properties of silver and silver oxide nanoparticles synthesized by cell‐free extract of a mangrove‐associated Pseudomonas aeruginosa M6 using two different thermal treatments’, Ind. Eng. Chem. Res., 2012, 51, (17), pp. 5976 –5985 [Google Scholar]
- 10. Negi H. Rathinavelu S. P. Agarwal T. et al.: ‘In vitro assessment of Ag2 O nanoparticles toxicity against gram‐positive and gram‐negative bacteria’, J. Gen. Appl. Microbiol., 2013, 59, pp. 83 –88 [DOI] [PubMed] [Google Scholar]
- 11. Wang X. Wu H.‐F. Kuang Q. et al.: ‘Shape‐dependent antibacterial activities of Ag2 O polyhedral particles’, Langmuir, 2009, 26, (4), pp. 2774 –2778 [DOI] [PubMed] [Google Scholar]
- 12. Martinez‐Guterirz F. Thi E. P. Silverman J. M.: ‘Antibacterial activity, inflammatory response, coagulation, and cytotoxicity effect of silver nanoparticles’, Nanomedicine, 2012, 8, (3), pp. 328 –336 [DOI] [PubMed] [Google Scholar]
- 13. Kar D. Bandyopadhyay S. Deba U. et al.: ‘Antibacterial effect of silver nanoparticles and capsaicin against MDR‐ESBL producing Escherichia coli: an in vitro study’, Microbiol. Res., 2016, 8, pp. 807 –810 [Google Scholar]
- 14. Jabeen N. Maqbool Q. Shamaila S. et al.: ‘Biosynthesis and characterization of nano‐silica as potential system for carrying streptomycin at nano‐scale drug delivery’, IET Nanobiotechnol., 2016, 11, pp. 557 –561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruden S. Hilpert K. Berditsch M. et al.: ‘Synergistic interaction between silver nanoparticles and membrane permeabilizing antimicrobial peptides’, Antimicrob. Agents Chemother., 2009, 53, pp. 3538 –3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi J. S. Lee H. Park Y.K. et al.: ‘Application of silver and silver oxide nanoparticles impregnated on activated carbon to the degradation of bromate’, J. Nanosci. Nanotechnol., 2016, 16, pp. 4493 –4497 [DOI] [PubMed] [Google Scholar]
- 17. Ji R. Wang L. Yu L. et al.: ‘Effective electrocatalysis based on Ag2 O nanowire arrays supported on a copper substrate’, ACS Appl. Mater. Interfaces, 2013, 5, pp. 10465 –10472 [DOI] [PubMed] [Google Scholar]
- 18. MacFaddin J. F.: ‘Biochemical tests for identification of medical bacteria’ (Williams and Wilkins, London, 2000) [Google Scholar]
- 19. Clinical and Laboratory Standards Institute : ‘Performance standards for antimicrobial susceptibility testing. Twenty second informational supplement update’, CLSI document M100‐S22 U (Clinical and Laboratory Standards Institute, Wayne, PA, 2012) [Google Scholar]
- 20. Drieux L. Brossier F. Sougakoff W. et al.: ‘Phenotypic detection of extended spectrum ‐lactamase production in Enterobacteriaceae: review and bench guide’, Clin. Microbiol. Infect., 2008, 14, (1), pp. 90 –103 [DOI] [PubMed] [Google Scholar]
- 21. Wilson K.: ‘Preparation of genomic DNA from bacteria’, Curr. Protoc. Mol. Biol., 2001, p. mb0204s56, DOI: 10.1002/0471142727 [DOI] [PubMed] [Google Scholar]
- 22. Edwards D. H. Errington J.: ‘The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division’, Mol. Microbiol., 1997, 24, (5), pp. 905 –915 [DOI] [PubMed] [Google Scholar]
- 23. Dakal T.C. Kumar A. Majumdar R.S. et al.: ‘Mechanistic basis of antimicrobial actions of silver nanoparticles’, Front. Microbiol., 2016, 7, p. 1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clark C. J. Kennedy W. A. Shortliffe L. D.: ‘Urinary tract infection in children: when to worry’, Urol. Clin. N. Am., 2010, 37, (2), pp. 229 –241 [DOI] [PubMed] [Google Scholar]
- 25. Gholipour A. Soleimani N. Shokri D. et al.: ‘Phenotypic and molecular characterization of extended‐spectrum beta‐lactamase produced by Escherichia coli, and Klebsiellapneumoniae isolates in an educational hospital’, Jundishapur J. Microbiol., 2014, 7, (8), p. 11758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mansor V. M. Shahid M. Evans J.T. et al.: ‘Occurrence, prevalence and genetic environment of CTX‐M b‐lactamases in Enterobacteriaceae from Indian hospitals’, J. Antimicrob. Chemother., 2006, 58, pp. 1260 –1263 [DOI] [PubMed] [Google Scholar]
- 27. Masroor H. Fariha H. Aamir S. A. et al.: ‘Prevalence of class A and AmpC b‐lactamases in clinical Escherichia coli isolates from Pakistan institute of medical science, Islamabad, Pakistan’, Jpn. J. Infect. Dis., 2011, 64, pp. 249 –252 [PubMed] [Google Scholar]
- 28. Canton R. Novais A. Valverde A. et al.: ‘Prevalence and spread of extended spectrum beta‐lactamase producing Enterobacteriaceae in Europe’, Clin. Microbiol. Infect., 2008, 14, (1), pp. 144 –153 [DOI] [PubMed] [Google Scholar]
- 29. Connor O.C. Roy K. Philip P. et al.: ‘The first occurrence of a CTX‐M ESBL producing Escherichia coli outbreak mediated by mother to neonate transmission in an Irish neonatal intensive care unit’, BMC Infect. Dis., 2017, 17, pp. 2 –7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schreurs W. J. Rosenberg H.: ‘Effect of silver ions on transport and retention of phosphate by Escherichia coli ’, J. Bacteriol., 1982, 152, (1), pp. 7 –13 [DOI] [PMC free article] [PubMed] [Google Scholar]


