Abstract
Mounting‐up economic losses to annual crops yield due to micronutrient deficiency, fertiliser inefficiency and increasing microbial invasions (e.g. Xanthomonas cempestri attack on tomatoes) are needed to be solved via nano‐biotechnology. So keeping this in view, the authors’ current study presents the new horizon in the field of nano‐fertiliser with highly nutritive and preservative effect of green fabricated zinc oxide‐nanostructures (ZnO‐NSs) during Lycopersicum esculentum (tomato) growth dynamics. ZnO‐NS prepared via green chemistry possesses highly homogenous crystalline structures well‐characterised through ultraviolet and visible spectroscopy, Fourier transform infrared spectroscopy, X‐ray diffraction and scanning electron microscope. The ZnO‐NS average size was found as small as 18 nm having a crystallite size of 5 nm. L. esculentum were grown in different concentrations of ZnO‐NS to examine the different morphological parameters includes time of seed germination, germination percentage, the number of plant leaves, the height of the plant, average number of branches, days count for flowering and fruiting time period along with fruit quantity. Promising results clearly predict that bio‐fabricated ZnO‐NS at optimum concentration resulted as growth booster and dramatically triggered the plant yield.
Inspec keywords: zinc compounds, II‐VI semiconductors, wide band gap semiconductors, nanostructured materials, nanofabrication, ultraviolet spectra, visible spectra, Fourier transform infrared spectra, X‐ray diffraction, scanning electron microscopy, crystallites, biomedical materials, nanomedicine
Other keywords: ZnO‐nanofertiliser, green chemistry, boosted growth dynamics, L. esculentum, mounting‐up economic losses, micronutrient deficiency, fertiliser inefficiency, microbial invasions, Xanthomonas cempestri, nanobiotechnology, zinc oxide‐nanostructures, Lycopersicum esculentum, high‐homogenous crystalline structures, ultraviolet spectroscopy, visible spectroscopy, Fourier transform infrared spectroscopy, X‐ray diffraction, scanning electron microscopy, crystallite size, morphological parameters, seed germination, germination percentage, plant leaves, ZnO
1 Introduction
The number of people below poverty line is increasing logarithmically and about 8 billion people around the globe are suffering from acute food shortage [1]. The traditional plant fertilisers applied exhibits multiple limitations such as high dosage requirement, environmental hazards such as soil and water pollution, cytotoxicity for other species etc. Excess use of nitrogen and phosphate‐rich fertilisers may cause water spills (due to higher organic contents and algal blooms), which ultimately affect the fresh water resources and make them contaminated. Each year agriculturalists have to plan huge amount of budget to manage fertiliser demands, resulting in loss of economic values [2].
Zinc being essential plant nutrients play a vital role in maintaining plant cellular metabolism. Biocatalysts which become functional through zinc ionic species are concerned with hydrocarbons metabolism, maintenance of cellular membranes, leaf morphology, physiology of membrane, protein production etc. [3, 4]. Zinc deficiency results in the arrested growth, loss of water uptake, chlorosis, reduction in leaves size, malfunctioning of flowers and some time loss of fertility [5]. It is being observed that zinc also plays an important role to tune the plant behaviour for in time response in several kinds of stresses by light energy, temperature and fungal infections and it also reduces the adverse effects of salt and heat shock [5]. However, in bulk form, zinc (Zn) performance was found limited due to the smaller specific surface area and lower surface energy ratio.
In modern biotechnological advancements, work on nanostructure (NS) is one of the eye‐catching and dynamic regions of research. NS encloses a wide range of modern applications due to their extremely sized, tuned structure and physio‐chemical properties in agriculture industries [6, 7]. Although there are so many routes to engineer application targeted NS but plants were proved to be the best choice due to least cytotoxicity and greater biocompatibility [8]. Evolution in green chemistry had resulted in the development of traditional agriculture methodologies to modern industrial agriculture in the last decade. Among all reported metallic oxide NSs so far, Zn oxide‐NS (ZnO‐NS) being a noteworthy type of metal oxide NS is broadly used in gas sensors, solar energy conversion, catalysis, bio‐medical applications and high‐temperature sensitive superconductors with sole features and superior efficiency [9].
Lycopersicum esculentum placed with the family Solanaceae (nightshade family), which is a diploid plant having chromosomes numbers 2n = 24 and fall into the category of perennial herbaceous plant. L. esculentum usually grows to the height of 2–3 m with weak and short woody stem having yellow flowers. Fruits vary in size from 1 to 10 cm in diameter showing red colour when fully ripen [10]. L. esculentum is as a significant nutritive crop around the globe holding the rich source of vitamin C, A and lycopene compound, which is a potent antioxidant having the capability to protect the cellular environment from free radicals (OH−, O2 − etc.) damage [11].
Keeping in view the agronomics of L. esculentum, our team has followed a facile green fabrication route for ZnO‐NS engineering. The purpose of this paper is to elaborate and understand the biocompatibility, cytotoxicity and supplementary behaviour of photo‐synthesised ZnO‐NS on L. esculentum. The main growth dimensions which were investigated include seed germination time/percentage, number of leaves, height of the plant and totality of number of branches, flowering time period and fruiting time period along with fruit quantity. All of the plants were also separately studied to observe any kind of morphological modifications. It is promisingly said that our work would open new horizons in the field of nano‐biotechnology.
2 Experimental Section
2.1 Facile bio‐synthesis of ZnO‐NS through Olea europaea leaf extract
The certified O. europaea leaves were collected from Barani Agricultural Research Institute, Chakwal. To make leaf extract, 40 g of shade‐dried leaves were finely grinded and put in 400 ml of dH2 O. The prepared mixture was put in shaking incubator for 2.5 h set at 40°C and 50 rpm. In the next step, mixture was filtered using Whatman filter paper no. 1. About 4.38 g of Zn(CH3 COO)2 · 2H2 O (Sigma‐Aldrich) was added in 200 ml of the leaf extract and placed at Scilogex‐magnetic stirrer set to 50°C at ∼1500 rpm for ∼3 h. Thereafter, the reaction mixture was repeatedly loaded in GR‐BioTek centrifuge at ∼12,000 rpm for 10 min; pellets were deposited at the bottom of the tubes and were collected. To completely washout uncoordinated bioactive compounds, washing with dH2 O was performed thrice. ZnO‐NS separated out was dried using Memmert‐hot air oven at 60°C for ∼6 h. Furthermore, to improve the crystallisation of as‐prepared ZnO‐nano‐powder, calcinations were performed in Gallenkamp‐furnace at 400°C for ∼3 h.
2.2 Characterisation of bio‐synthesised ZnO‐NS
Surface morphology of green‐synthesised ZnO‐NS was examined through scanning electron microscope (SEM) analysis using JOEL JSM 6490LASEM operating at 20 kV [together with energy dispersive X‐ray (EDX) spectroscopy]. Crystallographic nature of bio‐synthesised ZnO‐NS was accomplished using copper (Cu)‐Kα radiation [λ (wavelength) = 1.54060 Å] with nickel monochromator in the range of 2θ between 20° and 80° with step size 0.2°. Scherrer's formula (1) is used to calibrate the average crystallite size. In addition, to evaluate the optical and vibrational characterisation of as‐prepared ZnO‐NS samples, ultraviolet–visible (UV–vis) absorption spectroscopy [PerkinElmer Lambda 200 UV–vis/near infrared (IR)] and Fourier transform IR (FTIR) spectroscopy (SHIMADZU FTIR) of ZnO‐NS were carried out using potassium bromide pellet technology in the wave number ranging 400–4000 cm−1.
2.3 Collection and processing of plant material
Mature seeds of L. esculentum mill variety (money maker) were collected from National Agricultural Research Centre, Islamabad, Pakistan. First of all, seeds were soaked in ddH2 O for 24 h at room temperature to break their dormancy and then dried on filter paper. For sterilisation purpose, seeds were washed with detergent and distiled water for 1–2 min. After that seeds were washed with 70% ethanol for 2 min. Then, seeds were placed in 50% Clorox solution and washed again with ddH2 O for 3 min. All glassware and tools were autoclaved before seed surface sterilisation in order to avoid any contamination.
2.4 Treatment of L. esculentum seeds with ZnO‐NS
Treatment concentrations of ZnO‐NS suspensions were 0.01, 0.03 and 0.05% v/v along with the untreated seeds as controlled one. About 20 seeds were added in each suspension and placed for 7–8 h and were dried on filter paper.
After treatment with ZnO‐NS suspension, seeds were shifted to pots. About 40 pots were taken and filled with peat moss, soil and sand in a ratio of 1:1:1. The pots were kept in green house. Ten pots were taken for each treatment. About 10 seeds from each treatment were taken and sown in pots. There was one seed per pot. L. esculentum seeds were kept at a depth of 1–2 cm and seeds were covered with the soil. Pots were irrigated regularly. Seeds were germinated after 15 days. In ten pots, untreated seeds as controlled were sown. The pots were regularly irrigated with care.
Different parameters and morphological characters were observed, which includes germination percentage, time of germination, the height of the plant, number of leaves, number of branches per plant, number of days to flowering, number of days to fruiting and number of fruits per plant.
2.5 Statistical analysis
Statistical analysis of the data was performed by two factorial analysis of variance (Statistix 8.1 software).
3 Results
3.1 Bio‐fabrication process of ZnO‐NS, visual observation
Fig. 1 broadly elaborates green synthesis of ZnO‐NS to perform targeted application in agriculture biotechnology (nano‐fertiliser). Facile synthesis route lacks the use of hazardous acid–base chemicals which make the process environmentally friendly and eco‐friendly. ZnO‐NS prepared via green chemistry exhibits more biocompatibility and least cytotoxicity.
Fig. 1.
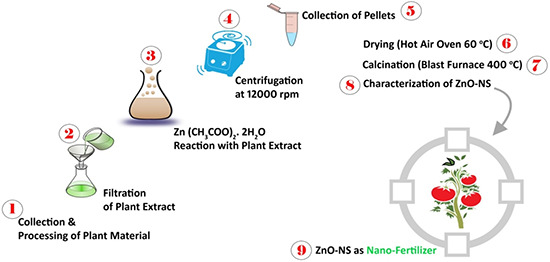
Step‐wise schematic explanation of ZnO‐NS green synthesis
3.2 Characterisation of prepared ZnO‐NS
As of the SEM micrograph investigations (Fig. 2), it is quite much obvious that ZnO‐NS prepared via green chemistry route are highly homogenous and spherical in shape owing average particle size of 18 ± 5 nm. EDX analysis is shown in Fig. 3 depicts the elemental percentage (i.e. by mass, 74.41% Zn and 25.59% O) of green‐synthesised ZnO‐NS. EDX findings further confirmed that only Zn and O ions are traced in the bio‐synthesised NS with the same composition as marked at the era of the experiment. No phase impurities of any kind were found in the prepared ZnO‐NS.
Fig. 2.
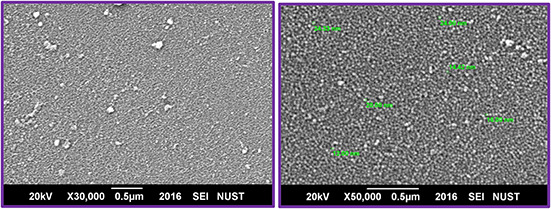
SEM micrographs of bio‐fabricated ZnO‐NS
Fig. 3.
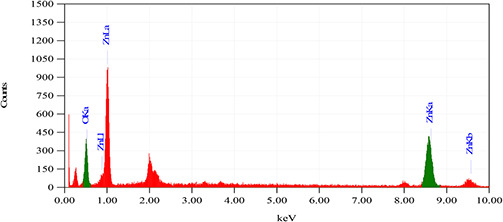
EDX analysis of ZnO‐NS
It is a well‐established fact that the reactivity of NS directly depends on the pure phase, crystallite size and shape, so in order to inspect the phase purity and typical crystallite size of the bio‐synthesised ZnO‐NS, X‐ray diffraction (XRD) procedure has been employed. Fig. 4 demonstrates crystallographic examinations of prepared crystallite size in the 2θ ranges from 20° to 80° with step size 0.2°. Numerous prominent Bragg peaks: (100), (002), (101), (102), (110), (103) and (112) are seen in the XRD blueprint. To measure microstructural crystallite size, Debye Scherrer formula is applied in the equation below:
| (1) |
where t is the crystallite size, λ represents the wavelength of incident Cu Kα X‐rays and β is the full width at half maximum, in radians, of the selected peak (101) at diffraction angle θ. It has been calibrated that average crystallite size is closer to 5 nm.
Fig. 4.
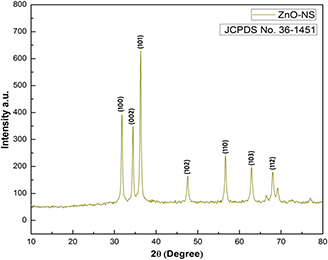
XRD spectrum of ZnO‐NS
Utilising non‐destructive methodology, i.e. UV spectroscopy, the optical as well as structural properties of as‐prepared ZnO‐NS have been shown in Fig. 5 a. The sharp absorption peak at 350 nm clearly indicates the presence of single‐phase ZnO‐NS and their visible light activation capabilities at room temperature. Fig. 5 b further strengthens the pure molecular structure of bio‐fabricated ZnO‐NS and shows vibrational modes of ZnO bonds present inside the prepared sample around 500 cm−1. Furthermore, presences of other functional group such as C–H, O–H (from surface–adsorbed water) and C–O (from capping agent) stretching vibrations can be traced between 2800 and 4000 cm−1.
Fig. 5.
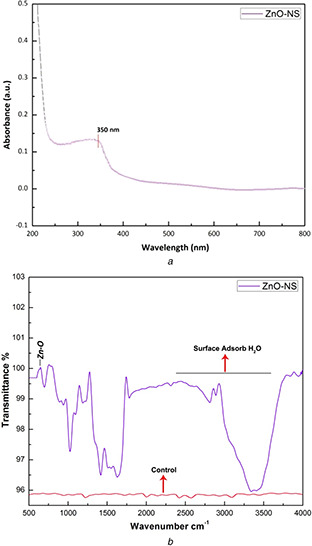
Utilising non‐destructive methodology
(a) UV plot, (b) FTIR spectrum analysis of bio‐fabricated ZnO‐NS
3.3 Effect of different concentrations of ZnO‐NS on L. esculentum growth parameters
Time of germination was recorded when the first seedling appeared in the pot. Pots have been regularly irrigated and observed. In untreated (controlled) pots, germination started after 15 days of sowing. After 15 days of sowing, germination percentage was also determined by using the following formula as shown in Table 1 :
Table 1.
Germination percentage in controlled and ZnO‐NS‐treated seeds of L. esculentum
| Treatment | Time of germination | Germination, % |
|---|---|---|
| controlled | 15 days | 50 |
| 0.01 g/l | 13 days | 80 |
| 0.03 g/l | 13 days | 60 |
| 0.05 g/l | 14 days | 50 |
The height of the plant was measured through scale when the age of plant was 60 days. While numbers of leaves and branches of plants were counted when the age of plant is 90 days. Data was collected and manipulated statistically as illustrated in Table 2.
Table 2.
Variances of different morphological characters in ZnO‐NS‐treated and untreated plants of L. esculentum
| Treatment | Plant height | Total number of branches | Total leaves count | Days to flowering | Days to fruiting | Number of fruits/plant |
|---|---|---|---|---|---|---|
| controlled | 10.41 | 15 | 953.3 | 6.33 | 20.33 | 14.55 |
| 0.01 g/l | 60.57 | 7.25 | 1375.86 | 10.77 | 20.69 | 21.61 |
| 0.03 g/l | 42.75 | 7.92 | 833.96 | 17.90 | 41.3 | 19.26 |
| 0.05 g/l | 32.7 | 9.36 | 744.66 | 12.33 | 27.46 | 32.8 |
Fig. 6 a shows the average height of plants. In case of control, heights of eight plants were measured, whereas in case of 0.01 g/l ZnO‐NS treatment, the heights of seven plants were measured and ranged from 27 to 47 cm. The average height of the plant was 36.71 cm and variance were 60.571. In case of 0.03 g/l ZnO‐NS treatment, the heights of total nine plants were measured. Height ranged from 25 to 45 cm. In this case, the average height of plant was 36.66 cm and variance was 42.75. At concentration 0.05 g/l ZnO‐NS treatment, the heights of total eight plants were measured ranged from 20 to 35 cm. In this connection, average heights of plants were 29.25 cm and variance was recorded as 32.7.
Fig. 6.
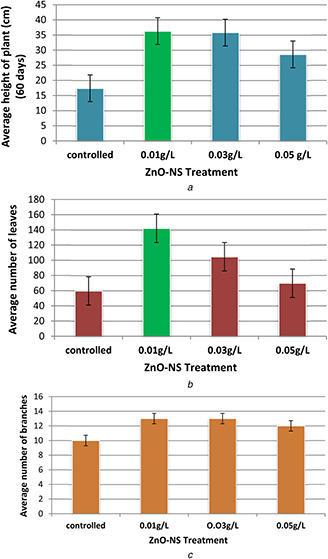
Overall Growth measurements in plants
(a) Average height of L. esculentum in controlled and ZnO‐NS‐treated seeds, (b) Average number of leaves per L. esculentum in controlled and ZnO‐NS‐treated plants, (c) Average number of branches per L. esculentum in controlled and ZnO‐NS‐treated plants
Numbers of leaves of plants were counted when the age of the plant was 90 days. Comparison of an average number of leaves per plant in controlled and ZnO‐NS‐treated plants are given in Fig. 6 b. In case of controlled, number of leaves of total five plants were counted and number ranged from 20 to 90. The average number was 59.6. At concentration 0.01 g/l ZnO‐NS‐treated plants, numbers of leaves of total nine plants were counted. A number of leaves, in this case,, ranged from 80 to 178. The average number of this treatment was 142.11. On the other hand, in case of 0.03 g/l ZnO‐NS‐treated plants, number of leaves of total eight plants were calculated and this number ranged from 70 to 150. The average number, in this case,, was 104.625. In 0.05 g/l ZnO‐NS concentration treatment, leaves of total six plants were calculated. Numbers of leaves, in this case,, ranged from 35 to 104. An average number of leaves in this connection were recorded as 69.66.
Numbers of branches were also calculated over 90 days. Comparison of an average number of branches per plant in controlled and ZnO‐NS‐treated plants is given in Fig. 6 c. In untreated (controlled) plants, number of branches of five plants were calculated. A number of branches in controlled plants ranged from 5 to 14. In case of the controlled group, an average number of branches were ten. Meanwhile, in case of 0.01 g/l ZnO‐NS‐treated plants, number of branches of total eight plants were calculated. The number ranged from 10 to 18. So, in this case, average was 13.66. At concentration of 0.03 g/l ZnO‐NS treatment group, numbers of branches of eight plants were calculated, and in this case number of branches ranged from 9 to 17.
Days to flowering were observed and recorded from the date of sowing for the appearance of the first flower on the plant as shown in Fig. 7 a. In a controlled group, flowering starts after 120 days. Days to flowering in controlled group ranged from 120 to 125 days in three plants. Average days to flowering were 122.66 days. At concentrations (ZnO‐NS) of 0.01 g/l, flowering starts after 90 days. Flowering ranged from 90 to 99 days in nine plants. In this case, average was 95.55 days. While in case of 0.03 g/l ZnO‐NS‐treated plants, flowering started after 93 days. Days to flowering of seven plants, in this case, ranged from 93 to 105 days. Average days to flowering were 98.71 days. Meanwhile, at concentration 0.05 g/l, flowering starts after 95 days. Days to flowering of seven plants, in this case ranged from 95 to 105 days. Average days to flowering were 100 days.
Fig. 7.
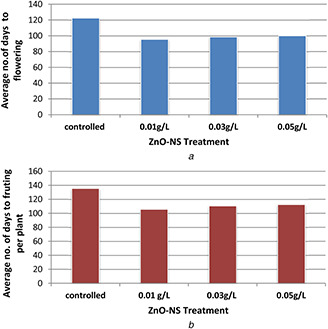
Flowering and fruits formation in plants
(a) Average number of days to flowering in ZnO‐NS‐treated and controlled L. esculentum, (b) Average number of fruit per plant in ZnO‐NS‐treated and controlled L. esculentum
A number of fruits of treated and untreated plants were counted and represented in Fig. 7 b. In case of controlled group, numbers of fruits of eight plants ranged from 4 to 15. In this case, an average number of fruit per plant was recorded as 9.37. In case of 0.01g/l, numbers of fruits of nine plants were counted. The number ranged from 9 to 22. In this case, average numbers of fruits were 15.88. On increasing the concentration of ZnO‐NS to 0.03 g/l, numbers of fruits of eight plants were ranged from 8 to 20. The average number in this case was 14.12. At 0.05 g/l ZnO‐NS‐treated plants, numbers of fruits of six plants ranged from 5 to 19. Average number of fruits was 12 in observed plants.
During the morphological study of L. esculentum, it was noted that flower of 0.01 g/l treatment group has six petals, whereas the controlled and other treatment groups, i.e. 0.03 and 0.05 g/l have five petals. This interesting investigation shows that ZnO‐NS at lower concentration had affected the plant at the genetic level and this effect produced the six petalled flowers, which in turn produced the big‐sized fruit on the plant. Comparison of flowers of 0.01, 0.03, 0.05 gl and controlled group are shown in Fig. 8.
Fig. 8.

Comparison of flower morphology of controlled and ZnO‐NS‐treated plants
4 Discussion
4.1 Characterisation analysis of prepared ZnO‐NS
As reported in our previous investigations that O. europaea leaves extract owns plenty of bioactive molecules involved in reducing metallic compounds into uniformed and stable NS [12]. Same in this case, highly homogenous and spherical morphology of green‐synthesised ZnO‐NS (Fig. 2) confirms successful capping action of bio‐molecules from O. europaea aqueous extract around ZnO‐NS. All of the peaks in XRD prints are found accurate to JCPDS Card No. 36–1451, which clearly hints the formation of single‐phase crystallised ZnO‐NS without any other impurities. Meanwhile, optical properties are of worth importance in tailoring the behaviour of NS at room temperature under visible light [13]. UV prints (Fig. 5 a) show a sharp peak at 350 nm, which reflects a dramatic change in band gap energies of prepared ZnO‐NS. This photo‐induced activation of ZnO‐NS is a key factor for enhanced cellular activity. The molecular stability, mode of vibrational energies and single pure phase of bio‐fabricated ZnO‐NS show vibrational modes of ZnO bonds at 500 cm−1 (Fig. 5 b). At extremely small size, similar stretching modes for ZnO have been reported previously [14, 15].
4.2 Investigations of green‐synthesised ZnO‐NS on L. esculentum (growth parameters)
It has been reported in prior observations that commonly used plant fertilisers required large dosage with higher economic values. Apart from that they possess lower performance. This is because of low cellular absorption, small surface area to size ratio, lower ionic discharge at cellular levels, higher activation energy and lower molecular stability [2, 16]. The plant metabolic machinery has to charge a tremendous amount of cellular adenosine triphosphate to deal with these limitations. This ultimately results in higher disease susceptibility, lower pest resistance and poor annual yield [2]. On the other hand, our experimental findings show that nano‐grade optimisation of ZnO‐NS as nano‐fertiliser using green chemistry has successfully removed these shortcomings. As shown in Fig. 9, as compared with bulk applied traditional fertilisers, enzyme activity can easily be boosted with nano‐fertiliser. Owing to the extremely small particle size of <50 nm, ZnO‐NS exhibits larger surface area to charge ratio. This will help ZnO to disassociate rapidly in the cytosol of the targeted plant. It can be predicted that rapid discharge of highly photo‐induced ZnO‐NS helps cellular enzymes to perform precise and efficient photosynthesis or photorespiration.
Fig. 9.
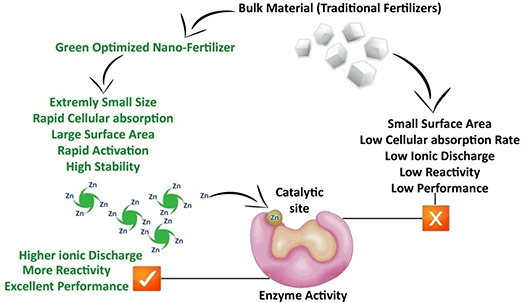
Nano‐fertiliser and comparison of enzyme activity in plant
It is a well‐established fact that the plant cellular metabolism is purely dependent on enzyme performance [17]. Plants such as Punica granatum and Saccharum officinarum show reduced photosynthetic carbon metabolism due to Zn deficiency [18, 19]. Zn being vital plant nutrients regulate the catalytic behaviour of carbonic anhydrase, a biocatalyst that monitors the conversion of carbon dioxide to reactive bicarbonate ions for fixation to variety of carbohydrates in plants. Zn is also part of numerous other enzymes such as superoxide catalase and dismutase, which prevents reactive oxygen species (ROS) stress in plant cells [20, 21]. Enzymes activation energy relies on binding of more energised and accurate co‐factor during catalysis. This co‐factor will facilitate the successful binding of the substrate molecule (target) to the enzyme catalytic site [22]. Zn is one of the vital metallic ions, which are utilised by enzymes (polymerases, kinases, dehydrogenases, phasphatases etc.) during the process of photosynthesis. It is well‐understood that most of the enzymes required metallic ions (Zn+, Cu+ etc.) as co‐factor for their proper functionality. Availability and reactivity of these metallic ions at the cellular level are important indicators for normal plant growth [23, 24, 25]. In our presented ZnO‐NS‐based nano‐fertiliser system, maximum plant growth is resulted, which reflects the supreme performance of cellular enzymes (Fig. 9).
In a recent investigation, zinc improves the cation‐exchange capacity of the roots, which thus upgrades assimilation of basic supplements, particularly nitrogen which is in charge of higher protein content. Zn assumes imperative part in sugar and protein digestion system and also it controls plant development hormone, i.e. indole acetic acid [26]. These realities show that the accessibility Zn to seed or high Zn content inside the seeds amid seed germination has essential physiological parts in seed germination and early seedling development. In our paper, green‐synthesised ZnO‐NS may also act as a positive chemical inducer for both cellular and genomic alterations (such as targeting ‘zinc finger transcription factor’ etc.) in L. esculentum cells. Interestingly, ZnO‐NS at a smaller dosage of 0.01 g/l brings genetic alterations with the appearance of an additional petal than normal in L. esculentum. Similar growth improvement with an extremely low concentration of 30 µg/l of NS was reported for Allium cepa [27]. Same in case of Arachis hypogaea plant, root length, seed germination, seedling vigour index and yield at 1000 ppm showed a significant increment as compared with untreated seed. However, at higher concentration of NS around 2000 ppm, all these growth parameters showed a significant decrease [26]. Metallic NS at higher concentration may cause cellular damage, which may be due to genotoxicity, ROS (mainly hydroxyl and free O radicals) etc. [28, 29, 30]. So, ZnO‐nano‐fertiliser should not accede to the optimum level.
5 Conclusion
Green synthesis has proved to be the most dependable, biocompatible and preferably economical procedure to fabricate extremely fine‐sized metallic oxide NS. This greater biocompatibility is important for metallic oxide NS in connection for safer mode of trails on living tissues. Owing to the homogenous size of 18 ± 5 nm, smaller crystallite size, photo‐induced activation energy, high reactivity with the large surface area and ZnO‐nano‐fertiliser has successfully supplemented the growth parameters in L. esculentum. We have experimentally proved that low dosage with higher efficiency can save time and economics. Antimicrobial potential of ZnO‐NS is also very promising and will provide dual effect along with supplementary behaviour. This will keep plant seedlings infection free and will provide the optimum environment for rapid growth. We do not yet have appropriate findings about the interaction of ZnO‐NS during transcription or post‐transcription, so, molecular level studies required more research. Keeping in view the importance of ZnO‐nano‐fertiliser, our team will perform size dependent and bimetallic (CuO/ZnO) NS in future studies to provide a complete package to farmers for higher economic yield.
6 Acknowledgment
One of co‐author NJ highly appreciates the intuitive efforts made by TB in the accomplishment of this research and her collaboration with other authors to take part.
7 References
- 1. Rockström J.: ‘Resilience building and water demand management for drought mitigation’, Phys. Chem. Earth, A/B/C, 2003, 28, pp. 869 –877 [Google Scholar]
- 2. Savci S.: ‘An agricultural pollutant: chemical fertilizer’, Int. J. Environ. Sci. Dev., 2012, 3, p. 73 [Google Scholar]
- 3. Mori A. Kirk G.J. Lee J.S. et al.: ‘Rice genotype differences in tolerance of zinc‐deficient soils: evidence for the importance of root‐induced changes in the rhizosphere’, Front. Plant Sci., 2016, 6, p. 1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hafeez B. Khanif Y.M. Saleem M.: ‘Role of zinc in plant nutrition – a review. Am. J. Exp. Agric., 2013, 3, p. 374 [Google Scholar]
- 5. Cakmak I.: ‘Tansley review no. 111 possible roles of zinc in protecting plant cells from damage by reactive oxygen species’, New Phytol., 2000, 146, (2), pp. 185 –205 [DOI] [PubMed] [Google Scholar]
- 6. Anwaar S. Maqbool Q. Jabeen N. et al.: ‘The effect of green synthesized CuO nanoparticles on callogenesis and regeneration of Oryza sativa L’, Front. Plant Sci., 2016, 7, p. 1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nie Z. Petukhova A. Kumacheva E.: ‘Properties and emerging applications of self‐assembled structures made from inorganic nanoparticles’, Nat. Nanotechnol., 2010, 5, (1), pp. 15 –25 [DOI] [PubMed] [Google Scholar]
- 8. Maqbool Q. Iftikhar S. Nazar M. et al.: ‘Green fabricated CuO nanobullets via Olea europaea leaf extract shows auspicious antimicrobial potential’, IET Nanobiotechnol., 2017, 11, (4), pp. 463 –468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X. Yang X. Chen S. et al.: ‘Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis’, Front. Plant Sci., 2016, 6, pp. 1243 –1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mirnezhad M. Romero‐González R.R. Leiss K.A. et al.: ‘Metabolomic analysis of host plant resistance to thrips in wild and cultivated tomatoes’, Phytochem. Anal., 2010, 21, (1), pp. 110 –117 [DOI] [PubMed] [Google Scholar]
- 11. Ilahy R. Hdider C. Lenucci M.S. et al.: ‘Phytochemical composition and antioxidant activity of high‐lycopene tomato (Solanum lycopersicum L.) cultivars grown in Southern Italy’, Sci. Horticulturae, 2011, 127, (3), pp. 255 –261 [Google Scholar]
- 12. Maqbool Q. Nazar M. Naz S. et al.: ‘Antimicrobial potential of green synthesized CeO2 nanoparticles from Olea europaea leaf extract’, Int. J. Nanomed., 2016, 11, p. 5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baruah S. Sinha S.S. Ghosh B. et al.: ‘Photoreactivity of ZnO nanoparticles in visible light: effect of surface states on electron transfer reaction’, J. Appl. Phys., 2009, 105, (7), p. 074308 [Google Scholar]
- 14. Tariq Jan J.I. Ismail M. Zakaullah M. et al.: ‘Sn doping induced enhancement in the activity of ZnO nanostructures against antibiotic resistant S. aureus bacteria’, Int. J. Nanomed., 2013, 8, p. 3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khalil M.I. Al‐Qunaibit M.M. Al‐Zahem A.M. et al.: ‘Synthesis and characterization of ZnO nanoparticles by thermal decomposition of a curcumin zinc complex’, Arab. J. Chem., 2014, 7, (6), pp. 1178 –1184 [Google Scholar]
- 16. Fernández‐Escobar R. Benlloch M. Herrera E. et al.: ‘Effect of traditional and slow‐release N fertilizers on growth of olive nursery plants and N losses by leaching’, Sci. Horticulturae, 2004, 101, (1), pp. 39 –49 [Google Scholar]
- 17. Henriques A.R. Chalfun–Junior A. Aarts M.: ‘Strategies to increase zinc deficiency tolerance and homeostasis in plants’, Braz. J. Plant Physiol., 2012, 24, (1), pp. 3 –8 [Google Scholar]
- 18. Hasani M. Zamani Z. Savaghebi G. et al.: ‘Effects of zinc and manganese as foliar spray on pomegranate yield, fruit quality and leaf minerals’, J. Soil Sci. Plant Nutr., 2012, 12, (3), pp. 471 –480 [Google Scholar]
- 19. Singh A. Gupta A.K. Srivastava R.N. et al.: ‘Response of zinc and manganese to sugarcane’, Sugar Tech, 2002, 4, (1–2), pp. 74 –76 [Google Scholar]
- 20. Badger M.R. Price G.D.: ‘The role of carbonic anhydrase in photosynthesis’, Annu. Rev. Plant Biol., 1994, 45, (1), pp. 369 –392 [Google Scholar]
- 21. Hacisalihoglu G. Hart J.J. Wang Y.H. et al.: ‘Zinc efficiency is correlated with enhanced expression and activity of zinc‐requiring enzymes in wheat’, Plant Physiol., 2003, 131, (2), pp. 595 –602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maret W.: ‘Zinc biochemistry: from a single zinc enzyme to a key element of life’, Adv. Nutr., Int. Rev. J., 2013, 4, (1), pp. 82 –91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winkel B.S.: ‘When an enzyme isn't just an enzyme anymore’, J. Exp. Bot., 2017, 68, (7), pp. 1387 –1389 [Google Scholar]
- 24. Sillanpää M. Chaker N.: ‘Biofuels and bioenergy’, ‘A sustainable bioeconomy’ (Springer International Publishing; ), 2017, pp. 79 –139 [Google Scholar]
- 25. Westfall C.S. Muehler A.M. Jez J.M.: ‘Enzyme action in the regulation of plant hormone responses’, J. Biol. Chem., 2013, 288, (27), pp. 19304 –19311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prasad T.N.V.K.V. Sudhakar P. Sreenivasulu Y. et al.: ‘Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut’, J. Plant Nutr., 2012, 35, (6), pp. 905 –927 [Google Scholar]
- 27. Raskar S.V. Laware S.L.: ‘Effect of zinc oxide nanoparticles on cytology and seed germination in onion’, Int. J. Curr. Microbiol. Appl. Sci., 2014, 3, (2), pp. 467 –473 [Google Scholar]
- 28. Chang Y.N. Zhang M. Xia L. et al.: ‘The toxic effects and mechanisms of CuO and ZnO nanoparticles’, Materials, 2012, 5, (12), pp. 2850 –2871 [Google Scholar]
- 29. Fard J.K. Jafari S. Eghbal M.A.: ‘A review of molecular mechanisms involved in toxicity of nanoparticles’, Adv. Pharm. Bull., 2015, 5, (4), p. 447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bahadar H. Maqbool F. Niaz K. et al.: ‘Toxicity of nanoparticles and an overview of current experimental models’, Iran. Biomed. J., 2016, 20, (1), p. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]


