Abstract
The aqueous extract of Chinese winter jujube (Ziziphus jujuba Mill. cv. Dongzao) was used as reducing and capping agents for the synthesis of silver nanoparticles (AgNPs) for the first time. The resulting AgNPs were characterised by UV/Visible (UV–Vis) spectroscopy, atomic force microscope, transmission electron microscopy, selected area electron diffraction, X‐ray diffraction, scanning electron microscopy, energy‐dispersive X‐ray and Fourier transform infrared spectroscopy (FTIR). The colloidal solution of AgNPs gave a maximum UV–Vis absorbance at 446 nm. The synthesised nanoparticles were almost in the spherical shapes with an average size of 11.5 ± 4. 8 nm. FTIR spectra were applied to identify the functional groups which were possibly responsible for the conversion of metal ions into nanoparticles. The results showed that the prepared AgNPs were coated with the biomolecules in the extract. The biosynthesised AgNPs showed a remarkable catalytic activity at room temperature, and they also showed good antibacterial properties against Escherichia coli and Staphylococcus aureus.
Inspec keywords: silver, nanoparticles, nanofabrication, antibacterial activity, biomedical materials, nanobiotechnology, scanning electron microscopy, X‐ray diffraction, transmission electron microscopy, ultraviolet spectra, visible spectra, X‐ray chemical analysis, Fourier transform infrared spectra, catalysis
Other keywords: wavelength 446 nm, temperature 293 K to 298 K, Ag, Escherichia coli, Staphylococcus aureus, biomolecules, catalytic activity, metal ions, colloidal solution, FTIR spectra, UV‐vis absorbance, TEM, SEM, XRD, Fourier transform infrared spectroscopy, energy‐dispersive X‐ray spectroscopy, scanning electron microscopy, X‐ray diffraction, selected area electron diffraction, transmission electron microscopy, atomic force microscopy, UV‐visible spectroscopy, catalytic properties, antibacterial properties, Chinese winter jujube extract, silver nanoparticles, facile phyto‐mediated synthesis
1 Introduction
Silver nanoparticles (AgNPs) are the clusters of silver atoms with the size in the range of 1–100 nm at least in one dimension. In recent years, AgNPs attract more and more attentions due to its unique physical, chemical properties and their widely applications in many fields [1]. Their excellent antibacterial activities make AgNPs popular for medical and clinical applications [2] and they can inhibit or even kill both Gram‐positive and Gram‐negative bacteria [3].
Biosynthesis methodology is considered as a cost‐effective and eco‐friendly alternative route to chemical and physical methods for the preparation of nanoparticles [4]. Following this strategy, nanomaterials can be synthesised using both microorganism [5] and plant extracts [1]. Various natural products had been used to prepare nanoparticles, such as tea leaves [6], leaves of lemon [7], aloe vera plant extract [8] and others [9, 10]. The biochemicals in the biota, such as vitamins, sugars, phenolics and flavonoids, were considered to be responsible for the reduction of Ag+ to AgNPs [11].
Jujube (Ziziphus jujuba Mill.) belongs to Rhamnaceae deciduous shrubs and widely distributed in the temperate and subtropical areas of the Northern Hemisphere. It is popularly and widely consumed as a tasty and highly nutritious food in Asian countries. China is now the largest producer of this common fruit in the world [12]. The nutritional value of jujube comes from its rich content of vitamins (vitamins C, B1 and B2) as well as other bioactive nutrients, such as sugars, phenolics, flavonoids, amino acids, triterpenoids, carotenoids and saponins [13, 14]. These bioactive compounds in the fruit not only result in the good taste of jujube but also contribute to its functional values for other utilisations [15]. The bioactive compounds in jujube are considered to benefit the health and decrease the risk of diseases caused by oxidative stress with its high natural antioxidant capacity [13, 14]. In addition to the fruit of jujube, the other parts of jujube, including bark, leaves, flowers and seeds, have also been used as Chinese traditional medicines [14]. The interest in the functional application of jujube rather than food is substantially increased considering its huge production, wide distribution and easy accessibility [15]. Recently, jujube has also been successfully used as an eco‐friendly adsorbent to remove heavy metals from industrial wastewater, which greatly expands the utilisation scope of jujube beyond food and traditional medicine [16].
Chinese winter jujube (Z. jujuba Mill. cv. Dongzao) was found to be of the highest content of total flavonoids (1851.96 mg QE/100 g DW) compared with other cultivars and is popularly cultivated in north China with huge annual production [17]. Therefore, Chinese winter jujube can be potentially employed as a natural and economic reductant to biosynthesise AgNPs. In this study, we reported a novel and green approach for the synthesis of AgNPs by reducing aqueous Ag+ ions to nanoparticles using Chinese winter jujube extract without any added toxic chemicals. The biological compounds in the extract were used as both reducing and stabilising agents. This simple procedure has several advantages (easy to operate, cost‐effective and eco‐friendly) over the conventional methods. The antibacterial activities of the as‐obtained AgNPs were tested against Escherichia coli and Staphylococcus aureus. The catalytic activity of the AgNPs was also confirmed by the degradation of 4‐nitrophenol (4‐NP) in water solution. This study will help us to explore the possible applications of jujube, a popular, cheap and natural biomass, in the field of nanoscience and technology.
2 Materials and methods
2.1 Materials
Chinese winter jujube was purchased from a supermarket in Baoding, China. Silver nitrate (Tianjin Reagent Factory, China) was used as the precursors for the synthesis of AgNPs. Sodium borohydride (NaBH4) was purchased from Tianjin Kemiou Chemical Reagent Co. Ltd. All other chemicals, such as NaOH and HNO3, were of analytical grade and all aqueous solutions were prepared in deionised water (>18 MΩ·cm).
2.2 Preparation of jujube extract
The fresh Chinese winter jujube was washed three times with deionised water to remove any impurities present on its surface before use. The cleaned Chinese winter jujube was then crushed into small pieces with a sterilised knife. Approximately 5.0 g of the biomass was weighed in a beaker and boiled for 10 min in 150 ml of distilled water. Then, the extracts were cooled down to room temperature. The mixture was then filtrated with 0.1 μm filter to remove the residual biomass in the extract. The filtered extract was stored in a refrigerator at 4°C for use. From an economic prospective, the extract was also obtained at room temperature (Supplementary Material). The extract was also applied to compare with the boiled extract.
2.3 Synthesis of AgNPs using Chinese winter jujube extract
In a typical reaction procedure, 8.0 ml of the Chinese winter jujube extract (pH 4.81) was mixed with 2.0 ml of the AgNO3 aqueous solution (10 mM, pH 5.27) and kept at room temperature (25°C) for hours. The pH of mixture solution was monitored by a pH meter and the value was 4.85. The change of colour took place within 20–30 min from colourless to reddish in the presence of silver nitrate, whereas no colour change was observed either in the extract solution without silver nitrate nor in the AgNO3 solution alone. The colour change preliminarily indicated the formation of AgNPs in the solution [18]. To investigate the effect of the AgNO3 concentration, 2.0 ml of different concentrations of AgNO3 were added into 8.0 ml extract, which gave the final concentration of AgNO3 to 1, 2, 4 and 5 mM. Then the mixture stood for about 24 h at room temperature. In order to study the effect of the incubation temperature, the optimum concentration of AgNO3 aqueous solution was added into 8.0 ml of Chinese winter jujube extract, then the mixture was incubated at 40, 50, 60 and 70°C, respectively. To investigate the possible effects of pH on the synthesis process, the various pH values of extract (2, 4, 6, 8, 10, 12 and 14) were adjusted by 0.1 M NaOH or 0.1 M HNO3. Then the optimum concentration of AgNO3 was added to 8.0 ml of the treated extract at the selected temperature to start the biosynthesis procedure. To obtain the powder of AgNPs, the AgNPs suspensions synthesised under the optimum conditions were centrifuged at 14,000 rpm for 30 min. The pellet was collected and washed by deionised water for several times to remove the remained bioactive compounds on the surface of particles. Then the final pellet was freeze‐dried and collected for further characterisations.
2.4 Characterisations
2.4.1 UV/Visible spectroscopy (UV/Vis)
The surface plasmon resonance (SPR) peak is the primary characteristic for the identification of AgNPs. The SPR peaks were monitored by a UV/visible spectrophotometer (T6, Persee General, Beijing, China). The reaction mixture was diluted for 1.0–10 times (according to the concentration of the mixture) and then poured into a 1.0 cm cuvette. The UV–Vis spectra were recorded at a resolution of 1 nm in the wavelength range of 300–800 nm at room temperature.
2.4.2 Conversion of Ag+ ions
The synthesised AgNPs suspensions were subjected to high‐speed centrifugation at 14,000 rpm for 30 min to separate the remained Ag+ from the produced AgNPs. The concentration of Ag+ in supernatant represented the concentration of the remained or unreacted Ag+. The reacted Ag+ was calculated by deducting the remained Ag+, which measured using inductively coupled plasma‐mass spectrometry (Agilent 7500ce, America). The conversion of Ag+ was determined by comparing the mass of the reacted Ag+ with the mass of the initial Ag+.
2.4.3 Atomic force microscope (AFM)
The reduced mixed solution was dropped few drops onto the silicon pellet, and then fasten it on the sample platform of AFM. The morphology of the nanoparticles was scanned by an AFM (CSPM5500, Being Nano‐instruments, Ltd., China) under the tapping mode.
2.4.4 Fourier transform infrared spectroscopy (FTIR) analysis
FTIR analyses were carried out to identify the potential biomolecules in Chinese winter jujube extract mainly responsible for the reduction, stabilisation and capping of the bioreduced AgNPs and predict their roles in the biosynthesis of AgNPs. In brief, the samples for FTIR analysis were prepared by mixing the AgNPs with KBr powder, and dried for 30 min. Then, the samples were measured using a FTIR spectrophotometer (Tensor FTIR spectrophotometer from Bruker Optics) with wavelength range between 4000 and 400 cm−1.
2.4.5 Scanning electron microscopy (SEM) and energy‐dispersive X‐ray analysis (EDX)
A 1.0 ml of the AgNPs colloid and the extract were coated on the silicon support by placing small drops of nanoparticles suspension and eliminating the moisture under vacuum condition at room temperature before adding another drop. The morphologies of the AgNPs were characterised using a SEM, which was attached with phoenix EDX for the identification of the elemental composition. The size distribution of AgNPs was estimated using ImageJ software.
2.4.6 X‐ray powder diffraction (XRD) analysis
XRD meter (D8 ADVANCE, Bruker/Switzerland) with Cu‐Kα radiation (λ = 1.5418 Å) with a scan speed of 2°/min was used to analyse the structure of AgNPs. The diffraction pattern was obtained with conditions at 40 kV and 30 Ma, and the particle sizes of silver were calculated using Debye–Scherrer equation: L = 0.9λ /β cosθ, where λ is the wavelength of the X‐ray, β is full‐width at half‐maximum and θ is the Bragg's angle.
2.4.7 Transmission electron microscopy (TEM) and particle size distribution
The TEM images of biosynthesised AgNPs were observed by using a Tecnai G2 F20 S‐TWIN (FEI, the United States of America) operated at an accelerating voltage of 200 kV. The samples for TEM were sonicated for 15 min. A drop of solution was loaded on a carbon‐coated copper grid, and the solvent was allowed to evaporate under infrared light for 10 min. The AgNPs in the photograph of TEM was counted by Sigma scan Pro software. The average particle size of each AgNP in the pictures was measured. Then, the particle size distributions were calculated by Origin software.
2.5 Antibacterial activity of AgNPs
The antibacterial activity of AgNPs was tested against E. coli and S. aureus. The two strains of bacteria were cultured in LB (Luria‐Bertani broth) medium at 37°C for 18 h. Then 100 μl of bacterial suspension was smeared to the LB solid medium and five Oxford cups were put on it. A 50 μl of Chinese date extract, AgNPs colloid solution (1.0, 2.0 and 4.0 mM) and gentamicin were added into the above oxford cup, respectively. The LB solid medium was then incubated in an incubator at 37°C for 24 h. The zones of inhibition were measured and the antibacterial properties were evaluated. Three replicates were maintained for each bacterium during the tests.
2.6 Catalytic activity of AgNPs
The catalytic activity of AgNPs were evaluated by the degradation of 4‐NP in the presence of NaBH4. A 20 μl of the AgNPs colloid was added to the mixture solution of deionised water, freshly prepared NaBH4 (5 mM) and 4‐NP (20 mM). The solutions were mixed by mild magnetic stirring and then transferred to a quartz cuvette. The process of the degradation was monitored by a UV–Vis spectrometer at specific time intervals. The degradation of 4‐NP in the absence of AgNPs was used as the control in our experiment.
3 Results and discussion
3.1 Biosynthesis of AgNPs
The starting point of the reaction was observed when the colour of the mixture turned to yellowish brown even black from transparent [18]. Comparing with the AgNPs synthesised using the extract prepared by boiling, the AgNPs synthesised by the extract obtained at room temperature was turbid. The UV–Vis spectrum of AgNPs was broader and more asymmetry (Supplementary Material Figure S1). These phenomena indicated the agglomeration of AgNPs in the solution. The results indicated that boiling is a more appropriate way to obtain the extract for the biosynthesis procedure. Fig. 1 showed the UV–Vis absorption spectra of the AgNPs produced at different AgNO3 concentrations. From the four pictures, we can preliminarily draw a conclusion that the characteristic SPR band centred at ∼415 nm.
Fig. 1.
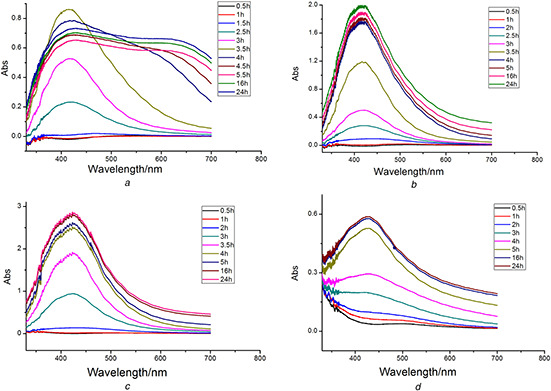
UV–Vis spectra of AgNPs synthesised by Chinese winter jujube extract at different AgNO3 concentrations
(a) 1 mM, (b) 2 mM, (c) 4 mM, (d) 5 mM
When 1.0 mM of AgNO3 was used, there was no reaction occurred in the mixture during the initial 1.5 h (Fig. 1 a). The colour of the mixture changed to yellow after 2.5 h. The characteristic SPR band stably centred at 415 nm during the synthesis process and the absorbance intensity gradually increased with reaction time until 4 h; but the absorbance decreased after 4 h and the peak became flat, which indicated that aggregation of the as‐produced AgNPs occurred. The yield of the produced AgNPs was calculated according to the procedure demonstrated in Section 2.4.2. A total of 42.54 ± 0.36% of Ag+ was converted into AgNPs after 24 h reaction.
When 2 mM of AgNO3 was applied, the synthesis reaction started after 2 h. The reaction speed looked stable during the initial 4 h (Fig. 1 b). After 4 h, the reaction speed slowed down and finally completed in 24 h. ICP‐MS measurement indicated 51.03 ± 0.43% of the total Ag+ ions was converted into AgNPs after 24 h and then kept constant with time increasing. The characteristic SPR bands were narrow and sharp during the whole reaction process, which indicated that the sizes of AgNPs were uniform. When 4 mM AgNO3 was used, the reaction started after 2 h (Fig. 1 c). The concentration of the precursor was high compared with the reactive components in the extract. The absorption peak of biosynthesised nanoparticles became wider with the reaction time. The particle sizes of the biosynthesised nanoparticles at this concentration of AgNO3 were larger than that obtained using 2 mM. If higher concentration of AgNO3 was used (Fig. 1 d), the reaction obviously slowed down and the adsorption peak became broad and flat. The intensity of peaks significantly decreased compared with the lower concentrations. The conversion proportion of Ag+ were only 43.30 ± 0.81% and 37.31 ± 1.15% which slightly increased with time increasing for 4 and 5 mM AgNO3. Such low conversion proportion of Ag+ and broad peaks illustrated that too high concentration of precursors would not benefit the reaction. The reactive components used as reducing and capping reagents in the extract were limited and they will be used up when too many precursors are present in the solution. The residual precursors promoted the aggregation between as‐prepared AgNPs. When the initial concentration of AgNO3 was 2 mM, the sizes of the nanoparticles were uniform, and the yield of nanoparticles was larger than the other concentrations.
The effects of different temperatures on the synthesis of the AgNPs were also investigated. The experiments were carried out at four different reaction temperatures (40, 50, 60 and 70°C) (Fig. 2). When the temperature was higher than 70°C, the reaction speed became too fast to measure the UV–Vis spectra. The narrow and stable adsorption peaks recorded at different temperatures suggested that the optimal reaction temperature was 50°C. The conversion proportion of Ag+ was 71.28 ± 0.85% under these conditions.
Fig. 2.
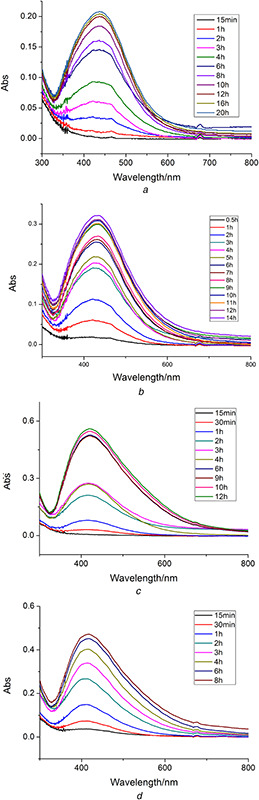
UV–Vis spectra of AgNPs synthesised by Chinese winter jujube extract at different temperatures
(a) 40°C, (b) 50°C, (c) 60°C, (d) 70°C
The absorption spectra of the mixed solution under different pH were recorded at specified time intervals and were shown in Fig. 3. When the mixture solution is acidic, the reaction rate decreased with pH decreasing. The biosynthesis reaction could not occur at pH 2. It could be interpreted that the reducing agent was destructed when the condition was strongly acidic. Although the reaction occurred at the higher pH values (4 or 6), the SPR peaks were broad and flat. The results indicated that AgNPs could not be synthesised under acidic conditions in the system. When the reaction was carried out under basic conditions (pH 8, 10, 12 and 14), the UV peaks of the solution became narrow. pH 12 was chosen as the optimised value for the following experiments. AgNO3 concentration of 2 mM, incubation temperature of 50°C, and extract pH of 12 were selected as the optimum conditions. The enhanced conversion proportion of Ag+ (97.49 ± 0.57%) was obtained under these conditions. The samples for the further characterisations were obtained under the optimum conditions.
Fig. 3.
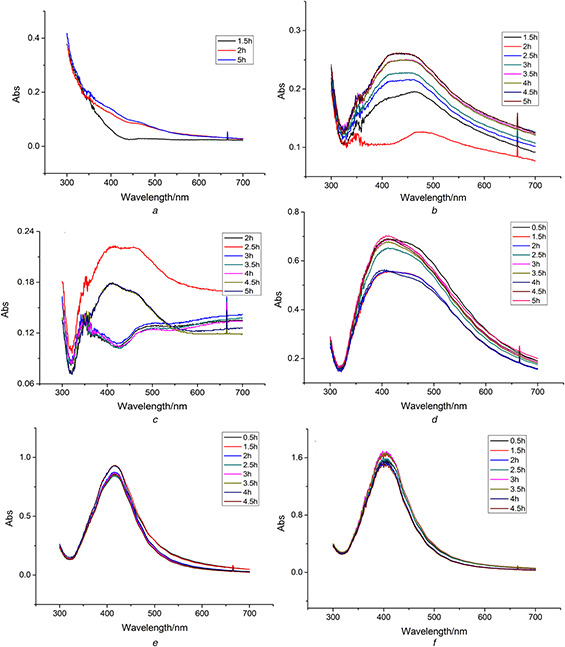
UV–Vis spectra of AgNPs synthesised by Chinese winter jujube extract at different pH
(a) 2, (b) 4, (c) 6, (d) 8, (e) 10, (f) 12
3.2 Characterisations
FTIR analysis was carried out to identify the possible functional groups responsible for the reduction of silver nitrate and stabilisation of the AgNPs. Fig. 4 showed the FTIR spectra of Chinese‐date powder (a) and AgNPs synthesised using Chinese winter jujube extract (b) (in the range of 4000–400 cm−1). The results revealed that there were six peaks at 3410, 2925, 1610, 1384, 1105 and 610 cm−1, respectively. The absorption bands at 2925 and 1610 cm−1 may be attributed to the stretching vibration of υ (=C–H) and υ(C = C) [19]. The peak at 1610 cm−1 could also be related to the surface adsorbed water molecule. The peak at 610 cm−1 may be assigned to δ (C–H) bending vibration or C–S, R–C–CH3 stretching for sulphur compounds [20]. Similarly, the bands at 3410 and 1384 cm−1 may be assigned to the stretching vibration of υ (O–H) and in‐plane bending vibration of δ (O–H) [21], respectively. Moreover, the band at 1105 cm−1 may be contributed by skeletal C–O and C–C vibration bands of glycosidic and pyrinoid ring [22]. The FTIR spectra of Chinese‐date powder were similar with that of the biosynthesised AgNPs, which demonstrated that AgNPs were capped with the bioactive compounds from the extract. The biological compounds in the extract played a very important role during the synthesis. FTIR spectra indicated the possible presence of the residual vitamin complexes, amino acids, flavonoids and phenolics on the surface of the obtained AgNPs.
Fig. 4.
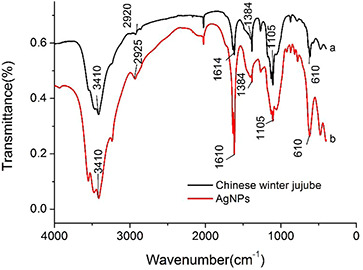
Characterisation by FTIR analysis
(a) FTIR spectra of Chinese‐date powder (b) AgNPs synthesised using Chinese winter jujube
AFM, SEM and EDX were employed to gain further insights of the biosynthesised AgNPs. The micrographs of the AgNPs were shown in Fig. 5. The SEM images revealed that most of biosynthesised AgNPs were spherical. A few of AgNPs were present in angular and nubby/clumpy shapes (Fig. 5 a). The diameters of synthesised nanoparticles from SEM images were about 10–35 nm, and the average diameter was 14.2 ± 4.7 nm based on the measurements of more than 150 particles.
Fig. 5.
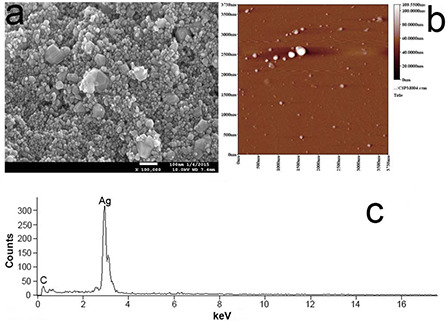
Micrographs of the silver nanoparticles
(a) SEM image, (b) AFM image, (c) EDX patterns of AgNPs synthesised using Chinese winter jujube
The AFM images further confirmed the separate distribution of the obtained nanoparticles (Fig. 5 b). The EDX analysis showed a strong signal of elemental silver, which confirmed the presence of AgNPs (Fig. 5 c).
Fig. 6 showed the TEM images of AgNPs with a size distribution range from 3.0 to 31.0 nm (Fig. 6). The average size of synthesised AgNPs was found to be 11.5 ± 4.8 nm via counting 150 particles. (Supplementary Material Figure S2) The shapes of the particles were spherical and the size distribution was uniform. High‐resolution TEM (HRTEM) analysis indicated that the biosynthesised AgNPs were in crystalline nature (Fig. 7).
Fig. 6.
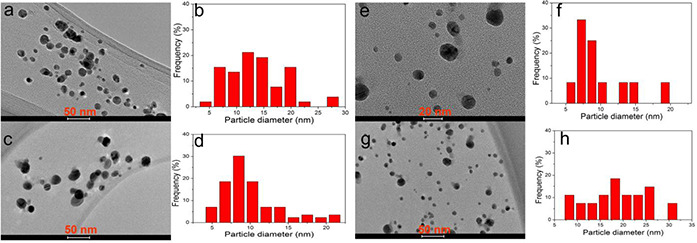
Transmission electron microscopy (TEM) images of synthesised AgNPs and particles size distribution. HV = 80 kV; direct magnification 200,000 × X: 33.2, Y: 27.5, T: 0.4. Bar = 20–50 nm (marked on bottom of every picture)
Fig. 7.
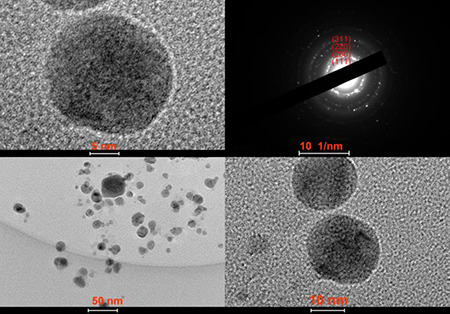
HRTEM images of the biosynthesised AgNPs
HV = 80 kV; direct magnification 200,000 × X: 33.2, Y: 27.5, T: 0.4. Bar = 5–50 nm
Fig. 8 showed the XRD patterns of the AgNPs prepared by the biosynthesis method using the Chinese winter jujube extract. The diffraction peaks at 2θ = 38°, 44°, 64°, 77° which assigned to the (111), (200), (220) and (311) planes were observed in samples obtained under the optimum conditions. The results indicated that the biosynthesised NPs were crystalline in nature with fcc phase [8]. According to the intensity ratio between (200) and (111) diffraction peaks, we could learn that the (111) plane was the predominant orientation in the silver crystal structure of the biosynthesised AgNPs. The size of AgNPs calculated using Debye–Scherrer's equation was about 14.9 nm, which was in agreement with the results of TEM and SEM analyses.
Fig. 8.
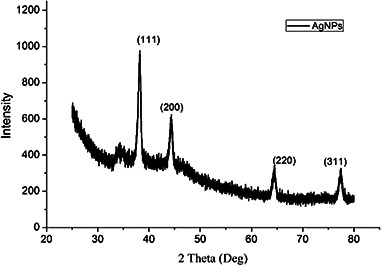
XRD patterns of the biosynthesised AgNPs
3.3 Antibacterial activity
The culture supernatant was used as control in the assay. The clear zone indicated that the bacterial growth was significantly inhibited by the biosynthesised AgNPs. The antibacterial activity of AgNPs was shown in Fig. 9. From the zones of inhibition, we observed that AgNPs had the good inhibitory effect on both E. coli and S. aureus. The inhibitory zones of S. aureus were bigger than E. coli, which meant AgNPs had the stronger antibacterial activity against S. aureus than E. coli. The antibacterial effect of AgNPs colloid increased with the concentration of AgNPs increasing.
Fig. 9.
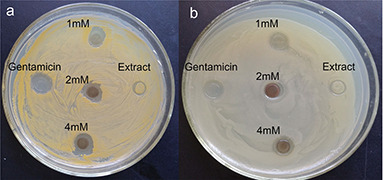
Antibacterial activity test
Antibacterial activity of AgNPs assayed by the agar diffusion method in petri plates. AgNPs poured in the discs showed the zone of inhibition against Staphylococcus aureus (a) and Escherichia coli (b)
3.4 Catalytic property
Nanoparticles have been widely used as excellent catalysts in many fields, which promote the researchers to develop more eco‐friendly and green methods for the preparation of nanomaterials, especially from biomass [23]. In our study, the catalytic ability of the biosynthesised AgNPs was also evaluated by the reaction of the 4‐NP reduction to 4‐aminophenol with the excess NaBH4 at room temperature as a probe. The degradation of 4‐NP in the absence of AgNPs was investigated as control in our experiment. The UV–Vis spectra were recorded at real time during the reaction (Figs. 10 a and c). The peak intensity of 4‐NP decreased with the reaction time, while a new peak at 313 nm attributed to 4‐AP appeared. Fig. 10 demonstrated that 4‐NP was hardly to be degraded in the absence of AgNPs even after 240 min, while the degradation of 4‐NP was immediately performed in the presence of AgNPs only in 20 min. The reaction of nitrophenol reduction by excess NaBH4 was described by a first‐order rate law with respect to nitrophenolate ion concentration. Thus, the value of the apparent reaction rate constant was estimated from a linear slope of the relative nitrophenolate ion content (A /A 0) in logarithmic form versus reaction time (Figs. 10 b and d). Obviously, the rate of degradation markedly increased from 0.00109 to 0.174 min−1 due to the addition of AgNPs as catalyst. AgNPs synthesised by the Chinese winter jujube extract showed a strong catalytic activity to 4‐NP.
Fig. 10.
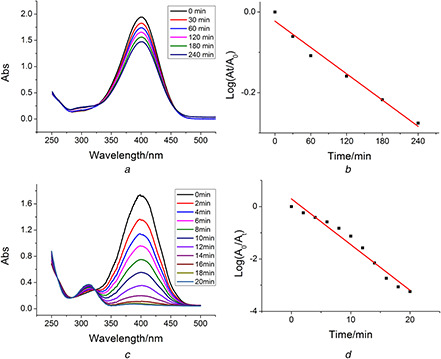
Catalytic reduction of 4‐NP by NaBH4
(a) In the absence of AgNPs and (c) In the presence of AgNPs. The absorbance intensity corresponding to 4‐NP consumption in logarithmic form versus reaction time (b) In the absence of AgNPs and (d) In the presence of AgNPs
4 Conclusion
The development of low‐cost, reliable, effective and eco‐friendly synthesis of AgNPs is an important aspect in current nanotechnology research and applications. Recently, plant extracts have been used as a potential biofactory for synthesis of AgNPs. In our study, AgNPs were successfully synthesised using the Chinese winter jujube extract as reducing and capping agents. The biological method showed in this study for synthesising AgNPs has several unique advantages over the chemical and physical methods, such as easy, simple, non‐toxic and eco‐friendly. The sizes of nanoparticles were found to be 3.0–31 nm. Morphological analysis showed the shapes of the AgNPs were spherical. Furthermore, the biosynthesised AgNPs displayed a significant antibacterial activity against E. coli and S. aureus. The catalytic experiment of AgNPs for hydrogenation of 4‐NP to 4‐aminophenol showed a remarkable high activity at room temperature. These results indicated that the biosynthesised AgNPs by Chinese winter jujube could be used as the bacterial inhibitors and the catalyst for degradation of organic pollutants in water solution.
5 Acknowledgments
This work was kindly co‐funded by the National Natural Science Foundation of China (Grant numbers 21277043 and 21620102008), the Beijing Natural Science Foundation (Grant number 8132038) and the Fundamental Research Funds for the Central Universities. The authors thank Ms Weiping Cao in Plant Protection Institute, Hebei Academy of Agricultural and Forestry Sciences for her kindly help on our antibacterial tests.
6 References
- 1. Ahmed S. Ahmad M. Swami B.L. et al.: ‘A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise’, J. Adv. Res., 2016, 7, (1), pp. 17 –28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peng J. Lin J. Chen Z. et al.: ‘Enhanced antimicrobial activities of silver‐nanoparticle‐decorated reduced graphene nanocomposites against oral pathogens’, Mater. Sci. Eng. C, 2017, 71, pp. 10 –16 [DOI] [PubMed] [Google Scholar]
- 3. Mohanta Y.K. Singdevsachan S.K. Parida U.K. et al.: ‘Green synthesis and antimicrobial activity of silver nanoparticles using wild medicinal mushroom Ganoderma applanatum (Pers.) Pat. from Similipal Biosphere Reserve, Odisha, India’, IET Nanobiotechnol., 2016, 10, pp. 184 –189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Revati A.K. Pandey B.D.: ‘Microbial synthesis of iron‐based nanomaterials—a review’, Bull. Mater. Sci., 2011, 34, (2), pp. 191 –198 [Google Scholar]
- 5. Park T.J. Lee K.G. Sang Y.L.: ‘Advances in microbial biosynthesis of metal nanoparticles’, Appl. Microbiol. Biotechnol., 2016, 100, (2), pp. 521 –534 [DOI] [PubMed] [Google Scholar]
- 6. Sun Q. Cai X. Li J. et al.: ‘Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity’, Colloid Surf. A, 2014, 444, pp. 226 –231 [Google Scholar]
- 7. Vankar P.S. Shukla D.: ‘Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric’, Appl. Nanosci., 2012, 2, pp. 163 –168 [Google Scholar]
- 8. Zhang Y. Cheng X. Zhang Y. et al.: ‘Biosynthesis of silver nanoparticles at room temperature using aqueous aloe leaf extract and antibacterial properties’, Colloid Surf. A, 2013, 423, pp. 63 –68 [Google Scholar]
- 9. Yuan C. Huo C. Gui B. et al.: ‘Green synthesis of gold nanoparticles using Citrus Maxima peel extract and their catalytic/antibacterial activities’, doi:10.1049/iet‐nbt.2016.0183 [DOI] [PMC free article] [PubMed]
- 10. Yuan C.G. Huo C. Yu S. et al.: ‘Biosynthesis of gold nanoparticles using Capsicum Annuum Var. Grossum pulp extract and its catalytic activity’, Phys. E., 2017, 85, pp. 19 –26 [Google Scholar]
- 11. Dauthal P. Mukhopadhyay M.: ‘Noble metal nanoparticles: plant mediated synthesis, mechanistic aspects of synthesis and applications’, Ind. Eng. Chem. Res., 2016, 55, (36), pp. 9557 –9577 [Google Scholar]
- 12. Zhao Z. Liu M. Tu P.: ‘Characterization of water soluble polysaccharides from organs of Chinese Jujube (Ziziphus jujuba Mill. cv. Dongzao)’, Eur. Food Res. Technol., 2008, 226, pp. 985 –989 [Google Scholar]
- 13. Guo S. Duan J.A. Qian D. et al.: ‘Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening’, Food Chem., 2015, 167, pp. 468 –474 [DOI] [PubMed] [Google Scholar]
- 14. Siriamornpun S. Weerapreeyakul N. Barusrux S.: ‘Bioactive compounds and health implications are better for green jujube fruit than for ripe fruit’, J. Funct. Foods, 2015, 12, pp. 246 –255 [Google Scholar]
- 15. Choi S.H. Ahn J.B. Kim H.J. et al.: ‘Changes in free amino acid, protein, and flavonoid content in Jujube (Ziziphus jujube) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts’, J. Agr. Food Chem., 2012, 60, pp. 10245 –10255 [DOI] [PubMed] [Google Scholar]
- 16. An B. Lee C.G. Song M.K. et al.: ‘Applicability and toxicity evaluation of an adsorbent based on jujube for the removal of toxic heavy metals’, React. Funct. Polym., 2015, 93, pp. 138 –147 [Google Scholar]
- 17. Ji X. Peng Q. Yuan Y. et al.: Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): a review’, Food Chem., 2017, 227, pp. 349 –357 [DOI] [PubMed] [Google Scholar]
- 18. Gopinath V. Mubarakali D. Priyadarshini S. et al.: ‘Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach’, Colloid Surf. B, 2012, 96, (1), pp. 69 –74 [DOI] [PubMed] [Google Scholar]
- 19. López‐Miranda J.L. Vázquez M. Fletes N. et al.: ‘Biosynthesis of silver nanoparticles using a Tamarix gallica leaf extract and their antibacterial activity’, Mater. Lett., 2016, 176, pp. 285 –289 [Google Scholar]
- 20. Nayak D. Ashe S. Rauta P.R. et al.: ‘Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma ’, Mater. Sci. Eng. C, 2016, 58, pp. 44 –52 [DOI] [PubMed] [Google Scholar]
- 21. Patra J.K. Das G. Baek K.H.: ‘Phyto‐mediated biosynthesis of silver nanoparticles using the rind extract of watermelon (Citrullus lanatus) under photo‐catalyzed condition and investigation of its antibacterial, anticandidal and antioxidant efficacy’, J. Phototochem. Photobiol. B, 2016, 161, pp. 200 –210 [DOI] [PubMed] [Google Scholar]
- 22. Vignesha V. Anbarasia K.F. Karthikeyenia S. et al.: ‘A superficial phyto‐assisted synthesis of silver nanoparticles and their assessment on hematological and biochemical parameters in Labeo rohita (Hamilton, 1822)’, Colloid Surf. A, 2013, 439, pp. 184 –192 [Google Scholar]
- 23. Meyabadi T.F. Dadashian F. Sadeghi G.M.M. et al.: ‘Spherical cellulose nanoparticles preparation from waste cotton using a green method’, Powder Technol., 2014, 261, (7), pp. 232 –240 [Google Scholar]


