Abstract
Different chemo‐physical methods are used to synthesise titanium oxide nanoparticles (TiO2 NPs), which are often expensive, unfriendly to the environment, toxic, not biocompatible, with a small yield. To resolve these problems, the researchers use green procedures to synthesise TiO2 ‐NPs by plant extracts of Capsicum annum L. and Allium cepa (onion) and characterise using atomic force microscopy, scanning electron microscopy, transmission electronic microscopy, X‐ray diffraction, ultraviolet (UV)–visible (Vis) spectra and Fourier transform infrared spectroscopy. The results indicate that most NPs synthesised by the first and second procedures of onion had an average diameter of 95.7 and 89.1 nm, while NPs synthesised by C. annum had an average diameter of 103.60 and 90.07 nm, respectively. In UV–Vis spectra, strong absorption was below 470 nm, and energy gap was 3.3 eV in each of the first procedure of A. cepa and the second procedure of C. annum compared with 270 nm, 6.3 eV for each of the second procedure of A. cepa and the first procedure of C. annum. The antimicrobial activities of NPs were evaluated and an attempt was made to enhance these activities by Eugenia caryophyllata plant's oil in combination therapies. There were synergistic effects between NPs and plant's oil.
Inspec keywords: scanning electron microscopy, visible spectra, nanofabrication, titanium compounds, ultraviolet spectra, X‐ray diffraction, nanoparticles, atomic force microscopy, antibacterial activity, transmission electron microscopy, Fourier transform infrared spectra, nanomedicine, semiconductor materials, semiconductor growth
Other keywords: cepa, green synthesis, titanium dioxide nanoparticles, volatile oil, eugenia caryophyllata, capsicum annum, atomic force microscopy, electron microscopy, transmission electronic microscopy, UV‐visible spectra, plant extracts, antimicrobial activities, chemophysical methods, Capsicum annum L., Allium cepa, scanning electron microscopy, X‐ray diffraction, Fourier transform infrared spectroscopy, onion, plant oil, TiO2
1 Introduction
The Latin names of clove or Kabsh Qarunfil are Syzygium aromaticum (L.) (Merr. & Perry), Eugenia caryophyllata (Thunb.), and Eugenia aromatica (Kuntze.), and they belong to Myrtaceae family [1].
Traditionally, Qarunfil is used as a carminative and anti‐inflammatory. Its aromatic bud flowers are used as anti‐inflammatory, an antiseptic and peripheral anti‐nociceptive activity [2], antiemetic, stimulant, treat dyspepsia, and gastric irritation. Oil employed as a local analgesic for hypersensitive dentine's and carious cavities; internally antispasmodic, topical anaesthesia [3]. Recently, an aqueous extract of E. caryophyllata (Qarunfil) was found to be the most efficient against Salmonella typhi, compared with Klebsiella pneumonia and Escherichia coli [4]. Essential oil (0.4%) was antibacterial (Bacillus subtilis, E. coli, Staphylococcus aureus and Pseudomonas aeruginosa), which are Gram‐negative and ‐positive bacteria [5, 6], and anaerobic bacteria Prevotella nigrescens and Porphyromonas gingivalis [7], and anti‐fungal activities against 53 clinical isolates of Candida [8]. The main chemical component in the buds’ oil, which was analysed by gas chromatography–mass spectrometry, are eugenol (81.13–84.44%), eugenyl acetate (11.60–15.02%) and β‐caryophyllene (3.45–4.60%), respectively, these compounds are present in leaves oil at 81.06–86.04%, 2.02–3.05% and 11.95–16.16%, and in stems oil eugenol is 97.20–98.83% [9, 10]. These activities of oil are due to eugenol. Eugenol and acetyleugenol exhibit cholagogue activity and could inhibit arachidonate‐, adrenalin‐ and collagen‐induced platelet aggregation. Qarunfil terpenes induced detoxifying enzyme, glutathione‐S‐transferase in mouse liver and intestine and could act as carcinogen detoxification. Whole qarunfils could exhibit chemoprotective activity against liver and bone marrow toxicity [3].
Nanoparticles (NPs; <100 nm) have unique properties compared with their bulk particles. TiO2 ‐NPs are very important because they exhibit low cost, stability, high refractive index, high optical properties, high ultraviolet (UV) absorbance, low toxicity, and strong redox ability, TiO2 has a high energy gap (i.e. 3.2–5.2 eV) [11], and has good electrical, optical and magnetic properties [12, 13]. Different chemo‐physical methods succeeded in synthesising TiO2 NPs, which depends on the top down and down top method [14, 15, 16]. They are often high cost, unfriendly to the environment, toxic, biocompatible, with a small yield etc. [17].
To resolve these problems, the researchers tend to use biosynthesis, bio‐nanotechnology or green synthesis, which usually refers to intersection between biotechnology and nanotechnology, including biomolecules produced by the plant, bacteria, virus, fungi etc.) to synthesise materials of nano‐size, which are used in different nanotechnological applications [18]. In other words, it means, synthesis of nanomaterials by living organisms or biological systems [19, 20]. Aloe vera leaves extract was successfully used in synthesising TiO2 ‐NPs with a tetragonal structure, their crystallite size was 12 nm as per X‐ray diffraction (XRD) analysis and 30 nm (using a particle size analyser). The absorption spectrum of the anatase phase of TiO2 ‐NPs is around 393 nm with 3.2 eV band gap [21]. In addition, spherical titanium dioxide NPs produced from nyctanthes leaves extract with a size range of 100 and 150 nm [22]. Curcuma longa is a good TiO2 ‐NPs biosynthesising agent by two procedures. The average sizes were 76.36 and 92.6 nm, respectively, with good optical properties. The crystals had the following shapes: anatase, rutile, and brookite when the first procedure was used and was pure anatase when the second procedure was used [23].
TiO2 NPs are used in different applications such as pigments, adsorbents, catalysts, supports, paints, sunscreens, toothpaste, ointments etc. [24, 25]. They also have biomedical applications such as anti‐parasitic properties [26], anti‐cancer treatment [27], reduce growth and sporulation of Fusarium graminearum and reduce damping‐off due to these fungi in two varieties of wheat plants [23], with less effect on plant germinations [28]. TiO2 NPs exhibit good antimicrobial activity, especially when exposed to visible light they absorb photocatalysts which successful kill S. aureus and E. coli [29], P. aeruginosa, Enterococcus hire and Bacteroides fragilis. This antimicrobial feature of TiO2 ‐NPs could also be activated by UV light to kill bacteria in 60 min, so, it is easy to be used as antibacterial coatings in the mixture with paint in hospital [30, 31] and wastewater disinfection processes [32, 33] but these NPs had either no toxicity in the dark [34] or a small anti‐bacterial effect at 100 mg/ml when it is used alone, other studies found that it could be stimulated using combination therapies of mixing them (50 mg/ml) with 50 mg/ml of methanol extraction of Citrullus colocynthis which showed synergistic effects in each of S. aureus and E. coli [35].
The goals of the present study are to synthesise TiO2 NPs via different green syntheses of Allium cepa and Capsicum annum plant extracts and characterised their crystallinity, crystallite size, band gap, functional group, and structural properties. The antimicrobial activities of green‐synthesised NPs alone and volatile oil of E. caryophyllata alone and their combination therapy were, also, evaluated to understand their biological properties and if there are any synergistic effects between them.
2 Materials and methods
2.1 Green synthesis of TiO2 ‐NPs using plant extraction
2.1.1 Plant extraction
Leaves of A. cepa (onions) and C. annum L were used for green synthesis. They were obtained from the Herbarium of Iraqi Ministry of Health who identified plants by a taxonomic method. Eighty grams of fresh parts of onion leaves were mixed with 250 ml of distal water by homogenised blender for 3 min. The extract was put on a hot plate with a magnetic stirrer at 50–60°C for 4–5 h, filtered by Whatman number four filter paper. The residue was removed. Ninety grams of fruit were mixed with 250 ml of distal water by using a homogenised blender for 3 min. The extract C. annum was put on a hot plate with a magnetic stirrer at 50–60°C for 4–5 h and filtered as described above. The filtrates were directly used for the green synthesis of NPs.
2.1.2 Synthesis
A. cepa : 50 ml of plant extract were mixed with 1 ml of (50 mg/ml) of bulk TiO2 particles (Sigma‐Aldrich, China; anatase crystal form, their size was >2 µm) in a flask, placed in a magnetic stirrer hot plate at 50C° with 1000 rpm for 4–5 h, this was the first procedure while in the second procedure, a magnetic stirrer plate at 25 ± 3°C with 1000 rpm for 48 h.
C. annum : for the first procedure, 50 ml of plant extract were mixed with 1 mm of (50 mg/ml) of bulk TiO2 particles in the flask, placed in a magnetic stirrer hot plate at 50C° with 1000 rpm for 4–5 h. In the second procedure, 50 ml of plant extract were mixed with 200 µl of (100 mg/ml) of bulk TiO2 particles in the flask, placed in a magnetic stirrer plate at 25 ± 3°C with 1000 rpm for 48 h.
The supernatant was neglected. The precipitate was washed with double distilled water, centrifuged at 1500 rpm for 10 min. This was repeated three times. The obtained precipitate was dried at 25 ± 2°C for 24 h. It is characterised as follows [23].
Characterisation: The following techniques were used for the characterisation of NPs: atomic force microscopy (AFM), (Shimadzu‐Japan, AA3000), at the Center of Nanotechnology and Advanced Materials, the University of Technology, Iraq [36]. A UV–visible spectrum (Schimadzu 1601 spectrophotometer) in the 200–800 nm range [37]. Transmittance measurements, coefficients of absorption and gap energy were calculated for the optical properties and described according to [38]. XRD (Shemadzu, Japan) was used to confirm crystal phases and sizes of each phase. XRD analysis was performed using an X‐ray diffractometer with Cu‐Kα crystal radiation (λ = 1.54056 Å) scanning at a rate of 5°/min) for (2θ) range of 20°–70°. The samples’ diffraction peaks were identified by comparison (00‐021‐1272 card, variable slit intensity). The full width at half maximum (FWHM) and Scherer's equation were used to determine the crystallite size [39], The strain value η [40] and the dislocation densities δ values [41] were also calculated. Scanning electron microscope (SEM) (Vega Tescan, USA), at the University of Technology, the Centre for Nanotechnology and Advanced Materials, Iraq, was utilised to analyse NPs. To determine the size and morphology of NPs, transmission electronic microscopy (TEM) analysis (Philips CM10 electron microscope operating at 60 kV) was used according to the procedure of College of Medicine, Al Nahrin University. Fourier transform infrared spectroscopy (FT‐IR, Shemadzu, Germany) was utilised to determine the various functional groups present in TiO2 in the Central Service Laboratory, University of Baghdad, College of Education and Pure Sciences, Ibn Al‐Haitham, Iraq.
2.2 Antimicrobial activities
2.2.1 Preparation
Volatile oil of E. caryophyllata was brought from Al‐Emad factory, Iraq. Tween 5% was used to dilute it. Sterilised distilled water used to prepare several concentrations of bulk particles of TiO2, standard NPs, green synthesis of nanoparticles, which were synthesised by the second procedure of A. cepa and C. annum plant extracts.
2.2.2 Experiments
The antimicrobial activities were estimated against four species of bacteria: S. aureus, K. pneumonia, Staphylococcus epidermidis, and E. coli and one isolate of Candida albicans, which were obtained from the Department of Biology, College of Science, AL‐Mustainsiriyah University, Baghdad, Iraq.
The dilution broth susceptibility procedure was used for the antimicrobial activity [42]. Six treatments were found, each treatment deals with various concentrations. These were TiO2 bulk particles, TiO2 NPs produced by the second procedure of A. cepa alone, TiO2 NPs produced by the second procedure of C. annum alone, volatile oil of E. caryophyllata alone, a combination therapy of NPs synthesised by the second procedure of A. cepa with volatile oil and finally combination therapy of NPs synthesised by the second procedure of C. annum with volatile oil.
One drop of each tested culture strain was added to 10 ml of nutrient broth, these were serially diluted five times. One drop (from dilute 5) was added to solution A (which was made up of 1 ml of nutrient broth and 1 ml sample), incubated at 37°C for 24 h, then diluted seven times. The seventh dilution was seeded by streaking the surface of nutrient agar medium and incubated at 37°C for 24 h. A negative control and three replicates of each treatment were found. The percentage of reduction was calculated by using the following equation:
| (1) |
where R is the percentage of reduction of the number of colonies, A is the colony number of bacteria in control treatment and B is the number of colonies of bacteria after the transaction article NPs.
The determination of the synergistic effects of TiO2 ‐NPs and volatile oil of E. caryophyllata combination treatment was carried out according to [43]. The Compusyn Computer Software (version 2011) was used for determining the combination index (CI) from drug cytotoxicity and a combination of synergistic, additive, or antagonistic was determined.
3 Results and discussion
3.1 Green synthesis
AFM: the calculated TiO2 NPs’ sizes were calculated using the software of the AFM. For the first and second procedures of A. cepa, size particles ranged 20–170 and 20–160 nm with an average diameter of 95.7 and 89.1 nm (Fig. 1 a), while the size of NPs produced by the first and second procedures of the C. annum plant extract was 103.60 and 90.07 nm, respectively (Fig. 1 b). Figs. 2 a –d show AFM topographic images of green synthesised TiO2 ‐NPs. Roughness averages (Ra) were 3.18 and 1.98 nm, root mean squares (Sq) were 3.72 and 2.3 nm for the first and second procedures of C. annum plant extract. SEM analysis found the formation of agglomerates containing spherical nano‐size particles with an approximate diameter of ∼100 nm for the first and second procedures of A. cepa (Figs. 3 a and b) compared with 90 nm for the second procedure of C. annum plant extract (Figs. 3 c and d). Figs. 4 a and b show TEM images of green synthesised NPs by the first procedure of A. cepa with an average particle size of 68.7 nm compared with particle size around 20 and 40 nm by the second procedure of A. cepa. Figs. 4 c and d show TEM images of TiO2 produced by the first procedure (size: 15 and 46 nm) and second procedure (size: 38 and 108 nm) of C. annum plant extracts. All particles were spherical
Fig. 1.
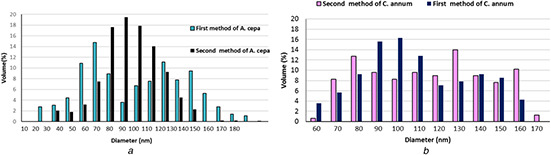
Granularity volume distribution chart of TiO2 ‐NPs synthesised by the first and second procedures of
(a) A. cepa, (b) C. annum
Fig. 2.
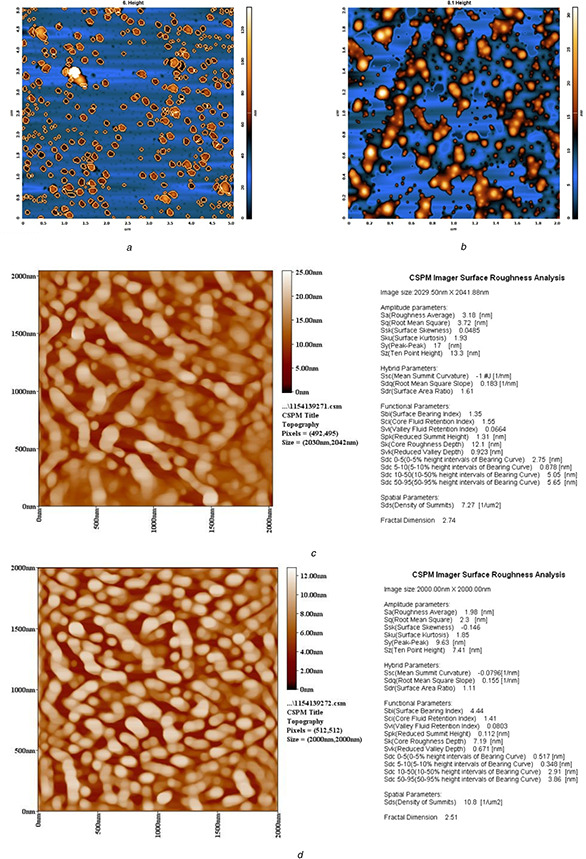
AFM topographic images of TiO2 NPs synthesis by
(a) First procedure of A. cepa, (b) Second procedure of A. cepa, (c) First procedure of C. annum, (d) Second procedure of C. annum
Fig. 3.
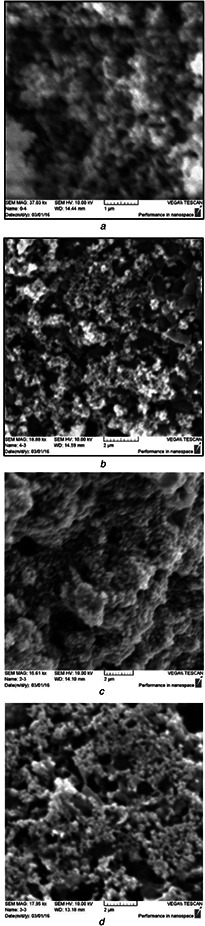
SEM images of TiO2 ‐NPs synthesised by
(a) First procedure of A. cepa (<100 nm), (b) Second procedure of A. cepa (<100 nm), (c) First procedure of C. annum (<95 nm), (d) Second procedure of C. annum (<90 nm)
Fig. 4.
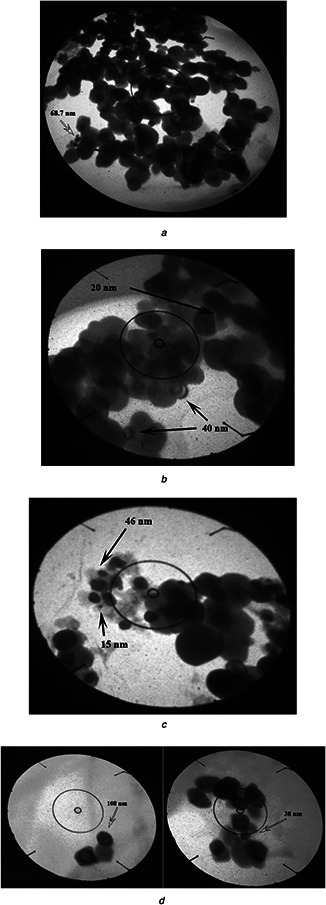
TEM of TiO2 produced by using
(a) First procedure of A. cepa plant extracts (68.7 nm) at 92,000× magnification, (b) Second procedure of A. cepa plant extracts (20, 40 nm) at 130,000× magnification, (c) First procedure of C. annum plant extracts (15, 46 nm), (d) Second procedure of C. annum plant extracts (38, 108 nm) at 130,000× magnification
The data of XRD of all samples indicated that the structures were rutile and anatase. The XRD pattern of the first and second procedures of A. cepa NPs showed six and five peaks, respectively. Strong diffraction peaks were 25.305° (101), 48.052° (200), 55.09° (323), 37.8° (004), 53.88° (105), which were anatase crystals and 36.2° (101), rutile crystals, for the first procedure of A. cepa (Fig. 5 a) and 25.217° (101), 47.946° (200), 37.684° (004), 37.8° (004), 53.3° (105), for the second procedure of A. cepa which were anatase and 36.04° (101), rutile crystals (Fig. 5 b).
Fig. 5.
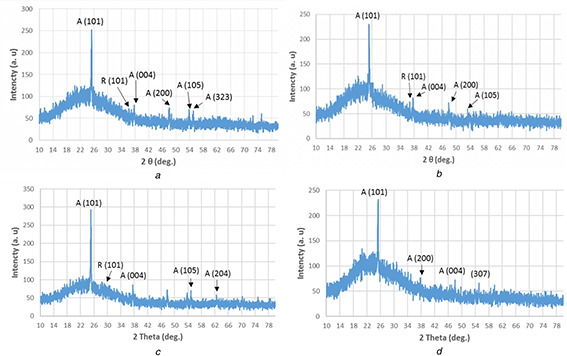
X‐ray pattern of TiO2 ‐NPs synthesised by
(a) First procedure of A. cepa, (b) Second procedure of A. cepa, (c) First procedure of C. annum, (d) Second procedure of C. annum
Strong peaks produced by the first procedure of C. annum were 25.1103° (101), 37.5897° (004), 54.9° (105), 62.48° (204) which were anatase crystals and 27.6° (101), 27.88° (101) for rutile crystals (Fig. 5 c). Strong peaks produced by the second procedure of C. annum were 25.324° (101), 48.054° (200), 37.832° (004), and 55.08° (307), their crystal shapes were anatase (Fig. 5 d). The average crystallite sizes were 39.16, 44.54 and 43.164, and 54.867 nm, respectively (Table 1).
Table 1.
Summary of X‐ray characterisation of green synthesised TiO2 NPs by the first and second procedures of A. cepa and C. annum plant extracts
| Sample | Planes (hkℓ) | 2θ, ° | FWHM, ° | D, nm | Strain XE‐4 | DIS X1014 |
|---|---|---|---|---|---|---|
| first procedure of A. cepa | 101 | 25.305 | 0.225 | 36.027 | 38.471 | 7.704 |
| 200 | 48.052 | 0.193 | 44.791 | 30.944 | 4.985 | |
| 323 | 55.094 | 0.243 | 36.671 | 37.796 | 7.436 | |
| second procedure of A. cepa | 101 | 25.217 | 0.199 | 40.630 | 34.113 | 6.058 |
| 200 | 47.946 | 0.167 | 51.916 | 26.697 | 3.710 | |
| 004 | 37.684 | 0.203 | 41.084 | 33.736 | 5.925 | |
| first procedure of C. annum | 101 | 25.110 | 0.219 | 36.967 | 37.493 | 7.318 |
| 200 | 47.838 | 0.197 | 43.979 | 31.515 | 5.17 | |
| 004 | 37.589 | 0.172 | 48.547 | 28.55 | 4.243 | |
| second procedure of C. annum | 101 | 25.324 | 0.195 | 41.598 | 33.319 | 5.779 |
| 200 | 48.054 | 0.15 | 57.721 | 24.012 | 3.002 | |
| 004 | 37.832 | 0.128 | 65.282 | 21.231 | 2.347 |
(hkℓ) planes: crystallographic plane; FWHM: full width at half maximum; D : dimension of crystal in nm; η × 10−4 : strain value; δ × 1014 : dislocation density.
The data of UV–Vis spectra found that the absorption spectra and optical band gaps of TiO2 ‐NPs produced by the first procedure of A. cepa were exactly the same in NPs produced by the second procedure of C. annum, strong absorption below 470 nm, and energy gap was 3.3 eV (Fig. 6 a). There was another similarity found between the second procedure of A. cepa and the first procedure of C. annum, their strong absorption and energy gaps were 270 nm and 6.3 eV respectively (Fig. 6 b).
Fig. 6.
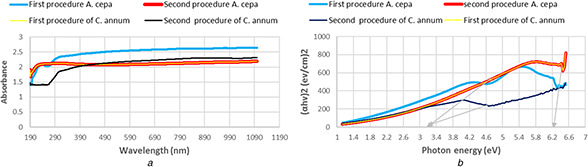
UV‐vis study of green synthesis TiO2 NPs using the first and second procedures of A. cepa and the first and second procedures of C. annum plants extracts.
(a) absorptions spectrum, (b) (ahv)2 versus photon energy and estimated optical absorption bandgap.
In FT‐IR spectra, samples exhibited absorption peaks at 528, 685, and 536, 685 cm−1 for the first and second procedures of A. cepa, respectively, which indicates the presence a Ti–O stretching bond. The 1637 and 1639 cm−1 for the first and second procedures of A. cepa, respectively, indicated the presence of an O–Ti–O stretching bond, in addition to 1458 and 1541 cm−1 (the second procedure of A. cepa) which indicates the presence Ti–O–Ti (Figs. 7 a and b).
Fig. 7.
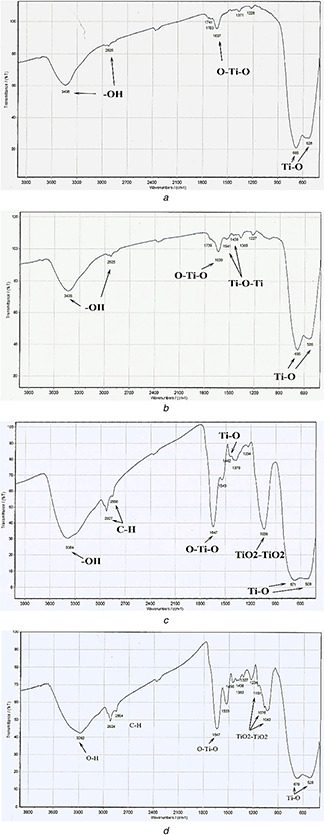
FT‐IR spectra of TiO2 produced by using
(a) First procedure of A. cepa plant extracts, (b) Second procedure of A. cepa plant extracts, (c) First procedure of C. annum plant extracts, (d) Second procedure of C. annum plant extracts
In the first procedure of C. annum, the strong peak located at 509 and 671 cm−1 in addition to 1442 cm−1 indicated the presence of Ti–O stretching while the peak at 1036 cm−1 corresponds to Ti–O/Ti–O–C and 1647 cm−1 indicating O–Ti–O (Fig. 7 c). In the second procedure of C. annum, the strong peak located at 528 and 679 cm−1 corresponds to the Ti–O stretching bond. The peaks at 1043, 1076 and 1151 cm−1 indicated Ti–O/Ti–O–C; moreover, the peak at 1647 cm−1 corresponds to the O–Ti–O stretching bond (Fig. 7 d).
The current study succeeded in synthesising TiO2 ‐NPs by A. cepa and C. annum plant extracts. The authors of [44] showed that these NPs had a high energy gap and high absorption coefficient than the traditional physiochemical procedure, they were around 351–362 nm and about 3.54–3.43 eV, which was prepared via two different routes: (i) the sol–gel route and (ii) the hydrothermal procedure, respectively [44], and compared with TiO2 fabricated by the hydrazine procedure with an energy gap of 3.36 eV for sample N, 3.32 eV for sample H, and 3.2 eV for sample C [45]. The band gap changes in the present results mainly due to the presence of stress applied along the weak direction of the anatase crystal [46].
TiO2 ‐NPs were fabricated by different plant extracts, A. vera leaves’ extract is an example of a synthesised tetragonal structure with crystallite size of 12 nm in XRD analysis. The absorption spectrum of the TiO2 sample was 393 nm with a band gap of the anatase phase (∼3.2 eV) [21]. Moreover, C. longa succeeds in biosynthesising TiO2 ‐NPs by two procedures with average sizes of 76.36 and 92.6 nm, respectively, and crystal shapes were of three forms: brookite, rutile, and anatase when the first procedure was used and it was pure anatase when the second procedure was used, their average sizes were 22.881 and 43.088 nm for colloidal solution and nano‐powder, respectively, in the first procedure and 45.808 nm for nano‐powder in the second procedure while in the current study, the average crystallite sizes were 39.16, 44.54 and 43.22 nm for the first and second procedures of A. cepa and C. annum, respectively [23]. Similar observations were made on spherical TiO2 ‐NPs produced from nyctanthes leaves extract, particles’ sizes in the range of 100–150 nm [22] and by Ailanthus alttissima plant extract [47].
Onion (family: Amaryllidaceae) contains phenolic substances especially quercetin, phenolic acids, sulphur, compounds (allicin), alkaloids, flavonoids, terpenes, steroids, vitamins and minerals [48], fructose, quercetin‐3‐glucoside, isorhamnetin‐4‐glucoside, mannose, galactose, glucose, xylose, organosulphur, compounds, thiosulphinates, S‐alk(en)yl cysteine sulphoxides, flavonoids, flavonols, selenium, cycloalliin, allylsulphides, seleno compounds and sulphur [49, 50, 51]. It can reduce gold to NPs due to vitamin C in onion extract [52]. Abdul Jalill and others in 2017 [53] had reported using A. cepa and C. annum plant extracts, acting as the reducing agent for fabricating ZnO NPs of well‐defined dimensions [53].
3.2 Antimicrobial activities
3.2.1 NPs and bulk particles alone
In this experiment, three treatments were found: TiO2 ‐NPs synthesised by A. cepa, TiO2 ‐NPs synthesised by C. annum and bulk particles of TiO2, each of them was used alone. K. pneumonia was not affected by all concentrations of bulk particles alone while bacteria were either not affected or stimulated by NPs alone (Figs. 8 a and b), while there were good activities against S. epidermidis, S. aureus, E. coli and C. albicans with significant differences (P ≤ 0.05) between the means of NPs compared with control except for S. epidermidis which had low reduction by the NPs synthesised by C. annum. All concentrations of NPs synthesised by A. cepa were more reducer to S. aureus compared with each of the bulk particles and NPs synthesised by C. annum, while in E. coli these effects were seen at lower concentrations (0.01–1 mg/ml) and the opposite was, also, seen at higher concentrations (Fig. 8 c). NPs synthesised by A. cepa were more reducer to all tested organisms than bulk particles and it was more reducer than NPs synthesised by C. annum. This might be due to the presence of the rutile crystal in NPs synthesised by C. annum and absence of NPs synthesised by A. cepa as seen in XRD analysis.
Fig. 8.
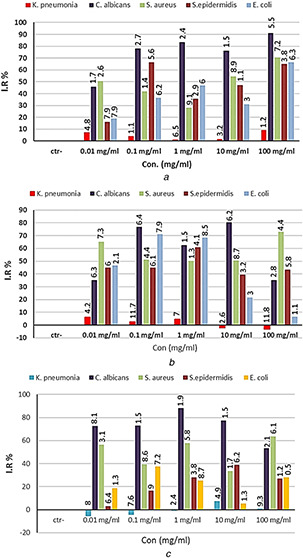
Antibacterial activity of
(a) Bulk TiO2 particles alone, (b) TiO2 NPs alone synthesised by A. cepa, (c) TiO2 NPs alone synthesised by the second procedure of C. annum. IR%: inhibition rate, Con.: concentration. The numbers above the bars are standard errors
3.2.2 Volatile oil of E. caryophyllata alone
There were good activities of all dilutions of oil alone against C. albicans and S. epidermidis with significant differences (P ≤ 0.05) between the means of NPs compared with control. The inhibition rate ranges from 79.74 to 93.16 and 79.07 to 86.3, respectively. K. pneumonia was less effective with IR% ranging from 31.82 to 45.45. S. aureus was reduced by most concentrations of oil except 12.5%, which induced growth (Fig. 9).
Fig. 9.
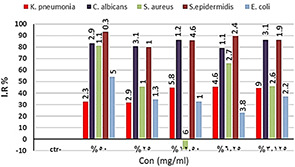
Antibacterial activity of E. caryophyllata oil alone. IR%: inhibition rate, Con.: concentrations. The numbers above the bars are standards errors
All dilutions of oil alone were more reducer to K. pneumonia, S. epidermidis and C. albicans than NPs alone, while other bacteria were more reducer in dose‐dependent manner. The toxicity of this plant alone is probably because of some components which were found in oils, especially eugenol 81.13 to 84.44% and eugenyl acetate (11.60–15.02%) or β‐caryophyllene (3.45–4.60%) [10].
3.2.3 Combination therapy
In combination therapy, different dilutions of volatile oils of E. caryophyllata were performed with various concentrations of NPs synthesised by A. cepa and C. annum. Results proved statistically significant difference (P ≤ 0.05) between all concentrations of combination therapies of NPs synthesised by A. cepa with oil plants on all different organisms under study compared with control (Fig. 10 a). The same results were obtained using combination therapy of NPs synthesised by C. annum with volatile oil (Fig. 10 b).
Fig. 10.
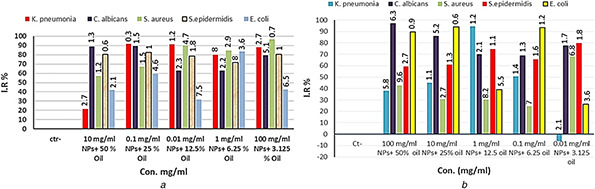
Antibacterial activity of combination therapy between oil of E. caryophyllata and each NP synthesised by the second procedure of
(a) A. cepa, (b) C. annum. IR%: inhibition rate, Con.: concentrations. The numbers above the bars are standards errors
3.2.4 Synergistic effect
In combination therapy between NPs synthesised by A. cepa and volatile oil, the results found four synergistic combination points in S. aureus, one synergistic combination point in E. coli and three synergistic combination points in C. albicans, the combination was tested at the 50% inhibitory concentration level. All combination points were located in the antagonism area in K. pneumonia and S. epidermidis (Table 2). While from the CI of combination therapy between NPs synthesised by C. annum with volatile oil there was one synergistic combination point in each of K. pneumonia, S. epidermidis, and S. aureus while there were four synergistic combination points in each of C. albicans and E. coli (Table 3).
Table 2.
CI data for non‐constant combination (oil plants with NPs synthesised by the second procedure of A. cepa) on different bacteria species
| Point | Dose | I.C. | |||||
|---|---|---|---|---|---|---|---|
| NPs, mg/ml | Plant oil, % | K. pneumonia | S. aureus | S. epidermidis | E. coli | C. albicans | |
| 1 | 10 | 50 | 98.4767 | 85.3019 | 7.02 × 1043 | 1.54155 | 40,051.8 |
| 2 | 0.1 | 25 | 436,871 | 2.45 × 10−4 | 1.96 × 1045 | 115.284 | 38,626.1 |
| 3 | 0.01 | 12.5 | 166,536 | 1.89 × 10−6 | 4.42 × 1037 | 1.84991 | 2.11 × 10−9 |
| 4 | 1 | 6.25 | 1409.76 | 3.61 × 10−6 | 2.17 × 1029 | 1.50 × 1022 | 1.32 × 10−7 |
| 5 | 100 | 3.125 | 17,006 | 1.49 × 10−8 | 3.98 × 1044 | 0.08953 | 0.0013 |
Bold I.C. (<1): synergistic effect, I.C. (>1): antagonism effect, I.C. (=1): additive.
Table 3.
CI data for non‐constant combination (oil plant with NPs synthesised by the second procedure of C. annum) on different bacteria species
| Point | Dose | I.C. | |||||
|---|---|---|---|---|---|---|---|
| NPs, mg/ml | Plant oil, % | K. pneumonia | S. aureus | S. epidermidis | E. coli | C. albicans | |
| 1 | 100 | 50 | 11,938.4 | 3.69 × 1013 | 4.54 × 1016 | 1.08 × 1010 | 0.02445 |
| 2 | 10 | 25 | 684.146 | 7819.84 | 3.47 × 1016 | 0.05994 | 6.00 × 10−13 |
| 3 | 1 | 12.5 | 525.967 | 339.669 | 144,690 | 0.02587 | 6.29 × 10−4 |
| 4 | 0.1 | 6.25 | 39.3592 | 0.00227 | 0.26187 | 0.00543 | 3.26 × 10−4 |
| 5 | 0.01 | 3.125 | 6.51 × 10−4 | 4.70 × 1031 | 5,302,729 | 7.90 × 10−4 | 1.20 × 10−10 |
Bold I.C. (<1): synergistic effect, I.C. (>1): antagonism effect, I.C. (=1): additive.
Combination therapy is the standard of care since it is a rational strategy to raise responses and tolerability's and to reduce resistance. Many different combination therapies are under investigation to facilitate some synergistic interactions between therapies which may permit lower concentration of agents to be used to minimise both cost and toxicity.
Some studies found that these NPs had either no toxicity in the dark [34] or a small anti‐bacterial effect at 100 mg/ml when they were used alone, but they could stimulate their antimicrobial activities especially under visible light absorbing photocatalysts which successfully kill E. coli and S. aureus [29], P. aeruginosa, E. hire, E. coli, B. fragilis and S. aureus or by UV light to kill bacteria in 60 min, so, it is easy to have antibacterial coatings mixed with paint in hospital. Recently, Abdul Jalill and Alli [35] found that it could be stimulated by the combination therapies of mixing them (50 mg/ml) with 50 mg/ml of methanol extraction of C. colocynthis which showed synergistic effects on E. coli and S. aureus [35].
In the current study, combinations of TiO2 ‐NPs with E. caryophyllata oil showed four synergistic combination points in S. aureus, one synergistic combination point in E. coli and three synergistic combination points in C. albicans. This is the first time that TiO2 ‐NPs with E. caryophyllata oil have been used as an antibacterial combination therapy.
Combination therapy is a common treatment for antimicrobial treatments, such as bee venom, its components, melittin and many plant’ secondary metabolites like benzyl isothiocyanate, sanguinarine, carvacrol, and berberine, which are mixed with antibiotics mostly more efficient than each of the treatment alone [54, 55].
The data of [56] suggest that the plants (Emblica officinalis and Nymphae odorata), which may be used with the antibiotic, amoxicillin, against methicillin resistant S. aureus infection alone or with combination [56]. Moreover, there was the highest and strong synergism between Carica papaya methanolic extract in combination with some antibiotics against bacterial strains [57]. Moreover, essential oils in addition to their combination with antibiotics/plant extracts have novel antimicrobial agents against multidrug resistant pathogenic microorganisms [58]. In addition, S. aureus killed by was potentiate when enterocin AS‐48 was used in combination therapies with the phenolic compounds carvacrol, geraniol, eugenol, terpineol, caffeic acid, p ‐coumaric acid, citral, and hydro‐cinnamic acid and these treatments were more efficient than each of them alone [59]. Recently, there was the synergistic activity of ZnO‐NPs with some antibiotics to resist: fungi, negative and positive gram bacteria [60].
4 Conclusions and recommendations
TiO2 ‐NPs could be produced by plant extracts of A. cepa and C. annum, most of these particles (but not all) were smaller than 100 nm, they had antimicrobial properties against some species of microbes when they were used alone. There were different antimicrobial activities of bulk particles and NPs alone in dose‐dependent manner against E. coli, S. aureus, C. albicans, and S. epidermidis. In addition, K. pneumonia was not affected by all concentrations of bulk particles alone while bacteria were either not affected or stimulated by NPs alone. Most dilutions of volatile oil of E. caryophyllata alone had antimicrobial activities.
Combination therapies reduced all studied organisms with different inhibition rates. There were four synergistic combination points of NPs synthesised by A. cepa with oil in S. aureus, one synergistic combination point in E. coli and three synergistic points in C. albicans. All points located in the antagonism area in K. pneumonia and S. epidermidis. While in the combination therapy of NPs synthesised by C. annum with oil, there was one synergistic point in each of K. pneumonia, S. aureus and S. epidermidis and four synergistic points in each of C. albicans and E. coli. In addition, all points in S. aureus are located in the antagonism area. Optimised other conditions for synthesising NPs such as charge, pH, pressure, and light, the exposure time will be helpful and more studies on detection of mechanical effects will be more beneficial.
5 Acknowledgments
The authors would like to thank Al‐Mustansiriyah University (www.uomustansiriyah.edu.iq) Baghdad, Iraq for its support of the current work.
6 References
- 1. Milind P. Deepa K.: ‘Clove: a champion spice’, Int. J. Res. Ayurveda Pharmacy, 2011, 2, (1), pp. 47 –54 [Google Scholar]
- 2. Daniel A.N. Sartoretto S.M. Schmidt G. et al.: ‘Anti‐inflammatory and antinociceptive activities of eugenol essential oil in experimental animal models’, Braz. J. Pharmacognosy, 2009, 19, (1B), pp. 212 –217 [Google Scholar]
- 3. Khare C.P.: ‘Indian medicinal plants: an illustrated dictionary’ (Springer‐Verlag; Berlin/Heidelberg: 2007), p. 836 [Google Scholar]
- 4. Joshi B. Sah G.P. Basnet B.B. et al.: ‘Phytochemical extraction and antimicrobial properties of different medicinal plants: Ocimum sanctum (Tulsi), Eugenia caryophyllata (Clove), Achyranthes bidentata (Datiwan) and Azadirachta indica (Neem)’, J. Microbiol. Antimicrobials, 2011, 3, (1), pp. 1 –7 [Google Scholar]
- 5. Fagere Z.O. Al Magbou A.Z.: ‘Antibacterial activity of clove oil against some microorganisms at Khartoum state, Sudan’, Adv. Med. Plant Res., 2016, 4, (4), pp. 122 –128 [Google Scholar]
- 6. Nuñez L. Aquino M.D.: ‘Microbicide activity of clove essential oil (Eugenia caryophyllata)’, Braz. J. Microbiol., 2012, 43, (4), pp. 1255 –1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sousa R.M.F. Morais S.A.L. Vieira R.B.K. et al.: ‘Chemical composition, cytotoxic, and antibacterial activity of the essential oil from Eugenia calycina cambess leaves against oral bacteria’, Ind. Crops Prod., 2015, 65, pp. 71 –78 [Google Scholar]
- 8. Chaieb K. Zmantar T. Ksouri R. et al.: ‘Antioxidant properties of the essential oil of Eugenia caryophyllata and its antifungal activity against a large number of clinical Candida species’, Mycoses, 2007, 50, pp. 403 –406 [DOI] [PubMed] [Google Scholar]
- 9. Kennouche A. Benkaci‐Ali F. Scholl G. et al.: ‘Chemical composition and antimicrobial activity of the essential oil of Eugenia caryophyllata cloves extracted by conventional and microwave techniques’, J. Biol. Act. Prod. Nat., 2015, 5, (1), pp. 1 –11 [Google Scholar]
- 10. Sohilait H.J.: ‘Chemical composition of the essential oils in Eugenia caryophylata, (Thunb.) from Amboina Island’, Sci. J. Chem., 2015, 3, (6), pp. 95 –99 [Google Scholar]
- 11. Bhakat C.: ‘Uniform TiO2 nanoparticles synthesis and characterization by hemolysis process’, Int. J. Eng. Sci. Technol., 2012, 4, (07), pp. 3081 –3085 [Google Scholar]
- 12. Ramakrishna G. Ghosh H.N.: ‘Optical and photochemical properties of sodium dodecylbenzenesulfonate (DBS)‐capped TiO2 nanoparticles dispersed in nonaqueous solvent’, Langmuir, 2003, 19, (3), pp. 505 –508 [Google Scholar]
- 13. Sahni S. Reddy S.B. Murty B.S.: ‘Influence of process parameters on the synthesis of nano‐titania by sol‐gel route’, Mater. Sci. Eng., 2007, A, 452–453, pp. 758 –762 [Google Scholar]
- 14. Nguyen T.V. Nguyen T.A. Dao P.H. et al.: ‘Effect of rutile titania dioxide nanoparticles on the mechanical property, thermal stability, weathering resistance and antibacterial property of styrene acrylic polyurethane coating’, Adv. Nat. Sci., Nanosci. Nanotechnol., 2016, 7, (4), pp. 1 –9 [Google Scholar]
- 15. Lucky R.A.: ‘Synthesis of TiO2 ‐based nanostructured materials using a soil‐gel process in supercritical CO2 ’. Thesis for the Degree of Doctor of Philosophy, School of Graduate and Postdoctoral Studies, The University of Western Ontario, London, Ontario, Canada, 2008. [Google Scholar]
- 16. Ahmed M.A. El‐Shennawy M. Althomali Y.M. et al.: ‘Effect of titanium dioxide nano particles incorporation on mechanical and physical properties on two different types of acrylic resin denture base’, World J. Nano Sci. Eng., 2016, 6, pp. 111 –119 [Google Scholar]
- 17. Narayanan K.B. Sakthivel N.: ‘Biological synthesis of metal nanoparticles by microbes’, Adv. Colloid Interface Sci., 2010, 156, pp. 1 –13 [DOI] [PubMed] [Google Scholar]
- 18. Douglas T. Strable E. Willits D. et al.: ‘Protein engineering of a viral cage for constrained nanomaterials synthesis’, Adv. Mater., 2002, 14, (6), pp. 415 –418 [Google Scholar]
- 19. Makarov V.V. Love A.J. Sinitsyna O.V. et al.: ‘Green nanotechnologies: synthesis of metal nanoparticles using plants’, Acta Naturae, 2014, 6, (1), pp. 35 –44 [PMC free article] [PubMed] [Google Scholar]
- 20. Gowramma B. Keerthi U. Mokula R. et al.: ‘Biogenic silver nanoparticles production and characterization from native stain of corynebacterium species and its antimicrobial activity’, 3 Biotech, 2015, 5, (2), pp. 195 –201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khadar A. Behara D.K. Kumar M.K.: ‘Synthesis and characterization of controlled size TiO2 nanoparticles via green route using Aloe vera extract’, Int. J. Sci. Res., 2016, 5, (11), pp. 1913 –1916 [Google Scholar]
- 22. Sundrarajan M. Gowri S.: ‘Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor‐tristis leaves extracts’, Chalcogenide Lett., 2011, 8, (8), pp. 447 –451 [Google Scholar]
- 23. Abdul Jalill R.DH. Nuaman R.S. Abd A.N.: ‘Biological synthesis of titanium dioxide nanoparticles by Curcuma longa plant extract and study its biological properties’, World Sci. News, 2016, 49, (2), pp. 204 –222 [Google Scholar]
- 24. Seabra A.B. Duran N.: ‘Nitric oxide‐releasing vehicles for biomedical applications’, J. Mater. Chem., 2010, 20, pp. 1624 –1637 [Google Scholar]
- 25. Goh P.S. Ng B.C. Lau W.J. et al.: ‘Inorganic nanomaterials in polymeric ultrafiltration membranes for water treatment’, Purification Rev., 2015, 44, pp. 216 –249 [Google Scholar]
- 26. Allahverdiyev A.M. Abamor E.S. Bagirova M. et al.: ‘Antimicrobial effects of TiO2 and Ag2 O nanoparticles against drug‐resistant bacteria and Leishmania parasites’, Future Microbiol., 2011, 6, (8), pp. 933 –940 [DOI] [PubMed] [Google Scholar]
- 27. Chen X. Mao S.S.: ‘Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications’, Chem. Rev., 2007, 107, pp. 2891 –2959 [DOI] [PubMed] [Google Scholar]
- 28. Abdul Jalill R.DH. Yousef A.M.: ‘Comparison the phytotoxicity of TiO2 nanoparticles with bulk particles on amber 33 variety of rice (Oryza sativa) in vitro ’, Sch. Acad. J. Biosci., 2015, 3, (3), pp. 254 –262 [Google Scholar]
- 29. Hu C. Lan Y. Qu J. et al.: ‘Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria’, J. Phys. Chem. B, 2006, 110, pp. 4066 –4072 [DOI] [PubMed] [Google Scholar]
- 30. Abass R.H. Haleem A.M. Hamid M.K. et al.: ‘Antimicrobial activity of TiO2 NPs against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923’, Int. J. Comput. Appl. Sci., 2017, 2, (1), pp. 6 –10 [Google Scholar]
- 31. Stankic S. Suman S.S. Haque F. et al.: ‘Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties’, J. Nanobiotechnol., 2016, 14, p. 73: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perez‐Espitia P.J. Ferreira‐Soares N.F. dos Reis Coimbra J.S. et al.: ‘Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications’, Food Bioprocess Technol., 2012, 5, pp. 1447 –1464 [Google Scholar]
- 33. Duran N. Seabra A.B.: ‘Metallic oxide nanoparticles: state of the art in biogenic syntheses and their mechanisms’, Appl. Microbiol. Biotechnol., 2012, 95, (2), pp. 275 –288 [DOI] [PubMed] [Google Scholar]
- 34. Muranyi P. Schraml C. Wunderlich J.: ‘Antimicrobial efficiency of titanium dioxide‐coated surfaces’, J. Appl. Microbiol., 2010, 108, (6), pp. 1966 –1973 [DOI] [PubMed] [Google Scholar]
- 35. Abdul Jalill R.DH. Ali A.M.: ‘Stimulate the antimicrobial activity of TiO2 nanoparticles (NPs) using methanol extract of Citrullus colocynthis ’, Sch. Acad. J. Pharm., 2016, 5, (8), pp. 326 –332 [Google Scholar]
- 36. Naveen H.K.S. Kumar G. Karthik L. et al.: ‘Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp.’, Arch. Appl. Sci. Res., 2010, 2, (6), pp. 161 –167 [Google Scholar]
- 37. Ba‐Abbad M.M. Kadhum A.H. Mohamad A.B. et al.: ‘Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation’, Int. J. Electrochem. Sci., 2012, 7, pp. 4871 –4888 [Google Scholar]
- 38. Meshram R.S. Suryavanshi B.M. Thombre R.M.: ‘Structural and optical properties of CdS thin films obtained by spray pyrolysis’, Adv. Appl. Sci. Res., 2012, 3, p. 1563 [Google Scholar]
- 39. Cullity B.D.: ‘Elements of X‐ray diffraction’ (Addison Wesley, London, 1978, 2nd edn.), p. 531 [Google Scholar]
- 40. Wei W. Mao X. Ortiz L.A. et al.: ‘Oriented silver oxide nanostructures synthesized through a template‐free electrochemical route’, J. Mater. Chem., 2011, 21, (2), pp. 432 –438 [Google Scholar]
- 41. Jobst P.J. Stenzel O. Schürmann M. et al.: ‘Optical properties of unprotected and protected sputtered silver films: surface morphology vs. UV/VIS reflectance’, Adv. Opt. Technol., 2013, 3, pp. 91 –102 [Google Scholar]
- 42. National Committee for Clinical Laboratory Standards : ‘Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7‐A’ (NCCLS, Villanova, PA, 1986) [Google Scholar]
- 43. Bijnsdorp I.V. Giovannetti E. Peters G.J.: ‘Analysis of drug interactions’, Methods Mol. Biol., 2011, 731, pp. 421 –434 [DOI] [PubMed] [Google Scholar]
- 44. Vijayalakshmi R. Rajendran V.: ‘Synthesis and characterization of nano‐TiO2 via different methods’, Arch. Appl. Sci. Res., 2012, 4, (2), pp. 1183 –1190 [Google Scholar]
- 45. Reddya K.M. Manoramaa S.V. Reddyb A.R.: ‘Bandgap studies on anatase titanium dioxide nanoparticles’, Mater. Chem. Phys., 2002, 78, pp. 239 –245 [Google Scholar]
- 46. Yin W. Chen S. Yang J. et al.: ‘Effective band gap narrowing of anatase TiO2 by strain along a soft crystal direction’, Appl. Phys. Lett., 2010, 96, (22), pp. 221901 –221903 [Google Scholar]
- 47. Ganesan S. Babu I.G. Mahendran D. et al.: ‘Green engineering of titanium dioxide nanoparticles using Ageratina altissima (L.)’, King & H.E. Robines. medicinal plant aqueous leaf extracts for enhanced photocatalytic activity’, Ann. Phytomed., 2016, 5, (2), pp. 69 –75 [Google Scholar]
- 48. Bystrická J. Musilová J. Vollmannová A. et al.: ‘Bioactive components of onion (Allium cepa L.). A review’, Acta Alimentaria, 2013, 42, (1), pp. 11 –22 [Google Scholar]
- 49. Arnault I. Auger J.: ‘Seleno‐compounds in garlic and onion’, J. Chromatogr. A, 2006, 1112, (1–2), pp. 23 –30 [DOI] [PubMed] [Google Scholar]
- 50. Hubbard G.P. Wolffram S. de‐Vos R. et al.: ‘Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen‐stimulated platelet activation pathway in man: a pilot study’, Br. J. Nutr., 2006, 96, (3), pp. 482 –488 [PubMed] [Google Scholar]
- 51. Lanzotti V.: ‘The analysis of onion and garlic’, J. Chromatogr., 2006, 1112, (1–2), pp. 3 –22 [DOI] [PubMed] [Google Scholar]
- 52. Parida U.K. Bindhani B.K. Nayak P.: ‘Green synthesis and characterization of gold nanoparticles using onion (Allium cepa) extract’, World J. Nano Sci. Eng., 2011, 1, (4), pp. 93 –98 [Google Scholar]
- 53. Abdul Jalill R.DH. Jawad M.M.H.M. Abd A.N.: ‘Plants extracts as green synthesis of zirconium oxide nanoparticles’, J. Genet. Environ. Res. Conserv., 2017, 5, (1), pp. 6 –23 [Google Scholar]
- 54. Shipradeep Karmakar S. Khare R.S. et al.: ‘Development of probiotic candidate in combination with essential oils from medicinal plant and their effect on enteric pathogens: a review’, Gastroenterol. Res. Pract., 2012, (6), pp. 1 –6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Al‐Ani I. Zimmermann S. Reichling J. et al.: ‘Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi‐drug resistant microbial pathogens’, Phytomedicine, 2015, 22, (2), pp. 245 –255 [DOI] [PubMed] [Google Scholar]
- 56. Mandal S. Debmandal M. Pal N.K. et al.: ‘Synergistic anti–Staphylococcus aureus activity of amoxicillin in combination with Emblica officinalis and Nymphae odorata extracts’, Asian Pac. J. Tropical Med., 2010, 3, (9), pp. 711 –714 [Google Scholar]
- 57. Rakholiya K. Chanda S.: ‘ In vitro interaction of certain antimicrobial agents in combination with plant extracts against some pathogenic bacterial strains’, Asian Pac. J. Tropical Biomed., 2012, 2, (2), pp. S1466 –S1470 [Google Scholar]
- 58. Padalia H. Moteriya P. Baravalia Y. et al.: ‘Antimicrobial and synergistic effects of some essential oils to fight against microbial pathogens – a review’, in Méndez‐Vilas A. (Ed.): ‘The battle against microbial pathogens: basic science, technological advances and educational programs’ (Formatex Research Center, Zurbaran, Badajoz, Spain, 2015), pp. 34 –45 [Google Scholar]
- 59. Grande M.J. López R.L. Abriouel H. et al.: ‘Treatment of vegetable sauces with enterocin AS‐48 alone or in combination with phenolic compounds to inhibit proliferation of Staphylococcus aureus ’, J. Food Prot., 2007, 70, (2), pp. 405 –411 [DOI] [PubMed] [Google Scholar]
- 60. Sharma N. Jandaik S. Kumar S.: ‘Synergistic activity of doped zinc oxide nanoparticles with antibiotics: ciprofloxacin, ampicillin, fluconazole and amphotericin B against pathogenic microorganisms’, Ann. Br. Acad. Sci., 2016, 88, (3), pp. 1689 –1698 [DOI] [PubMed] [Google Scholar]


