Abstract
Currently, nanotechnology and nanoparticles (NPs) are recognised due to their extensive applications in medicine and the treatment of certain diseases, including cancer. Silver NPs (AgNPs) synthesised by environmentally friendly method exhibit a high medical potential. This study was conducted to determine the cytotoxic and apoptotic effects of AgNPs synthesised from sumac (Anacardiaceae family) fruit aqueous extract (AgSu/NPs) on human breast cancer cells (MCF‐7). The anti‐proliferative effect of AgSu/NPs was determined by MTT assay. The apoptotic properties of AgSu/NPs were assessed by morphological analysis and acridine orange/propidium iodide (AO/PI) and DAPI staining. The mechanism of apoptosis induction in treated cells was investigated using molecular analysis. Overall results of morphological examination and cytotoxic assay revealed that AgSu/NPs exert a concentration‐dependent inhibitory effect on the viability of MCF‐7 cells (IC50 of ∼10 µmol/48 h). AO/PI staining confirmed the occurrence of apoptosis in cells treated with AgSu/NPs. In addition, molecular analysis demonstrated that the apoptosis in MCF‐7 cells exposed to AgSu/NPs was induced via up‐regulation of Bax and down‐regulation of Bcl‐2. These findings suggested the potential use of AgSu/NP as cytotoxic and pro‐apoptotic efficacy and its possible application in modern medicine for treating certain disorders, such as cancer.
Inspec keywords: nanoparticles, silver, nanomedicine, biomedical materials, toxicology, cancer, molecular biophysics, proteins, biochemistry, cellular biophysics, nanofabrication
Other keywords: Ag, Bcl‐2 down‐regulation, Bax up‐regulation, MCF‐7 cell viability, concentration‐dependent inhibitory effect, cytotoxic assay, molecular analysis, DAPI staining, acridine orange‐propidium iodide staining, morphological analysis, MTT assay, human breast cancer cells, sumac fruit aqueous extract, Anacardiaceae family, cytotoxic effects, drug delivery function, diseases, Rhus coriaria L, silver nanoparticles, antiproliferative potential, apoptotic efficacy
1 Introduction
Cancer occurs as a result of genetic changes and cell abnormalities; cancer is also one of the most destructive diseases with a high mortality rate, and it is a severe problem in human health [1]. According to the World Health Organization, cancer rates will increase to 25 million annually in the next two decades [2]. Breast cancer is the most common cancer in women, despite efforts in the field of prevention and treatment; many women die from this disease annually [3]. Common treatments for breast cancer include surgery, hormone therapy, immunotherapy, and systemic chemotherapy; these treatments exhibit individual side effects [4]. For example, the main problems with cancer chemotherapy are severe shortage of active drugs, low solubility, instability, and biologically incompatible drugs [5]. Nanomedicine, a combination of nanotechnology with medicine, offers new opportunity in new therapeutic and medicinal areas. This novel field of nanoscience has created the possibility of using these nanomaterials in the treatment of many diseases, such as cancer [6]. The recent use of nanoparticles (NPs) in medical applications, particularly in imaging, treatment, and drug carriers, has attracted much attention [7]. NPs are used to prevent tumour formation and progression because of their intrinsic anti‐cancer properties [2]. Among various NP types, silver NPs (AgNPs) display wide applications of nanotechnology and medicine due to their distinct biological properties; these NPs are suitable candidates for the treatment of some malignancies [6, 8]. In recent years, the use of plant extracts in the preparation of metallic NPs as a convenient substitute for physical and chemical methods has been proposed. Biological synthesis of AgNPs using microorganisms, fungi, plants, and algae, which are suitable replacements of toxic chemicals, is safe, simple, durable [9], environmentally friendly, and nontoxic [10]. Plants display superiority over other biological agents. Sumac is a traditional herb; its leaves and fruits show therapeutic effects on a wide range of diseases, including diabetes mellitus, cancer, stroke, oral diseases, inflammation, diarrhoea, and dysentery [5]. NPs synthesised using plant extracts exhibit various pharmaceutical and therapeutic effects. Therefore, in the present study, we investigated the anti‐cancer effects of AgNPs synthesised using sumac fruit (Rhus coriaria L.) aqueous extract (AgSu/NPs). Sumac has phenolic compounds such as tannin, quercetin, myristin, anthocyanins, and organic acids. Polyphenols are very effective against treating and preventing cancer, and this can be considered as the importance of our research.
2 Material and methods
All cell cultured materials were obtained from Gibco (USA). RNA isolation kit was purchased from Roche Company. cDNA synthesis kit and SYBER green kit were purchased from Thermo Scientific Company.
2.1 Cell culture and maintenance
Human breast cancer cells (MCF‐7) were purchased from the Pasture Institute of Tehran, Iran. These cells were grown in RPMI medium complemented with FBS (%10) and antibiotic (%1) and incubated at 37°C in a humidified condition with CO2 (5%).
2.2 MTT assay
The proliferation of MCF‐7 cancer cells was evaluated by MTT reduction assay. Cells were seeded (1 × 105 cells/ml) in a 96‐well plate and incubated for 24 h. Afterwards, the cancer cells were exposed to appropriate concentrations of AgSu/NPs for 24, 48, and 72 h. Cell viability was assessed by MTT test after the exposure period. The absorbance was also measured at 570 nm by a spectrophotometer.
2.3 Cell morphological assessment
To investigate the effects of AgSu/NPs on the morphological properties of cancer cells, MCF‐7 cells were seeded in six‐well plates (3 × 104). After 24 h, the cancer cells were exposed to appropriate concentrations of AgSu/NPs for 48 h. The morphological characteristics of the treated and untreated MCF‐7 cells were investigated by using an inverted microscope.
2.4 DAPI staining
DAPI staining was used to detect the cell death induced by AgSu/NPs. Cells were seeded, treated for 48 h with various concentrations of AgSu/NPs, washed, and fixed (10 min each) with PBS and methanol. Finally, DAPI was added to the cells at a concentration of 0.5 µg/ml for 5 min. The apoptotic nuclei were examined under a fluorescent microscope.
2.5 Acridine orange (AO) and propidium iodide (PI) double staining
To determine the type of cell death, the MCF‐7 cells were stained with AO/PI, which was used as fluorescent probe. The cells were harvested, washed with PBS, and mixed with the fluorescent dye (1:1), which contained 10 μl of AO (100 μg/ml) and PI (100 μg/ml), after the treatment period; these cells were observed under fluorescence microscope [11].
2.6 Flow cytometry
The flow cytometric technique was used to detect the effect of AgSu/NPs on the percentage of apoptotic nuclei. MCF‐7 cells were treated with different concentrations (5, 10, and 15 µM) of NP. Afterwards, PI was used to stain cells. Apoptosis was evaluated by a FACScan laser flow cytometer after incubation (30 min, 37°C).
2.7 Assessment of alterations in gene expression of Bax and Bcl‐2
The alterations in the expression of Bax and Bcl‐2 mRNA were examined by real‐time PCR. The total RNAs of treated and untreated cells were extracted by the high‐pure RNA isolation kit (Roche, Germany) in accordance with the company's protocol and subsequently kept at − 80 °C. To synthesise the complementary DNA, reverted first‐strand cDNA synthesis kit (Thermo Scientific) was used. The obtained cDNA (2 µl) was added to SYBR Green master mix (10 μl) for real‐time PCR. Appropriate forward and reverse primers (2 µl) and water were also added to obtain a volume of 20 μl. Real‐time PCR was performed on a Bio‐Rad CFX96 detection system. The primers used are listed in Table 1.
Table 1.
Primer sequences
| Genes | Forward 5′→3′ |
|---|---|
| GAPDH | 5′TGAACGGGAAGCTCACTGG3′ |
| BAX | 5′TTTGCTTCAGGGTTTCATCCA3′ |
| Bcl‐2 | 5′CATGTGTGTGGAGAGCGTCAAC3′ |
| Genes | Reverse 5′→3′ |
| GAPDH | 5′GCTTCACCACCTTCTTGATGTC3′ |
| BAX | 5′CTCCATGTTACTGTCCAGTTCGT3′ |
| Bcl‐2 | 5′CAGATAGGCACCCAGGGTGAT3′ |
2.8 Data analysis
Data analyses were carried out using SPSS software. The level of P ≤ 0.05 was considered significant. All results are expressed as mean ± SD. Comparisons of multiple groups were conducted through ANOVA and LSD test.
3 Results
3.1 Cell proliferation assay
Assessment of the anti‐proliferative effect of AgSu/NPs on MCF‐7 cells revealed that with increased concentrations of AgSu/NPs, the cell survival was reduced. As shown in Fig. 1, AgSu/NPs inhibited the viability of cancer cells in a time‐ and dose‐dependent manner. Result showed that the median concentration (IC50) was ∼10 µM.
Fig. 1.
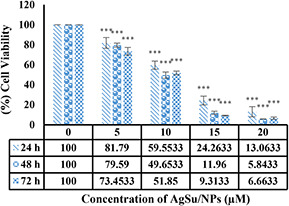
Assessment of the viability of MCF‐7 cells exposed to AgNPs synthesised using sumac fruit (Rhus coriaria L.) aqueous extract (AgSu/NPs) compared with that of untreated cells using MTT assay. ***P < 0.001 was considered significant, and results are presented as mean ± SD
3.2 Morphological observation
Morphological studies revealed that the toxicity of AgSu/NPs on MCF‐7 cancer cells caused changes in the size, shape, and volume of cells (Fig. 2 a). DNA fragmentation and nuclear apoptosis were confirmed by DAPI staining. Fig. 2 b shows that the nuclei in apoptotic cells are well condensed and those in untreated cells are ideally round. AO/PI staining demonstrated that with increased concentrations of AgSu/NPs, apoptosis was induced in the cells, and the cell colour was changed from green (living cells) to yellow and red (cell apoptosis) (Fig. 2 c).
Fig. 2.
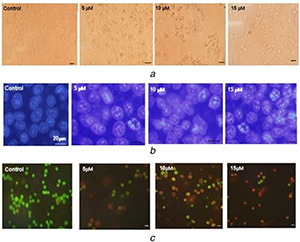
Assess the effect of AgSu/NPs on cell and nuclear morphology in MCF‐7 cells
(a) The cells were treated for 48 h with different concentrations of AgSu/NPs and then morphological studies were conducted using the inverted microscope, (b) Study changes in cell nuclei were done with DAPI staining, (c) AO/PI staining of cells, observed by fluorescence microscope
3.3 Flow cytometry
Flow cytometric analysis indicated that subG1 peak was significantly increased in treated cells compared with that in the control. Additionally, apoptosis was dose‐dependently induced in breast cancer cell line treated with AgSu/NPs (Fig. 3).
Fig. 3.
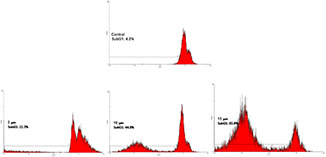
Effect of different concentrations of AgSu/NPs on the cell cycle of the MCF‐7 cells by flow cytometry
3.4 Effect of AgSu/NPs on Bax and Bcl‐2 gene expression in MCF‐7 cells
Real‐time PCR analysis indicated that the exposure of MCF‐7 cells to AgSu/NPs significantly reduced the gene expression of Bcl‐2 as an anti‐apoptotic gene and increased the expression of Bax as a pro‐apoptotic gene compared with those in the control group (Fig. 4).
Fig. 4.
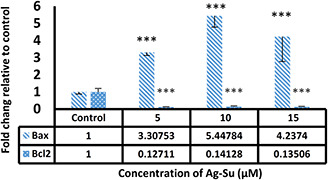
Assessment of alterations in expression of Bax and Bcl‐2 genes in MCF‐7 cells exposed to different doses of AgSu/NPs compared with untreated cells. ***P < 0.001 were considered significant and result are presented as mean ± SD
4 Case studies
Environmentally friendly synthesis of NPs using different plants is a simple and safe method of NP synthesis. One of the most considerable concerns in the field of nanotechnology is the use of environmentally friendly processes for the synthesis of NPs with specific properties [8]. Evidence showed that AgNPs synthesised by environmentally friendly method is important to a wide range of applications. Cancer therapy is the most important aspect in the use of these NPs. AgNPs synthesised by using different plant species can reduce the rate of tumour cell growth in a variety of cancer cell lines [12]. However, no information is available about the use of AgSu/NP as an anti‐malignancy agent against breast cancer cells. Hence, in the present study, the anti‐cancer properties of AgSu/NPs were investigated. Results showed that the AgSu/NPs can inhibit the MCF‐7 cells with IC50 of ∼10 µM after 48 h of incubation (Fig. 1). Morphological observation and fluorescent images also confirmed the occurrence of apoptosis in cells treated with NPs (Fig. 2). In addition, these NPs can induce apoptosis in MCF‐7 cells by decreasing the expression of Bcl‐2 and increasing the expression of Bax gene (Fig. 3). Previous studies demonstrated that AgNPs can also influence the expression of genes related to apoptosis and cell cycle; they exert inhibitory effect on cancer cells [13]. The innate toxicity of NPs is related to size, shape, and other characteristics, which can lead to the production of free radicals, DNA damage, and denaturation of some proteins [14]. The cytotoxic effects of AgNPs synthesised using environmentally friendly methods on certain cancer cells lines, such as human epithelioma cells (Hep‐2) [15], human epithelial carcinoma cells (HeLa) [13], fibrosarcoma cells (Wehi 164) [16], human breast cancer cells (MCF‐7) [17], and human ductal breast epithelial tumour cells (T47D) [18], have been reviewed and approved. In 2009, Miura et al. compared the cytotoxic effect of two compounds, namely nano‐Ag and AgNO3; their results showed that AgNO3 (IC50 : 17 μg Ag/ml) significantly inhibits the HeLa cells compared with that of nano‐Ag (92 μg Ag/ml). Flow cytometry results revealed that nano‐Ag induces apoptosis to suppress HeLa cells [19]. In 2014, Garvita et al. synthesised AgNPs from a kind of cyanobacteria (Ag‐CNPs) and examined their antibacterial and anti‐cancer properties. Their results showed that the Ag‐CNPs significantly inhibit the growth of cancer cells (Dalton's lymphoma and human carcinoma colo205 cells) even at low concentrations. This toxicity may be due to the production of reactive oxygen species and subsequent damage to DNA, which ultimately leads to the induction of apoptosis [20]. Heydari and Rashidipour synthesised AgNPs using oak fruit hull (jaft) extract and assessed the cytotoxic effects AgNPs and AgNPs with jaft extract on MCF‐7 cells. Their results indicated that the AgNPs with jaft extract (IC50 : 0.04 μg/ml) exert stronger cytotoxic effect on treated cells than that of AgNPs (IC50 : 50 μg/ml) (Heydari and Rashidipour, 2015). Another study confirmed the apoptotic effect of AgNPs on glioblastoma multiforme cells [21]. Govender et al. synthesised AgNPs from the extract of Albizia adianthifolia (AA‐AgNPs) and investigated their anti‐cancer properties on human lung cells (A549). Their findings showed that the AA‐AgNPs reduce cell viability in a dose‐dependent manner. Moreover, apoptosis is 1.5‐fold higher in the cells exposed to AA‐AgNPs than that in untreated cells; the expression of CD95 receptor in these cells is also significant. NP treatment on cells results in a 2.5‐fold decrease in the activity of caspase 8 and 1.7‐ and 1.4‐fold increase in caspase‐3‐7 and caspase 9, respectively. Results also showed that treatment with AA‐AgNPs causes a 16‐fold increase in the expression of Bax gene as a pro‐apoptotic gene [22]. The present study showed that the AgSu/NPs induced a six‐fold increase in the gene expression of Bax. Another study in 2015 presented similar result; this specific study investigated the anti‐cancer effects of Achillea biebersteinii AgNPs (Ab‐AgNPs) on MCF‐7 cell line by focusing on the apoptosis process. AgNPs decrease (IC50 : 20 µg/ml) the cell viability in a dose‐dependent manner. Morphological and fluorescent staining (DAPI and AO) results confirmed the occurrence of apoptosis. Furthermore, their results showed that the treatment of MCF‐7 cells with Ab‐AgNPs induces change in the expression of genes associated with apoptosis; this result was confirmed by a decrease in the Bcl‐2 gene expression and increase in the Bax gene expression [23], which is similar to our results. Previous results of human and animal cells exposed to AgNPs also indicated that these particles can induce apoptosis by altering the expression of genes involved in this process, such as Bax and Bcl‐2 [24]. Different strategies, such as induction of apoptosis and inhibition of angiogenesis, can be used to inhibit cancer cells and prevent their progression. AgNPs can cause cell death by targeting apoptotic pathways [25]. In 2015, Archana et al. showed that the CPS films that contain silver oxide NPs in combination with chitosan and poly vinyl pyrrolidone can strikingly reduce the viability of L929 cells. This study confirmed that silver NPs, with some suitable substrates also have cytotoxic effects [26]. According to these results and our study, AgSu/NPs exhibit remarkable cytotoxic activities. These NPs can also inhibit the growth of MCF‐7 cell line in a dose‐dependent manner and subsequently induce apoptosis. The toxicity properties of AgSu/NPs can be utilised for the development of therapeutic agents to eliminate cancer cells. AgSu/NPs can also be used as potential anti‐cancerous agents. Anti‐cancerous AgSu/NPs suggested the possible utilisation of biogenic AgNP as an effective and biocompatible biocide.
5 Acknowledgments
Our work was supported by the Science and Research Branch, Islamic Azad University, Tehran, Iran.
6 References
- 1. Parhi P. Mohanty C. Sahoo S.K.: ‘Nanotechnology‐based combinational drug delivery: an emerging approach for cancer therapy’, Drug Discov. Today Technol., 2012, 17, (17), pp. 1044 –1052 [DOI] [PubMed] [Google Scholar]
- 2. Vinardell M.P. Mitjans M.: ‘Antitumor activities of metal oxide nanoparticles’, Nanomaterials, 2015, 5, (2), pp. 1004 –1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mijan M.C. Longo J.P.F. de Melo L.N.D. et al.: ‘Vascular shutdown and pro‐inflammatory cytokine expression in breast cancer tumors after photodynamic therapy mediated by nano‐sized liposomes containing aluminium‐chloride‐phthalocyanine’, J. Nanomed. Nanotechnol., 2014, 5, (4), p. 1 [Google Scholar]
- 4. Tanaka T. Decuzzi P. Cristofanilli M. et al.: ‘Nanotechnology for breast cancer therapy’, Biomed. Microdevices, 2009, 11, (1), pp. 49 –63 [DOI] [PubMed] [Google Scholar]
- 5. Greco F. Vicent M.J.: ‘Combination therapy: opportunities and challenges for polymer–drug conjugates as anticancer nanomedicines’, Adv. Drug Deliv. Rev., 2009, 61, (13), pp. 1203 –1213 [DOI] [PubMed] [Google Scholar]
- 6. Carreon‐Alvarez C.D. Yanez‐Sanchez I. Zamudio‐Ojeda A. et al.: ‘Silver nanoparticles induce apoptosis in L5178Y lymphoma by lipoperoxide activity’, 2014.
- 7. Selim M.E. Hendi A.A.: ‘Gold nanoparticles induce apoptosis in MCF‐7 human breast cancer cells’, Asian Pac. J. Cancer Prev., 2012, 13, (4), pp. 1617 –1620 [DOI] [PubMed] [Google Scholar]
- 8. Rahimi Z. Yousefzadi M. Noori A. et al.: ‘Green synthesis of silver nanoparticles using Ulva flexousa from the Persian Gulf, Iran’, J. Persian Gulf, 2014, 5, (15), pp. 9 –16 [Google Scholar]
- 9. Merin D.D. Prakash S. Bhimba B.V.: ‘Antibacterial screening of silver nanoparticles synthesized by marine micro algae’, Asian Pac. J. Trop. Biomed., 2010, 3, (10), pp. 797 –799 [Google Scholar]
- 10. Ponarulselvam S. Panneerselvam C. Murugan K. et al.: ‘Synthesis of silver nanoparticles using leaves of catharanthus roseus Linn. G. Don and their antiplasmodial activities’, Asian Pac. J. Trop. Biomed., 2012, 2, (7), pp. 574 –580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Namvar F. Rahman H.S. Mohamad R. et al.: ‘Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract’, Int. J. Nanomed., 2014, 9, (1), pp. 2479 –2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Satapathy S.R. Mohapatra P. Preet R. et al.: ‘Silver‐bAased nanoparticles induce apoptosis in human colon cancer cells mediated through p53’, Nanomedicine, 2013, 8, (8), pp. 1307 –1322 [DOI] [PubMed] [Google Scholar]
- 13. Sukirtha R. Priyanka K.M. Antony J.J. et al.: ‘Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model’, Process. Biochem., 2012, 47, (2), pp. 273 –279 [Google Scholar]
- 14. Chaloupka K. Malam Y. Seifalian A.M.: ‘Nanosilver as a new generation of nanoproduct in biomedical applications’, Trends Biotechnol., 2010, 28, (11), pp. 580 –588 [DOI] [PubMed] [Google Scholar]
- 15. Jacob S.J.P. Finub J. Narayanan A.: ‘Synthesis of silver nanoparticles using piper longum leaf extracts and its cytotoxic activity against Hep‐2 cell line’, Colloids Surf. B: Biointerfaces, 2012, 91, pp. 212 –214 [DOI] [PubMed] [Google Scholar]
- 16. Safaepour M. Shahverdi A.R. Shahverdi H.R. et al.: ‘Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against fibrosarcoma‐wehi 164’, Avicenna J. Med. Biotechnol., 2009, 1, (2), pp. 111 –115 [PMC free article] [PubMed] [Google Scholar]
- 17. Vivek R. Thangam R. Muthuchelian K. et al.: ‘Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF‐7 cells’, Process. Biochem., 2012, 47, (12), pp. 2405 –2410 [Google Scholar]
- 18. Hekmat A. Saboury A.A. Divsalar A.: ‘The effects of silver nanoparticles and doxorubicin combination on DNA structure and its antiproliferative effect against T47D and MCF7 cell lines’, J. Biomed. Nanotechnol., 2012, 8, (6), pp. 968 –982 [DOI] [PubMed] [Google Scholar]
- 19. Miura N. Shinohara Y.: ‘Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells’, Biochem. Biophys. Res. Commun., 2009, 390, (3), pp.733 –737 [DOI] [PubMed] [Google Scholar]
- 20. Singh G. Babele P.K. Shahi S.K. et al.: ‘Green synthesis of silver nanoparticles using cell extracts of anabaena doliolum and screening of its antibacterial and antitumor activity’, J Microbiol. Biotechnol., 2014, 24, (10), pp. 1354 –1367 [DOI] [PubMed] [Google Scholar]
- 21. Urbańska K. Pająk B. Orzechowski A. et al.: ‘The effect of silver nanoparticles (AgNPs) on proliferation and apoptosis of in ovo cultured glioblastoma multiforme (GBM) cells’, Nanoscale Res. Lett., 2015, 10, (1), p. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Govender R. Phulukdaree A. Gengan R.M. et al.: ‘Silver nanoparticles of Albizia adianthifolia: the induction of apoptosis in human lung carcinoma cell line’, Nanotechnology, 2013, 11, (1), p. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baharara J. Namvar F. Ramezani T. et al.: ‘Silver nanoparticles biosynthesized using Achillea biebersteinii flower extract: apoptosis induction in MCF‐7 cells via caspase activation and regulation of Bax and Bcl‐2 gene expression’, Molecules, 2015, 20, (2), pp. 2693 –2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gopinath P. Gogoi S.K. Sanpui P. et al.: ‘Signaling gene cascade in silver nanoparticle induced apoptosis’, Colloids Surf. B: Biointerfaces, 2010, 77, (2), pp. 240 –245 [DOI] [PubMed] [Google Scholar]
- 25. Hsin Y.‐H. Chen C.‐F. Huang S. et al.: ‘The apoptotic effect of nanosilver is mediated by a ROS‐and JNK‐dependent mechanism involving the mitochondrial pathway in NIH3T3 cells’, Toxicol. Lett., 2008, 179, (3), pp. 130 –139 [DOI] [PubMed] [Google Scholar]
- 26. Archana D. Singh B.K. Dutta J. et al.: ‘Chitosan‐PVP‐nano silver oxide wound dressing: in vitro and in vivo evaluation’, Int. J. Biol. Macromol., 2015, 73, pp. 49 –57 [DOI] [PubMed] [Google Scholar]


