Abstract
Borrelia burgdorferi infection in beagle dogs was studied quantitatively with skin punch biopsy samples and blood samples collected at 4- and 2-week intervals, respectively, over a 500-day period. Thereafter, 25 tissue samples of each dog were collected for further analysis. Starting at day 120 after tick challenge, 12 dogs were treated with antibiotics (azithromycin, ceftriaxone, or doxycycline) for 30 consecutive days. Four dogs received no antibiotic therapy. Quantification of B. burgdorferi DNA was done with an ABI Prism 7700 Sequence Detection System with oligonucleotide primers and a fluorescence-labeled probe designed to specifically amplify a fragment of the ospA gene of B. burgdorferi strain N40. All 16 dogs became infected with B. burgdorferi after tick challenge. In skin biopsy samples, spirochete numbers peaked at day 60 postinfection (<1.5 × 106 organisms per 100 μg of extracted DNA), at the same time when clinical signs of arthritis developed in 11 of 16 dogs, and decreased to almost undetectable levels during the following 6 months. The number of B. burgdorferi organisms detected in skin biopsy samples was inversely correlated with the antibody levels measured by enzyme-linked immunosorbent assay. Antibiotic treatment reduced the amount of detectable spirochete DNA in skin tissue by a factor of 1,000 or more. At the end of the experiment, B. burgdorferi DNA was detectable at low levels (102 to 104 organisms per 100 μg of extracted DNA) in multiple tissue samples regardless of treatment. However, more tissue samples of untreated dogs than of antibiotic-treated dogs were positive, and tissue samples of untreated dogs also were positive by culture. Only 1.6% of 576 blood samples of all dogs were positive for B. burgdorferi by PCR.
Lyme borreliosis or Lyme disease is caused by a group of bacteria species called Borrelia burgdorferi sensu lato (2, 15). The spiral-shaped organism is known to induce a variety of clinical manifestations in humans, particularly acute and chronic skin lesions, arthritis, pericarditis, and inflammation of the central and peripheral nervous systems (25). Analogous clinical signs may develop in animals, although in dogs, cows, and horses lameness due to arthritis is the hallmark of the disease (1, 21). During the blood meal of hard-shelled ticks of the genus Ixodes, the infectious agent is injected into the skin of the mammalian host (9) and then B. burgdorferi migrates into adjacent tissues, where it establishes a persistent infection that is not eliminated by the host immune system (23). The mechanisms by which the disease is initiated and maintained are not well defined, but it is known that B. burgdorferi is present in inflamed and chronically infected tissues (22) and that host factors also contribute to the pathogenesis of the infection (7, 8, 14, 27, 32).
A large number of people and animals throughout North America, Europe, and Asia become infected every year, but not all infected individuals develop clinically apparent disease. Estimates of the proportion of individuals who develop clinical signs range from approximately 5 to 50% (4, 16, 26). It is not clear what specific factor determines the outcome of the infection, although data from experiments with mice suggest that the overall number of B. burgdorferi organisms in tissue might be crucial for the development of an inflammatory response (20). Mouse strains (C3H) susceptible to severe Lyme arthritis harbored more spirochetes in comparable tissue samples than mouse strains (BALB/c) that become infected but that are less susceptible to severe arthritis (34), and therefore, the dose of infecting organisms may be the initial cause of severe inflammation.
B. burgdorferi organisms can be detected in clinical and experimental tissue samples by several techniques, especially by culture or PCR. Detection of B. burgdorferi by culture is accomplished with liquid medium, in which tissue samples are incubated for several weeks (3). The number of floating B. burgdorferi organisms in liquid medium is not a measure of the number of spirochetes originally present in the test sample, because gravity and clumping of the spirochetes result in an uneven distribution of the organisms within the medium. Recently, a new quantitative PCR (q-PCR) system has become available, which can be used to quantify B. burgdorferi organisms (20) and other microorganisms (11) in various specimens. In contrast to conventional PCR assays, DNA amplification is monitored throughout the reaction (real-time PCR) rather than just at the end of the test, when amplification kinetics might no longer be exponential. This avoids the distortion of quantitative relationships.
In this study, q-PCR was used to quantify B. burgdorferi populations in skin tissue and blood samples of beagle dogs collected sequentially over a period of more than 500 days. To determine whether the number of borrelia organisms is correlated with clinical disease and whether antibiotic therapy eliminates the organisms in tissue, three groups of four dogs were each treated with different antibiotics for a 30-day period, and data for these animals were compared to those for untreated dogs. This experimental model was used because Lyme borreliosis in dogs is very similar to the disease in humans (1, 28). Our studies have shown that despite a vigorous immune response of the dog, B. burgdorferi is not eliminated and the bacterium establishes a persistent infection, particularly in collagen-rich tissue (10). Application of this q-PCR to the canine model of Lyme disease provided the opportunity to investigate and monitor changes in B. burgdorferi populations in situ for an unprecedented time period.
MATERIALS AND METHODS
Dogs and tick exposure.
Experiments were conducted in compliance with regulations of the Animal Welfare Act and of the New York State Department of Health and were approved by the Institutional Animal Care and Use Committee. Specific-pathogen-free beagles of both sexes from the breeding colony of the James A. Baker Institute for Animal Health, Cornell University, were used for the study. After weaning, 16 6- to 8-week-old puppies were transferred into P2 units. Dogs were exposed to B. burgdorferi-bearing ticks, which had been collected in Westchester County, N.Y., 1 week prior to the first exposure. On day 0 of the experiment, 15 female and 7 male Ixodes scapularis ticks were placed on the left chest of each dog as described elsewhere (1). Ticks were allowed to engorge completely and were removed on day 7. At the same time, the dogs were immunized against canine distemper virus and canine parvovirus. To ensure that all dogs became infected with B. burgdorferi, exposure to the same number of ticks was repeated starting on day 14. Dogs maintained in groups of four were fed commercial dog food and water ad libitum. Clinical signs and body temperature were recorded daily, and body weight was recorded weekly.
Antibiotic therapy.
Four dogs (dogs N1 to N4) received no antibiotic treatment and were used as controls. Starting on day 120 of the experiment, three groups of four dogs each were treated with a different antibiotic for 30 consecutive days. The first group, dogs Azi1 to Azi4, was treated with 25 mg of azithromycin (Zithromax; Pfizer Laboratories, New York, N.Y.) per kg of body weight orally once a day. The second group, dogs Cef1 to Cef4, was treated with 25 mg of ceftriaxone (Rocephin; Hoffmann-La Roche, Nutley, N.J.) per kg intravenously once a day. The third group, dogs Dox1 to Dox4, received 10 mg of doxycycline (doxycycline hyclate capsules; Danbury Pharmacal Inc., Danbury, Conn.) per kg orally twice a day.
Serology.
Blood samples were collected at 2-week intervals beginning at day 0 of the experiment. After centrifugation at 200 × g for 30 min, serum was collected and was stored at −20°C. Sera were tested for B. burgdorferi antibody levels by a computerized kinetic enzyme-linked immunosorbent assay (KELA) as described elsewhere (24). Sera from each dog were tested together to correct for any interassay variability.
Isolation of B. burgdorferi from tissue samples.
Skin punch biopsy specimens (diameter, 4 mm) were collected sterilely under local anesthesia at 4-week intervals. Additional skin biopsy samples were collected 14 days after the initiation of the antibiotic therapy. Twenty-five different tissues were collected from each dog at necropsy, with frequent changes of instruments to avoid cross-contamination. Tissues collected at necropsy included skin from the left and right sides, synovial membranes from six joints (shoulder, elbow, and knee), muscle and fascia from front limbs (musculus triceps, fascia antebrachii), and hind limbs (musculus adductor, fascia lata), superficial cervical, axillary, and popliteal lymph nodes, pericardium, peritoneum, and meninges. Skin samples were ground in 0.2 ml of modified Barbour-Stoenner-Kelly medium containing kanamycin and rifampin (BSK-II plus KR) with a sterile pellet pestle and were placed into 6.5 ml of medium as described previously (1). Tissues retrieved postmortem were suspended in 3 ml of medium and were treated in a tissue homogenizer (Stomacher; Teckmar, Cincinnati, Ohio). The suspension was placed into 27 ml of prewarmed medium. The medium was incubated at 34°C for 5 weeks and was examined at 1, 3, and 5 weeks by dark-field microscopy for the presence of live spirochetes.
Addition of culture-derived B. burgdorferi organisms to WBCs of uninfected dogs.
To determine the number of spirochetes in tissue, a defined number of B. burgdorferi organisms was added to white blood cell (WBC) samples of uninfected dogs, which were used as standards for later PCR tests. Blood was drawn into EDTA-coated tubes, and the tubes were centrifuged at 200 × g for 30 min. WBCs were collected by aspiration. Aliquots of 100-μl amounts were dispensed into 1.5-ml microcentrifuge tubes. Borrelia grown in BSK-II plus KR medium at 34°C were counted with a Petroff-Hausser counting chamber. Dilutions that contained 106 to 10−1 spirochetes per ml were prepared with fresh medium. One hundred microliters of each dilution was added to WBC aliquots, resulting in a standard dilution series with from 105 to 10−2 B. burgdorferi organisms per 100 μl of canine WBCs.
Quantification of borrelia DNA in WBC and tissue samples. (i) DNA extraction.
To avoid a potential carryover contamination of the samples with previously amplified PCR products, DNA extractions, preparation of the amplification reactions, and amplifications were performed in three separate rooms with different sets of instruments. To monitor for potential carryover contamination among the test samples, tubes with water were distributed among the tubes with tissue samples and were handled in the same way as the tubes with tissue samples during DNA extraction and PCR. In addition, at the end of the test series DNA was extracted from 25 tissue samples of an uninfected dog with the instruments and reagents used for all other samples, and the DNA was subjected to PCR amplification. Total DNA from WBCs, tissues, and the positive control standards was extracted by the phenol-chloroform procedure. One hundred microliters of WBCs or tissue samples was digested in a solution containing 100 μl of proteinase K (1 mg/ml; Boehringer Mannheim, Mannheim, Germany), 150 μl of 1% sodium dodecyl sulfate solution (Sigma, St. Louis, Mo.), and 75 μl of β-mercaptoethanol (Sigma) in a 1.5-ml microcentrifuge tube. Digestion was carried out under constant shaking for 2 h at 55°C (WBCs) or 6 h at 55°C (skin biopsy and tissue samples). Subsequently, digests were transferred into phase-lock gel tubes (PLG I-H; Eppendorf 5 Prime → 3 Prime, Inc., Boulder, Colo.) and were mixed with 500 μl of 75% Tris-saturated phenol (pH 8.0; Sigma) and 25% chloroform-isoamyl alcohol (Fisher Scientific, Pittsburgh, Pa.). The organic phase was separated from the aqueous phase by centrifugation at 12,000 × g for 5 min, and the gel contained in the tube formed a barrier between the two phases. After transfer of the aqueous phase into new phase-lock gel tubes (PLG I-H), extraction was repeated with 500 μl of 50% phenol and 50% chloroform-isoamyl alcohol and once again with 500 μl of 100% chloroform-isoamyl alcohol. The supernatant was mixed with 50 μl of 3 M ammonium acetate (Sigma), and the DNA was precipitated with 2 volumes of cold 100% ethanol. The pellet was washed in 70% ethanol, dried under vacuum, and dissolved in 500 μl of water. DNA yield and purity were determined with a spectrophotometer (Beckman, Fullerton, Calif.) at three wavelengths: λ1 = 260 nm, λ2 = 280 nm, and λ3 = 320 nm.
(ii) DNA quantification.
B. burgdorferi-specific DNA was detected and quantified by using the ABI Prism 7700 Sequence Detection System (PE Biosystems, Foster City, Calif.). Primers and a probe were designed to anneal specifically to the ospA gene of B. burgdorferi strain N40, which was isolated regularly from the tick-challenged beagles. The design was done with Primer Express software, version 1.0, and oligonucleotides were synthesized by PE Biosystems (ospA-N40.seq-17F, 5′-AATGTTAGCAGCCTTGACGAGAA-3′; ospA-N40.seq-119R, 5′-GATCGTACTTGCCGTCTTTGTTT-3′; ospA-N40.seq-41T, 5′-FAM-AACAGCGTTTCAGTAGATTTGCCTGGTGA-TAMRA-3′). DNA was diluted 1:5 with distilled water. Titration of DNA showed that more than 1 μg of total DNA resulted in the inhibition of the PCR amplification. Five microliters of the DNA solution was used for subsequent PCR tests (approximately 30 to 300 ng of DNA per PCR tube). DNA amplification was carried out in 25-μl reaction volumes that, in addition to DNA and water, contained 1× Taqman Buffer A, 3.5 mM MgCl2, 900 mM each primer, 200 mM probe, 200 μM each deoxynucleoside triphosphate (dATP, dCTP, dGTP), 400 μM dUTP, 0.63 U of AmpliTaq Gold (0.025 U/μl), and 0.25 U of AmpErase UNG (uracil-N-glycosylase) (0.01 U/μl). All reagents except the primers and probe were included in the Taqman PCR Core Kit (PE Applied Biosystems). Amplification was performed in MicroAmp optical tubes by a standard amplification protocol recommended by the manufacturer (2 min at 50°C, 10 min at 95°C, and 40 cycles with 15 s at 95°C and 1 min at 60°C). During the reaction, signals of the FAM-label were recorded with a charge-coupled device camera controlled by Sequence Detection software, version 1.6. The amount of accumulated, free FAM in the reaction mixture, which the instrument can detect only in this form, is directly related to the amount of amplified DNA (17). The background noise of the fluorescent signals was monitored between cycles 3 and 15. The software calculated the mean and the standard deviation (SD) of the background noise and then placed a threshold 10 times the SD above the calculated mean. Every time that a linear ascent of an amplification plot crossed the previously calculated threshold, a new value defined as the threshold cycle (CT) was calculated. The CT value was inversely correlated to the amount of target DNA present in the test tube and was used by the software to calculate the number of B. burgdorferi organisms. The CT values for the unknown samples were compared to those obtained from the standard curve. Each 96-well test plate was equipped with a standard curve prepared with aliquots stored at −80°C. By using the spectrophotometric readings, the number of B. burgdorferi organisms was correlated with the amount of isolated DNA and was expressed as the number of spirochetes per 100 μg of extracted DNA.
(iii) Demonstration of amplified DNA by gel electrophoresis.
PCR products were separated in 12% polyacrylamide gels (Bio-Rad Laboratories, Hercules, Calif.), stained with ethidium bromide (Sigma), and visualized over a UV source.
Quantification of DNA from heat-killed B. burgdorferi organisms in skin punch biopsy samples by PCR.
To determine how long the DNA of B. burgdorferi organisms remains in mammalian tissue, heat-killed borrelia organisms were injected into the skin of an uninfected dog. Low-passage-number B. burgdorferi organisms (strain N40; a gift from A. R. Pachner, Newark, N.J.) were grown in culture for 7 days to a density of 1.2 × 107 bacteria per ml. Spirochetes in the culture medium were killed at 65°C for 1 h. Twenty-five 5-μl aliquots of this spirochete suspension (1.5 × 106 organisms total) were injected intradermally into the skin of an anesthetized dog. Injection sites were located on the left rib cage of the dog and were positioned 1 cm apart (area, 4 by 4 cm). Starting 1 day after inoculation, 4-mm skin punch biopsy samples were collected at weekly intervals under local anesthesia from the area where spirochetes had been injected previously. DNA extraction and PCR testing were done as outlined above.
RESULTS
Clinical signs.
After tick exposure, the dogs only rarely had an elevated temperature (≥39.4°C [103°F]), which lasted for 1 day and, on a single occasion, for 2 days. Six of 33 episodes of lameness were associated with elevated temperature. Following tick exposure, but before antibiotic treatment, 11 of 16 dogs developed clinically apparent arthritis in one or two limbs. The episodes of lameness lasted 3 to 6 days and resolved without treatment in all cases. The frequency of these episodes of joint inflammation varied among the dogs, with one to six episodes of lameness in the 11 clinically affected animals. The first episode of lameness occurred after a median incubation period of 71 days (range, 50 to 123 days after tick exposure) and developed in the left front leg in all dogs, the limb closest to the site of tick exposure. Subsequent bouts of lameness were observed in all extremities and were separated by 2- to 14-day intervals. After antibiotic therapy, only one ceftriaxone-treated dog (dog Cef3) showed two 1- and 2-day-long episodes of lameness starting on days 205 and 280 after tick exposure, while two of four untreated control dogs (dogs N3 and N2) developed one lameness episode each on days 123 and 169 after tick exposure, respectively.
Antibody response.
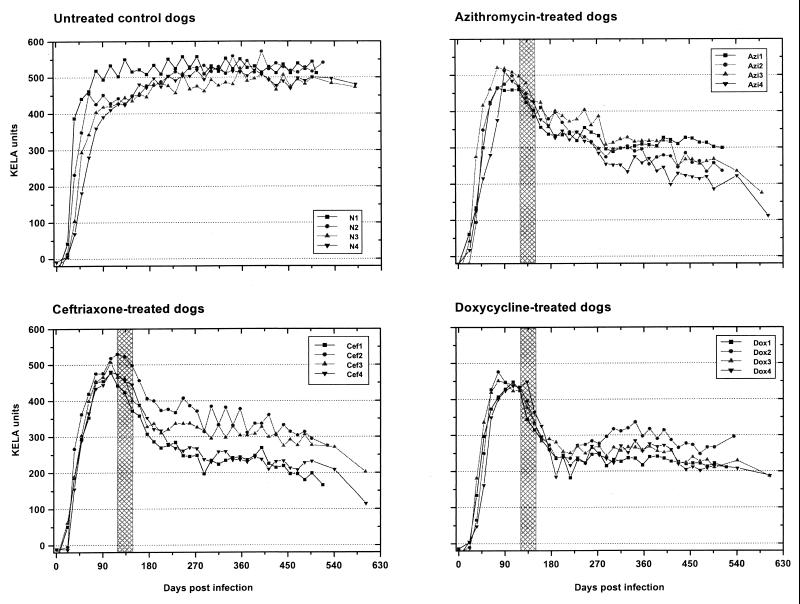
All dogs responded to the infection with B. burgdorferi and produced high antibody titers (400 to 500 KELA units) within 90 days of tick exposure (Fig. 1). Titers in the four untreated control dogs (dogs N1 to N4) continued to increase slightly thereafter, and the dogs retained these high levels throughout the experiment (505 to 581 days after the first tick exposure). All antibiotic-treated dogs, however, displayed a significant decrease in antibody titers due to antibiotic therapy. Seven of eight azithromycin- and ceftriaxone-treated dogs (the exception was dog Azi1) presented a steady persistent decline in antibody titers during the posttreatment period. Doxycycline-treated dogs responded to therapy, with a marked decrease in antibody titers during treatment and for another 30 days after cessation of treatment. Antibody levels then plateaued, with constant or slightly rising antibody titers throughout the remaining observation period.
FIG. 1.
KELA antibody responses of B. burgdorferi-infected beagles. Dogs were exposed to I. scapularis ticks on days 0 and 14. Subsequently, one group of four dogs was left untreated and three additional groups of four dogs were treated with either azithromycin (25 mg/kg orally once a day), doxycycline (10 mg/kg orally twice a day), or ceftriaxone (25 mg/kg intravenously once a day) for 30 consecutive days starting on day 120 after the first tick exposure (hatched area).
Culture.
Attempts were made to detect viable B. burgdorferi organisms by culture in 4-mm skin punch biopsy samples collected at 4-week intervals and in a set of 25 tissue samples taken from each dog during the necropsy. The results are summarized in Table 1 and 2. During the first 3 months after tick exposure, skin punch biopsy samples were uniformly positive by culture. By the 4th month and before the initiation of antibiotic treatment, only 50% of the skin punch biopsy samples were positive by culture. After antibiotic treatment, none of the skin punch biopsy samples and none of the postmortem tissue samples of dogs that had received azithromycin, ceftriaxone, or doxycycline were positive by culture. For all untreated control dogs (dogs N1 to N4), skin biopsy samples were sporadically positive by culture more than 120 days after tick exposure. At necropsy, 1 to 19 samples of 25 tissue samples were positive by culture for the four untreated control dogs (Table 2).
TABLE 1.
Comparison of culture and q-PCR results for skin punch biopsy samples taken close to the tick bite location at 4-week intervals
| Dog no. and testa | Skin punch biopsy samples collected after first tick challenge (in 4-wk intervals)
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before antibiotic treatment
|
During antibiotic treatment
|
After antibiotic treatment
|
|||||||||||||||||
| 1 | 2 | 3 | 4 | 4.5 | 5 | 5.5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| N1 | |||||||||||||||||||
| Culture | + | + | + | + | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 64,728 | 228,316 | 3,772 | 25,507 | 2,269 | 2,596 | 530 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N2 | |||||||||||||||||||
| Culture | + | + | + | + | + | + | − | + | − | + | + | + | − | − | − | − | − | − | − |
| q-PCR | 189,976 | 102,163 | 13,994 | 55,541 | 2,047 | 0 | 791 | 0 | 827 | 0 | 0 | 14,386 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N3 | |||||||||||||||||||
| Culture | + | + | + | + | − | − | − | + | − | − | − | − | − | − | − | − | − | + | − |
| q-PCR | 309,895 | 70,168 | 42,267 | 3,985 | 1,023 | 0 | 0 | 203 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N4 | |||||||||||||||||||
| Culture | + | + | + | − | + | − | − | − | + | − | − | + | − | − | − | − | − | − | − |
| q-PCR | 76,345 | 50,632 | 20,299 | 1,010 | 9,910 | 3,055 | 2,202 | 0 | 8,981 | 0 | 0 | 3,903 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Azi1 | |||||||||||||||||||
| Culture | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 80,526 | 105,656 | 13,819 | 5,704 | 0 | 0 | 0 | 0 | 0 | 643 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Azi2 | |||||||||||||||||||
| Culture | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 129,100 | 796 | 32,576 | 6,135 | 0 | 0 | 0 | 0 | 134 | 0 | 0 | 964 | 0 | 257 | 0 | 0 | 0 | 0 | 366 |
| Azi3 | |||||||||||||||||||
| Culture | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 369,877 | 61,001 | 6,444 | 25,519 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Azi4 | |||||||||||||||||||
| Culture | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 71,843 | 252,116 | 29,258 | 21,326 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1,826 | 0 | 0 | 0 | 0 | 0 | 0 | 539 |
| Cef1 | |||||||||||||||||||
| Culture | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 253,255 | 1,331,362 | 96,901 | 1,032 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cef2 | |||||||||||||||||||
| Culture | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 40,872 | 63,103 | 43,776 | 125,050 | 0 | 0 | 0 | 0 | 0 | 1,162 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cef3 | |||||||||||||||||||
| Culture | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 321,326 | 194,361 | 328 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cef4 | |||||||||||||||||||
| Culture | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 34,096 | 19,335 | 80,335 | 679 | 0 | 0 | 0 | 0 | 0 | 1,150 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 679 |
| Dox1 | |||||||||||||||||||
| Culture | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 270,608 | 358,555 | 4,998 | 6,689 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 988 | 0 | 0 |
| Dox2 | |||||||||||||||||||
| Culture | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 193,840 | 141,264 | 13,609 | 3,361 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dox3 | |||||||||||||||||||
| Culture | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 109,402 | 133,418 | 979 | 1,994 | 0 | 0 | 0 | 0 | 0 | 0 | 824 | 0 | 0 | 0 | 1,709 | 0 | 0 | 0 | 0 |
| Dox4 | |||||||||||||||||||
| Culture | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| q-PCR | 978 | 214,680 | 79,491 | 9,403 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 809 | 0 | 0 | 0 | 0 | 0 |
Culture results, +, positive; −, negative. q-PCR results: numbers of B. burgdorferi organisms per 100 μg extracted DNA.
TABLE 2.
Comparison of culture and q-PCR with 25 tissue samples collected from untreated dogs at necropsy
| Tissue | Detection of B. burgdorferi in untreated control dogs
|
|||||||
|---|---|---|---|---|---|---|---|---|
| N1
|
N2
|
N3
|
N4
|
|||||
| Culturea | q- PCRb | Culture | q- PCR | Culture | q- PCR | Culture | q- PCR | |
| Left skin | − | 0 | − | 0 | − | 0 | + | 188,698 |
| Left axillary ln.c | − | 0 | − | 0 | − | 0 | − | 0 |
| Left cervical ln. | − | 0 | − | 0 | − | 73 | − | 0 |
| Left popliteal ln. | − | 0 | − | 4,667 | − | 0 | + | 282 |
| Left shoulder | + | 1,393 | − | 0 | − | 0 | + | 1,285 |
| Left elbow | + | 0 | − | 0 | + | 0 | − | 0 |
| Left knee | − | 0 | − | 0 | + | 2,775 | + | 20,645 |
| Left front muscle | + | 0 | − | 0 | − | 0 | + | 0 |
| Left hind muscle | + | 0 | − | 0 | + | 0 | + | 0 |
| Left front fascia | + | 0 | − | 0 | + | 6,266 | + | 0 |
| Left hind fascia | + | 0 | − | 0 | + | 0 | + | 0 |
| Right skin | − | 0 | − | 0 | − | 0 | + | 11,823 |
| Right axillary ln. | − | 0 | − | 0 | − | 0 | − | 0 |
| Right cervical ln. | − | 0 | − | 0 | − | 0 | + | 0 |
| Right popliteal ln. | − | 0 | − | 0 | − | 0 | + | 129 |
| Right shoulder | + | 6,116 | − | 0 | − | 0 | + | 3,293 |
| Right elbow | − | 0 | − | 0 | − | 0 | + | 0 |
| Right knee | + | 0 | − | 0 | + | 0 | + | 0 |
| Right front muscle | + | 4,615 | − | 0 | + | 0 | + | 0 |
| Right hind muscle | + | 0 | − | 0 | − | 0 | + | 966 |
| Right front fascia | + | 0 | − | 0 | + | 2,537 | + | 0 |
| Right hind fascia | + | 3,602 | + | 2,029 | + | 0 | + | 0 |
| Pericardium | + | 0 | − | 0 | − | 0 | − | 0 |
| Peritoneum | + | 0 | − | 0 | + | 343 | + | 324 |
| Meninges | − | 0 | − | 0 | − | 0 | − | 0 |
Culture results: +, positive; −, negative.
q-PCR results: numbers of B. burgdorferi organisms per 100 μg of extracted DNA.
ln., lymph node.
PCR.
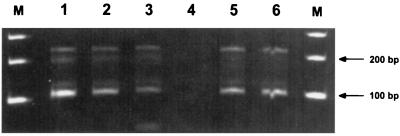
Gel electrophoresis of amplified products of the standard curve and skin tissues demonstrated that a fragment of the expected length of 104 bp was present and varied in signal strength according to the number of spirochetes present in the sample (Fig. 2). Interassay variation was assessed by calculating the means and the SDs of the CT values of the standard curve obtained from 16 separate tests. In a graph with a logarithmic x axis, the standard curve was described by a straight line: y = 46.913 − 4.170 · z [where y is the CT value, z is log10(x), and x is the number of B. burgdorferi organisms]. An inoculum of 105 B. burgdorferi organisms per 100 μl of WBCs resulted in a CT value of 25.92 ± 0.54 (mean ± SD), an inoculum of 104 organisms resulted in a CT value of 30.55 ± 0.71, an inoculum of 103 organisms resulted in a CT value of 34.20 ± 0.59, and an inoculum of 102 organisms resulted in a CT value of 38.61 ± 1.08. The detection limit of the test was reached with an inoculum of 100 spirochetes per 100 μl of WBCs, which equaled approximately one organism per PCR sample. At this spirochete concentration, CT values for duplicate samples ranged between 36 and 40 cycles, reflecting the fact that only single copies of B. burgdorferi DNA (∼36 cycles) or no DNA copies (40 cycles, indicating no specific DNA amplification) were present in the sample.
FIG. 2.
DNA signals for the ospA gene of B. burgdorferi after amplification with the ABI Prism 7700 Sequence Detection System. Lanes 1 to 4, signals produced with 105 to 102 B. burgdorferi organisms per 100 μl of WBCs. DNA was extracted from the entire sample, but only 0.2% of the total amount was used for a single PCR. Lanes 5 and 6, signals from two skin punch biopsy samples taken 4 and 8 weeks after tick exposure; lane M, 100-bp marker.
The amount of DNA present in the sample was determined by spectrophotometry, and the results were expressed as the numbers of B. burgdorferi per 100 μg of extracted total DNA, which naturally contained mainly canine DNA.
In skin punch biopsy samples, maximal numbers of B. burgdorferi organisms were found between 30 and 60 days after infection. Up to 1.3 × 106 spirochetes per 100 μg of DNA were detected during this period. During the following 4 weeks, spirochete density in skin samples decreased approximately 10-fold. PCR results for these samples paralleled the culture data with only one exception, in which PCR was positive and culture was negative (Table 1). At 4 months after tick exposure, the number of spirochetes in skin biopsy samples had declined and the spirochete concentration ranged between 102 and 104 spirochetes per 100 μg of DNA. Only 50% of the matching skin samples taken at the same time and from the same area were positive by culture (Table 1). For the untreated dogs, the numbers of spirochetes dropped to levels at which B. burgdorferi organisms were detected only sporadically by culture or PCR. Antibiotic treatment resulted in the temporary disappearance of B. burgdorferi DNA. Skin samples became positive by PCR starting 60 days after treatment had ended, and additional positive samples were detected later. No viable spirochetes were recovered by culture after antibiotic treatment.
Blood samples collected at 2-week intervals and analyzed in parallel with the other samples were an unreliable source for B. burgdorferi detection. Blood samples were found to be positive by PCR in only four dogs. For two of these dogs (dogs Dox3 and Azi4) three samples were positive, and the positive samples were found during all phases of the experiment. The numbers of spirochetes ranged from 610 to 15,295 per 100 μg of extracted DNA. Two samples from one azithromycin-treated dog (dog Azi2) were positive, with 3,677 and 584 organisms per 100 μg of DNA on days 35 and 176 after tick exposure, respectively, and one sample from one ceftriaxone-treated dog (dog Cef1) was positive, with 2,133 organisms on day 455 after tick exposure.
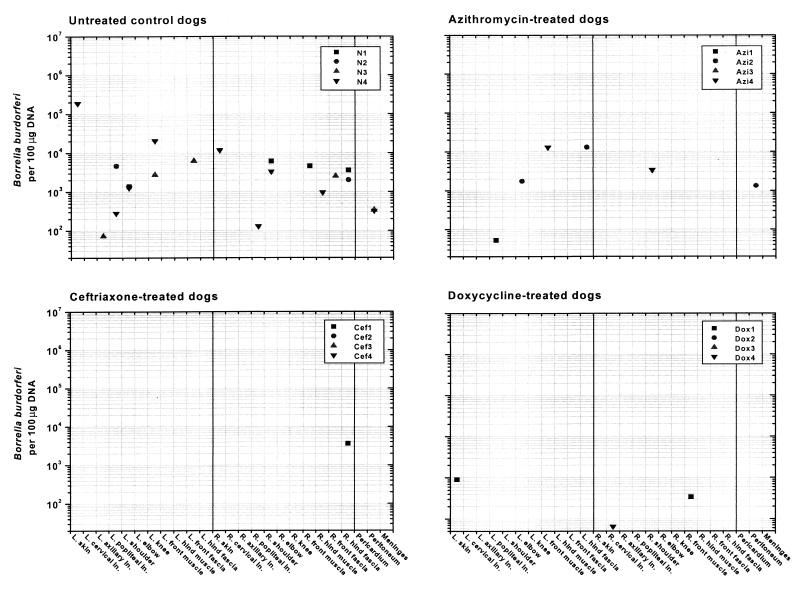
At necropsy, 505 to 604 days after tick exposure, untreated control dogs (dogs N1 to N4) harbored B. burgdorferi DNA in multiple tissues (Table 2 and Fig. 3). The numbers of organisms ranged from 73 to 188,698 spirochetes per 100 μg of DNA. In contrast, only 50% of the antibiotic-treated dogs were positive for B. burgdorferi by PCR, and only one to three tissue samples per dog were positive. The amount of spirochetal DNA detected in these samples was equivalent to 53 and 31,266 spirochetes per 100 μg of extracted total DNA.
FIG. 3.
Quantification of B. burgdorferi in tissues from untreated and antibiotic-treated dogs tested between 505 and 605 days after tick exposure. B. burgdorferi was frequently found in the untreated dogs, as shown by PCR and culture. Only a few samples from antibiotic-treated dogs were positive by PCR. Note that 0-value data (0 spirochetes per 100 μg of DNA) are not depicted. L., left; R., right; ln, lymph node.
Detection of heat-killed organisms by PCR.
A total of 1.5 × 106 heat-killed, low-passage B. burgdorferi organisms (strain N40) were injected into the skin of an uninfected beagle. Ten sequential skin punch biopsy samples were taken at weekly intervals starting 1 day after injection. PCR analysis revealed that B. burgdorferi DNA was detectable up to 3 weeks after injection. During that time the amount of detected DNA equaled 242 spirochetes (day 1 after injection) and 78 spirochetes (22 days after injection) per 100 μg of extracted DNA. No DNA was detected on days 8 and 15 after injection.
DISCUSSION
PCR has become a common tool for the detection of organisms that are present at low concentrations in tissue specimens. However, by conventional PCR techniques, which determine the amount of DNA after a certain number of amplification cycles, it is difficult and cumbersome to quantify the number of organisms present in the samples. Real-time PCR, as it was used in this study, monitors DNA amplification throughout the course of the reaction and therefore allows a rapid, consistent, reliable, and accurate quantification of DNA. In this study rapidness was achieved, because up to 86 samples (not considering positive and negative controls on a 96-well plate) were tested within 2 hours. The assay was consistent, because results were almost identical when aliquots of the samples were tested repeatedly. Unlike in conventional PCR techniques, no postamplification handling of DNA, such as loading, staining, and interpretation of gels, or even reamplification of DNA (nested PCR assays) was necessary. The assay demonstrated reliability, because this technique was less prone to the production of false-positive results. The assay incorporated a contamination prevention system based on the enzymatic activity of uracil N-glycosylase, which destroys carryovers of previously amplified DNA products containing uridine instead of thymidine (18). Amplified products remained in the reaction tubes, since they were not used for gel electrophoresis, and were less likely to contaminate further samples of subsequent tests. Finally, this new technique produced accurate results. The quantity of spirochetes per 100 μg of total extracted DNA appeared to range within biologically acceptable limits and is in accordance with results from other laboratories. Morrison et al. (19) found approximately 1 to 1,000 spirochetes in mouse tissue using 200 ng of DNA, and Pahl et al. (20) found up to 10,000 organisms per 106 mouse cells. However, numbers of organisms are influenced by the method by which the amount of DNA is normalized. Morrison et al. (19) and Pahl et al. (20) relied on the amplification of host genes such as nidogen (19) or β-actin (20) in separate reactions. In this study, DNA quantity and purity were determined spectrophotometrically, a method that produced reliable and repeatable results in my hands.
When large sets of tissues from untreated dogs were tested by PCR and culture, it became evident that PCR is not superior to culture in terms of sensitivity, especially after long-term infection. For untreated dogs more tissue samples were positive by culture than by PCR (Table 2). This result is not so surprising when the amount of sample used for a single test is considered. The entire tissue sample was suspended in culture medium, but only 0.2% of the total extracted DNA was added to a single PCR test tube, because larger amounts of DNA resulted in PCR inhibition. Considering the low-level infection at the end of the study, which stochastically resulted in the presence of DNA of a few organisms per PCR mixture, it is not surprising that larger sample size (tissues in culture) resulted in a higher frequency of detection of B. burgdorferi. Another point needs to be taken into consideration: in this study culture and PCR results for two different skin biopsy samples, although taken in close proximity, are compared. Whether one sample is positive and the other is negative and vice versa after long infection periods cannot be answered. For antibiotic-treated dogs, cultures were uniformly negative after treatment. Why there is such a discrepancy between culture and PCR needs further investigation. However, recent observations suggest that under certain conditions B. burgdorferi can convert into cysts (6), which probably are more difficult to culture than regular spiral-shaped organisms.
An important finding in this study is that shortly after tick exposure the number of B. burgdorferi organisms increased dramatically in skin punch biopsy samples which were taken close to the area where tick bites had occurred. Starting at day 90 after tick exposure, a progressive decrease in the number of organisms was observed in skin biopsy samples, while specific antibody levels against the spirochete increased steadily. Approximately 90 to 180 days after tick exposure, antibody levels had reached maximal levels and spirochetes were detected only sporadically in skin tissue samples. Interestingly, episodes of Lyme arthritis, the most common clinical sign observed in dogs, developed between 50 and 169 days after tick exposure or prior to antibiotic therapy. Remarkably, all dogs which became lame in this study (12 of 16 infected dogs) developed the first episode of lameness in the joint closest to the tick bite (the left front quadrant), namely, in the shoulder and elbow of the left front quadrant. Considering the general postulation that B. burgdorferi disseminates via the bloodstream (13, 33), it would be expected that arthritis would develop with the same probability for all joints. A preference for nearby joints might be the result of active migration of B. burgdorferi through tissue rather than passive dissemination by blood. Early during infection, large numbers of the organisms were detected in skin tissue, while organisms were rarely found in the blood of infected animals, indicating the local presence of many organisms during the early phase of the infection. Further spread of the organisms by migration probably results in an expansion of the skin lesion described as erythema migrans in humans and rabbits (12, 30). It can be expected that migration in all spatial dimensions results in the colonization of deeper tissue such as synovial membranes in joints. Our previous studies have shown that at the time when the numbers of B. burgdorferi organisms decline in skin biopsy samples (about 90 days after tick exposure) they have already reached the closest joints, but spirochetes are not evenly distributed in the body of the dog (27). Similar observations were made by Pahl et al. (20). They found, first, that B. burgdorferi organisms were not evenly distributed in the mouse body early after infection and, second, that spirochete concentrations were higher in certain tissues of C3H mice, a mouse strain more susceptible to arthritis than strains with a different genetic background. These data emphasize results published by Yang et al. (34), in which large numbers of spirochetes in tissues were found to be associated with arthritis in certain mouse strains.
Similar to the host immune response, therapy with different antibiotics seems to reduce the load of B. burgdorferi infection to a level of approximately 53 to 13,078 spirochetes per 100 μg of extracted total DNA but fails to eliminate the infection. This is not surprising and was documented by our and other groups previously (20, 29, 31). In this study organisms were not recovered by culture. However, live spirochetes may have been present in antibiotic-treated dogs. DNA of heat-killed borrelia was not detectable for very long in skin tissue of an uninfected dog, implying that during natural infection the DNA of killed organisms is removed quickly and completely within a few days. This was the first controlled study in which animals were treated with antibiotic after a relatively long infection period (120 days after tick exposure), at a time when high antibody titers were present. After antibiotic therapy had ended, in some treated dogs antibody titers remained at constant levels rather than decreasing further. This argues more for the persistence of the antigenic stimulus than for the complete elimination of B. burgdorferi. Whether B. burgdorferi survives antibiotic therapy by forming viable cysts (5, 6) or by other mechanisms merits further investigation.
In summary, real-time PCR allowed a quantitative insight into the host-bacterium interaction in canine Lyme borreliosis: (i) it was shown that the number of B. burgdorferi organisms changed over time in a given tissue sample, (ii) the data suggest that clinical signs of arthritis develop at a time when large numbers of B. burgdorferi organisms are present in the skin, and (iii) antibiotic therapy reduced the load of B. burgdorferi organisms in the host but failed to eradicate the agent. This technique will benefit future studies designed to solve the exact mechanisms by which B. burgdorferi establishes a persistent infection and triggers an inflammatory response in tissue.
ACKNOWLEDGMENTS
This study was supported by the Tick Borne Disease Institute, State of New York Department of Health (contract C011798).
The excellent technical assistance of Mary Beth Matychak and Patti Easton is greatly appreciated. I am also grateful to R. H. Jacobson, B. A. Summers, G. Lust, and J. N. MacLeod for support and helpful discussions.
REFERENCES
- 1.Appel M J G, Allen S, Jacobson R H, Lauderdale T-L, Chang Y-F, Shin S J, Thomford J W, Todhunter R J, Summers B A. Experimental Lyme disease in dogs produces arthritis and persistent infection. J Infect Dis. 1993;167:651–664. doi: 10.1093/infdis/167.3.651. [DOI] [PubMed] [Google Scholar]
- 2.Baranton G, Postic D, Saint Girons G, Boerlin P, Piffaretti J-C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme disease. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 3.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Berglund J, Eitrem R, Norrby S R. Long-term study of Lyme borreliosis in a highly endemic area in Sweden. Scand J Infect Dis. 1996;28:473–478. doi: 10.3109/00365549609037943. [DOI] [PubMed] [Google Scholar]
- 5.Brorson Ø, Brorson S H. Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection. 1997;25:240–246. doi: 10.1007/BF01713153. [DOI] [PubMed] [Google Scholar]
- 6.Brorson Ø, Brorson S H. In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection. 1998;26:144–150. doi: 10.1007/BF02771839. [DOI] [PubMed] [Google Scholar]
- 7.Brown C R, Reiner S L. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect Immun. 1999;67:1967–1973. doi: 10.1128/iai.67.4.1967-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J P, Zachary J F, Teuscher C, Weis J J, Wooten R M. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 10.Chang Y-F, Straubinger R K, Jacobson R H, Kim J B, Kim T J, Kim D, Shin S J, Appel M J G. Dissemination of Borrelia burgdorferi after experimental infection in dogs. J Spirochetal Tick-Borne Dis. 1996;3:80–86. [Google Scholar]
- 11.Desjardin L E, Chen Y, Perkins M D, Teixeira L, Cave M D, Eisenach K D. Comparison of the ABI 7700 system (TaqMan) and competitive PCR for quantification of IS6110 DNA in sputum during treatment of tuberculosis. J Clin Microbiol. 1998;36:1964–1968. doi: 10.1128/jcm.36.7.1964-1968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley D M, Gayek R J, Skare J T, Wagar E A, Champion C I, Blanco D R, Lovett M A, Miller J N. Rabbit model of Lyme borreliosis: erythema migrans, infection-derived immunity, and identification of Borrelia burgdorferi proteins associated with virulence and protective immunity. J Clin Invest. 1995;96:965–975. doi: 10.1172/JCI118144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman J L, Bradley J F, Ross A E, Goellner P, Lagus A, Vitale B, Berger B W, Luger S, Johnson R C. Bloodstream invasion in early Lyme disease: results from a prospective, controlled, blinded study using the polymerase chain reaction. Am J Med. 1995;99:6–12. doi: 10.1016/s0002-9343(99)80097-7. [DOI] [PubMed] [Google Scholar]
- 14.Härter L, Straubinger R K, Summers B A, Erb H N, Appel M J G. Up-regulation of inducible nitric oxide synthase mRNA in dogs experimentally infected with Borrelia burgdorferi. Vet Immunol Immunopathol. 1999;67:271–284. doi: 10.1016/s0165-2427(98)00231-1. [DOI] [PubMed] [Google Scholar]
- 15.Johnson R C, Schmidt G P, Hyde F W, Steigerwald A G, Brenner D J. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 16.Levy S A, Lissman B A, Ficke C M. Performance of a Borrelia burgdorferi bacterin in borreliosis-endemic areas. J Am Vet Med Assoc. 1993;202:1834–1838. [PubMed] [Google Scholar]
- 17.Livak K, Flood S, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- 18.Longo M C, Berninger M S, Hartley J L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990;93:125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- 19.Morrison T B, Ma Y, Weis J H, Weis J J. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissue by continuous fluorescent monitoring of PCR. J Clin Microbiol. 1999;37:987–992. doi: 10.1128/jcm.37.4.987-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahl A, Kühlbrandt U, Brune K, Röllinghoff M, Gessner A. Quantitative detection of Borrelia burgdorferi by real-time PCR. J Clin Microbiol. 1999;37:1958–1963. doi: 10.1128/jcm.37.6.1958-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker J L, White K K. Lyme borreliosis in cattle and horses: a review of the literature. Cornell Vet. 1992;82:253–274. [PubMed] [Google Scholar]
- 22.Picken M M, Picken R N, Han D, Cheng Y, Ruzic-Sabljic E, Cimperman J, Maraspin V, Lotric-Furlan S, Strle F. A two year prospective study to compare culture and polymerase chain reaction amplification for the detection and diagnosis of Lyme borreliosis. Mol Pathol. 1997;50:186–193. doi: 10.1136/mp.50.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiler K P, Weis J J. Immunity to Lyme disease: protection, pathology and persistence. Curr Opin Immunol. 1996;8:503–509. doi: 10.1016/s0952-7915(96)80038-0. [DOI] [PubMed] [Google Scholar]
- 24.Shin S J, Chang Y-F, Jacobson R H, Shaw E, Lauderdale T-L, Appel M J G, Lein D H. Cross-reactivity between B. burgdorferi and other spirochetes affects specificity of serotests for detection of antibodies to the Lyme disease agent in dogs. Vet Microbiol. 1993;36:161–174. doi: 10.1016/0378-1135(93)90137-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 26.Steere A C, Taylor E, Wilson M L, Levin J F, Spielman A. Longitudinal assessment of the clinical and epidemiological features of Lyme disease in a defined population. J Infect Dis. 1986;200:344–347. doi: 10.1093/infdis/154.2.295. [DOI] [PubMed] [Google Scholar]
- 27.Straubinger R K, Straubinger A F, Härter L, Jacobson R H, Chang Y-F, Summers B A, Erb H N, Appel M J G. Borrelia burgdorferi migrates into joint capsules and causes an up-regulation of interleukin-8 in synovial membranes of dogs experimentally infected with ticks. Infect Immun. 1997;65:1273–1285. doi: 10.1128/iai.65.4.1273-1285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straubinger R K, Straubinger A F, Summers B A, Jacobson R H, Erb H N. Clinical manifestation, pathogenesis, and effect of antibiotic treatment on Lyme borreliosis in dogs. Wien Klin Wochenschr. 1998;110:874–881. [PubMed] [Google Scholar]
- 29.Straubinger R K, Summers B A, Chang Y-F, Appel M J G. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol. 1997;35:111–116. doi: 10.1128/jcm.35.1.111-116.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Mierlo P, Jacob W, Dockx P. Erythema chronicum migrans: an electron-microscopic study. Dermatology. 1993;186:306–310. doi: 10.1159/000247384. [DOI] [PubMed] [Google Scholar]
- 31.Weber K. Treatment failure in erythema migrans: a review. Infection. 1996;24:73–75. doi: 10.1007/BF01780663. [DOI] [PubMed] [Google Scholar]
- 32.Weis J J, McCracken B A, Ma Y, Fairbairn D, Roper R J, Morrison T B, Weis J H, Zachary J F, Doerge R W, Teuscher C. Identification of quantitative trait loci governing arthritis severity and numeral responses in the murine model of Lyme disease. J Immunol. 1999;162:948–956. [PubMed] [Google Scholar]
- 33.Wormser G P, Liveris D, Nowakowski J, Nadelman R B, Cavaliere L F, McKenna D, Holmgran D, Schwartz I. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–725. doi: 10.1086/314922. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]