Abstract
Advancement in materials synthesis largely depends up on their diverse applications and commercialisation. Antifungal effects of phytogenic silver nanoparticles (AgNPs) were evident, but the reports on the effects of the same on agricultural crops are scant. Herein, we report for the first time, size dependent effects of phytogenic AgNPs (synthesised using Stevia rebaudiana leaf extract) on the germination, growth and biochemical parameters of three important agricultural crops viz., rice (Oryza sativa L), maize (Zea mays L) and peanut (Arachis hypogaea L). AgNPs with varied sizes were prepared by changing the concentration and quantity of the Stevia rebaudiana leaf extract. As prepared AgNPs were characterized using the techniques, such as high‐resolution transmission electron microscopy, particle size and zeta potential analyser. The measured (dynamic light scattering technique) average sizes of particles are ranging from 68.5 to 116 nm. Fourier transform infrared studies confirmed the participation of alcohols, aldehydes and amides in the reduction and stabilisation of the AgNPs. Application of these AgNPs to three agricultural crop seeds (rice, maize and peanut) resulted in size dependent effects on their germination, growth and biochemical parameters such as, chlorophyll content, carotenoid and protein content. Further, antifungal activity of AgNPs also evaluated against fungi, Aspergillus niger.
Inspec keywords: antibacterial activity, silver, nanoparticles, nanomedicine, biochemistry, crops, transmission electron microscopy, Fourier transform infrared spectra, electrokinetic effects, organic compounds, particle size, plant diseases, microorganisms
Other keywords: fungi, Aspergillus niger, size 68.5 nm to 116 nm, silver particles, phytoextract, amide functional groups, aldehydes, alcohols, Fourier transform infrared studies, zeta potential analyser, particle size, high‐resolution transmission electron microscopy, Stevia plant leaf extract, agricultural crops, antifungal effects, materials synthesis, Arachis hypogaea L, peanut, Zea mays L, maize, Oryza sativa L, rice, biochemical parameters, growth, germination, antifungal phytogenic silver nanoparticles, size dependent effects
1 Introduction
The demand for nanoscience and nanotechnology based products has been increasing rapidly in several fields as a solution to an array of critical problems which were not addressed effectively by any other technology or science so far. The phenomenal quantum effects exhibited by the matter at nanoscale (1–100 nm) and tapping of some of these effects resulted in the development of novel products. Agriculture, being a host of a variety of materials, could be one of the best platforms to find novel applications of a given material and is being one of the most attractive research fields of the materials scientists in the recent past, at nanoscale in particular. Silver is one of the best known antifungal agent from the ancient times, and it continues to attract the mankind even more at nanoscale. Nanoscale silver particles (AgNPs) found numerous avenues for their applications as medicine stands first followed by health care, cosmetics and home appliances. Though there are several number of methods available to produce AgNPs, chemical routes are often posing toxic effects [1, 2, 3, 4, 5, 6, 7, 8] leading to the restricted usage of the same. AgNPs synthesised through the green routes such as using plants and plant materials [9, 10, 11, 12, 13, 14, 15, 16, 17], microbes [12, 13, 14, 15], biotemplates [18, 19, 20, 21, 22, 23] not only having wide applications, but also are relatively less/non‐toxic encouraging these AgNPs for application in biology.
Recently, there has been a large focus on the nanoscience usage in the crop yield improvement in various varieties of staple and economic crops. Nanotechnology permits broad advances in agricultural research, such as reproductive science and technology, conversion of agricultural and food wastes to energy and other useful byproducts through enzymatic nanobioprocessing, disease prevention and treatment in plants using various nanocides [22]. Recent reports explain the usage of nanoparticles to increase the supply of pesticides and fertilisers through plant shoots and foliage [24].
Use of phytogenic metal nanoparticles in agriculture is attractive due to the ease of synthesis and cost effectiveness in making nanoparticles. Moreover, the stability of phytogenic nanoparticles is an interesting character in applied perspective where the size dependent effects play an important role in agricultural productivity and quality. The nanoparticles, which are capped by bio‐materials giving exceptional stability in the solution which is the most significant and desired property needs to be achieved for biological and commercial applications.
Several reports are available for synthesising nanoparticles using plant extracts such as geranium leaf, neem leaf, lemon grass and Aloe vera plant extracts. There are several reports of effective usage of AgNPs on various aquatic species such as fishes [8, 25, 26, 27], water fleas [28, 29, 30] and algae [31]. However, use of AgNPs in terrestrial ecological systems and efficacy studies related to staple agricultural crops is scant [32, 33, 34, 35].
Stevia rebaudiana (Asteraceae) is a wild perennial herb grown on a commercial scale in a number of countries [36] and reported about availability of sesquiterpenes‐based bisabolane, germacrane [37], flavonoids, diterpene [38], bis‐nor‐diterpene and sterols [39, 40, 41]. The presence of sweet diterpene glycosides in Stevia is an important ingredient for diabetic patients as an artificial sweetener [40, 41, 42, 43, 44] and dried leaves and/powdered leaves are being used as an alternative to sugar/carbohydrates.
Synthesis of AgNPs using Stevia rebaudiana was reported [45], however, control over size of AgNPs has not been found. In the present investigation, leaf extract of Stevia rebaudiana was prepared and used to synthesise varied size AgNPs. To the best of our knowledge it is the first report on the application of phytogenic AgNPs on important crops of cereal and pulse to assess their size dependent effects on germination, growth and biochemical parameters.
2 Experimental
2.1 Collection of plant material
Stevia rebaudiana plant leaves were purchased from the local market in Tirupati, Andhra Pradesh, India. The leaves were thoroughly washed for three times with double distilled water and then shade dried for 7 days.
2.2 Preparation of aqueous extract (AE)
The shaded dried Stevia rebaudiana plant leaves were ground to get fine powder. Then, 10 g of powder was mixed with 100 ml of distilled water and boiled for 30 min. After that, the extract was filtered using Whatman No. 1 filter paper and collected in plastic bottles and stored at 4°C for further characterisation and experimentation.
2.3 Preparation of Stevia rebaudiana AgNPs
To prepare AgNPs, a 90‐ml aqueous solution of 1 mM silver nitrate was mixed with 10 ml of 1 and 2% aqueous solution of Stevia rebaudiana leaf extract. The silver samples were mixed with Stevia rebaudiana leaf extract at different concentrations such as 2% – 20 (S1), 2% – 5 (S2), 1% – 20 (S3) and 1% – 5 (S4) and the bulk form of the silver nitrate solution were marked B. It was observed that the colour of the solution has been changed from yellow to dark brown which visually confirms the formation of AgNPs.
2.4 Characterisation of AgNPs
2.4.1 UV–vis is spectral analysis
The bioreduction of silver and the localised surface plasmon resonance (LSPR) of AgNPs was recorded by UV–vis spectrophotometer (UV‐2450, SHIMADZU) from 400 to 800 nm.
2.4.2 Fourier transform infrared (FTIR) spectroscopic measurements
The FTIR spectrum was taken in the mid‐IR region of 400–4000 cm−1. The spectrum was recorded using attenuated total reflectance technique. The dried sample was mixed with the KBr (1:200) crystal, and the spectrum was recorded in the transmittance mode.
2.4.3 High‐resolution transmission electron microscopy (HRTEM) measurements
The characterisation of the samples was done by transmission electron microscopy (HRTEM, JEOL, 3010). The TEM samples were prepared by drop casting the suspensions on carbon coated Cu grids.
2.4.4 Particle size and zeta potential analysis
The hydrosol containing AgNPs was filtered through a 0.22‐ml syringe‐driven filter unit, and the size and distribution of the nanoparticles were measured using dynamic light scattering technique (Nanopartica, HORIBA, SZ‐100).
2.4.5 Germination of seeds
The Arachis hypogaea (peanut), Zea mays (maize) and Oryza sativa (rice) seeds procured from Regional Agricultural Research Station, Tirupati and were soaked with 100 ml each of AgNPs S1, S2, S3, S4, bulk silver sample (B) and control with distilled water. Further, the soaked seeds were allowed to germinate in Petri dishes under continuous white illumination after 3 days and controlled room temperature. The seeds are supplied with 10 ml of distilled water daily to maintain moisture in the seeds and for uptake of nutrients from the seeds. After the appearance of sprouts during seventh day the shoot, root lengths and germination percentages were calculated as per the following equation [46]:
Root and shoot lengths were recorded and expressed in centimetres using a standard ruler. The shoot and root lengths were recorded after the appearance of the second leaves in monocots rice and maize on tenth day. In case of dicotyledonous peanut plant, the emergence of the second branch leaves was marked to record the shoot and root lengths were measured on 24th day. All germinated plants from each species were selected to record the average root and shoot lengths.
2.4.6 Determination of chlorophyll
During the 15th day of plantlets growth fresh 1 g second leaf was collected and immediately homogenised with chilled 80% acetone in motor and pestle. The absorbance of the extracts at wavelengths 480 (A480), 663 (A663) and 645 nm (A645) were measured using schimadzu‐2450 UV–vis spectrophotometer. The concentrations of chlorophyll‐a, chlorophyll‐b, total chlorophyll and carotenoids were then calculated using the (Arnon, 1949) as given below:
Using the above formula and dilution factor leaf pigment contents such as Chl‐t and Chl a/b ratios were determined and expressed as mg/g FW.
2.5 Total soluble protein estimation
Total soluble protein was estimated according to the method of [47] with few modifications during extraction. Fresh 1 g leaf samples were weighed and homogenised using 1% sulphosalicylic acid in motor and pestle, further it is incubated overnight in refrigerator. The pelleted samples were neutralised using 5 ml of basic 1%NaOH solution. In the above mentioned 1 ml sample, 5 ml of Lowry reagent (1% CuSO4 0.5H2 O + 1% sodium potassium tartarate + 2% Na2 CO3 in 0.1 N NaOH) was added, vertexed well and incubated at room temperature for 30 min. Then 0.5 ml of Folin‐phenol (Qualigens) reagent was added and incubated for 10 min at room temperature and the absorbance was read immediately at 680 nm using Schimadzu‐2450 UV–vis spectrophotometer. The total soluble protein content was calibrated against the standard graph obtained using bovine serum albumin (Sigma) as standard protein.
2.6 Antifungal activity
Aspergillus niger a severe contaminant of agricultural harvest was isolated from the peanut seeds and maintained from several years in the laboratory is used in the preparation of log phase cultures. The spores were suspended in potato dextrose agar (PDA) broth solution in 250 ml Erlenmeyer flask and incubated for 24–72 h. Spores were collected and used for assessing the antifungal properties of S1–S4 compounds. The fungal spores were inoculated in four corners of Petri plate, while a 40 µl solution of each compound S1–S4 was poured into well at the centre of each PDA plates. Similarly 40 µl streptomycin served as standard antifungal agent as a control for comparison and interpretation. Finally, the plates were incubated at 24 ± 4°C for 24–72 h. The radial growth and the diameter of the clear zones were measured on the fourth day.
2.7 Statistical analysis
The statistical significance of all the values was tested with F‐test at 5% level of probability and compared the treatmental means with critical difference. The mean values were separated from Duncans multiple range test.
3 Results and discussion
3.1 UV–vis spectral analysis
It is well known that AgNPs exhibit brown colour, which arises due to excitation of surface plasmon vibrations of the AgNPs. After addition of 1 mM silver nitrate solution to the Stevia leaf extract, the colour of the composition has been changed from yellow to dark brown. UV–vis spectroscopy was employed to record the LSPR of the AgNPs. The maximum absorbance peaks were observed at 445 nm – S3, 443 nm – S4, 441 nm – S2 and 434 nm – S1 (Fig. 1 a). During the reduction of silver ions by phytochemicals or other reducing agents present in Stevia leaf extract, the electrons are trapped in the small quantum boxes leading to quantum confinement of silver material. The direct consequence to this phenomenon is LSPR which is responsible for changes in the colour of the mixed solution. The colour change was observed immediately and to the best of our knowledge, this is the shortest time reported for the synthesis of AgNPs using plant extract.
Fig. 1.
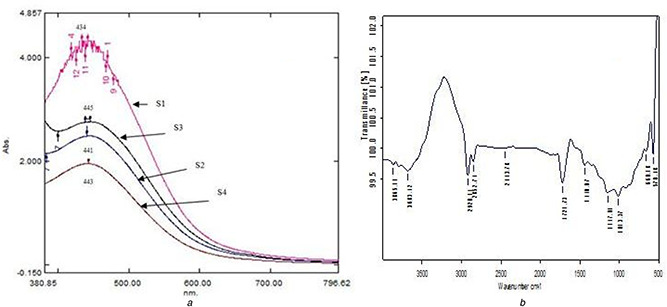
Maximum absorbance peaks
(a) UV–visible spectroscopic micrograph and showing the LSPR of AgNPs (434–443 nm) synthesised using Stevia rebaudiana leaf extract with different concentrations (S1–S4), (b) FTIR spectroscopic micrograph representing the functional groups responsible for the reduction and stabilisation of AgNPs synthesised using the AE of Stevia rebaudiana leaf extract at different concentrations (S1)
During phytogenic particle synthesis nucleation process is the fundamental step for the formation of AgNPs, and it is dependent on the solubility of silver nitrate in the phytoextract solution. If the solubility is low then there is a possibility of enhancement in the rate of nucleation to a greater extent [48]. The important step during the process of nucleation is the surface energies of AgNPs. Minimisation of the surface energies of nanoparticles reduces surface area and leads to the achievement of desired sizes of nanoparticles. The presence of high amount of phytochemicals and ions in the Stevia extract alters the surface energy of the silver ions and directs the growth of crystal faces leads to the formation of different size and shaped nanoparticles and structures [49, 50].
3.2 FTIR analysis
FTIR spectrum is used to identify the possible chemical interactions among the silver salts and functional groups present in the leaf extract. FTIR spectrum of the synthesised AgNPs using Stevia leaf extract is shown in Fig. 1 b. In the sample S1, the absorption peaks at 3865.11 and 3683.42 cm−1 reveal the presence of O–H stretches, indicating the presence of alcohols and phenols. The peaks at 2920.14, 2852.74 and 1440.07 cm−1 reveal the presence of C–H stretches, indicating the presence of alkanes. The peak at 2443.78 cm−1 reveals the presence of O–H stretch, indicating the presence of carboxylic acids. The peak at 1721.73 cm−1 reveals the presence of C=O stretch, indicating the presence of aldehydes. The peak at 1147.01 cm−1 reveals the presence of C–H wag (–CH2 X), indicating the presence of alkyl halides. The peak at 1013.37 cm−1 reveals the presence of C–N stretch, indicating the presence of aliphatic amines. The peak at 660.18 cm−1 reveals the presence of C–H band indicating the presence of aromatics. The peak at 570.48 cm−1 reveals the presence of C–Br stretch, indicating the presence of alkyl halides.
In S2, the absorption peak at 3680.39 cm−1 reveals the presence of O–H stretch, indicating the presence of alcohols and phenols. The peaks at 2921.11 and 2863.27 cm−1 reveal the presence of C–H stretch, indicating the presence of alkanes. The peak at 1725.59 cm−1 reveals the presence of C=O stretch, indicating the presence of aldehydes. The peak at 1528.02 and 1442.14 cm−1 reveals the presence of N–O asymmetric stretch, indicating the presence of nitro compounds. The peak at 1149.82 cm−1 reveals the presence of C–H wag (–CH2 X), indicating the presence of alkyl halides. The peak at 1008.24 cm−1 reveals the presence of C–N stretch, indicating the presence of aliphatic amines. The peak at 624.03 cm−1 reveals the presence of C–Br stretch, indicating the presence of alkyl halides.
In S3, the absorption peaks at 3785.20 and 3685.71 cm−1 reveal the presence of O–H stretches, indicating the presence of alcohols. The peaks at 2921.01 and 2853.63 cm−1 reveal the presence of C–H stretches, indicating the presence of alkanes. The peaks at 1816.43 and 1727.20 cm−1 reveal the presence of C=O stretches, indicating the presence of aldehydes. The peak at 1011.73 cm−1 reveals the presence of C–N stretch, indicating the presence of aliphatic amines. The peak at 581.38 cm−1 reveals the presence of C–Br stretch, indicating the presence of alkyl halides.
In S4, the absorption peak at 3684.55 cm−1 reveals the presence of O–H stretches, indicating the presence of alcohols. The peaks at 2920.49 and 2853.57 cm−1 reveal the presence of C–H stretches, indicating the presence of alkanes. The peak at 1727.94 cm−1 reveals the presence of C=O stretch, indicating the presence of aldehydes. The peak at 1012.40 cm−1 reveals the presence of C–N stretch, indicating the presence of aliphatic amines. The peak at 562.72 cm−1 reveals the presence of C–Br stretch, indicating the presence of alkyl halides [51].
Whereas bio‐reduced AgNPs showed the peaks corresponding to the same organic functional groups confirms the involvement of proteins in reduction and stabilisation of silver ions. The silver ions are bound to the functional organic groups (carboxyl and amine) that are present in the Stevia rebaudiana plant leaves extract, and these functional groups could act as template for reducing and capping of AgNPs.
3.3 Dynamic light scattering analysis
Dynamic light scattering technique has been used to measure hydrodynamic diameter (HDD) of the hydrosol. The HDD was found to be S1 – 77.5 nm, S2 – 111.7 nm, S3 – 68.6 nm and S4 – 98.9 nm (Fig. 2). The recorded zeta potentials were −39.8, 37.0, −126.0 and −87.1 mV for S1–S4, respectively. If the hydrosol has a large negative or positive zeta potential (>25 mV), then the particles tend to repel with each other and show no tendency to agglomerate resulted in highly dispersed particles.
Fig. 2.
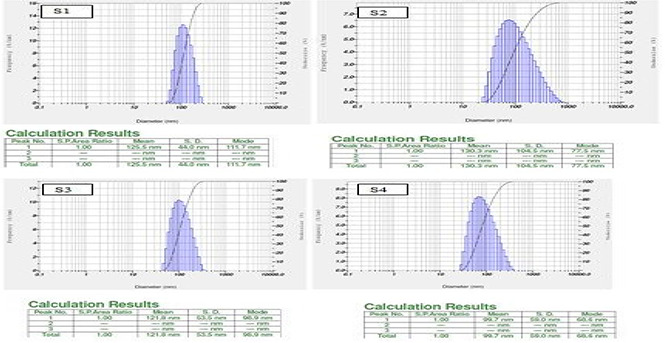
Histograms represents particle size distribution of silver nanoparticles synthesized using different concentrations (S1, S2, S3 and S4) of Stevia rebaudiana leaf extract
The surface morphology, size and shape of AgNPs were shown in HRTEM micrograph (Fig. 3) and it is evident that AgNPs were spherical in shape and were polydispersed. The measured average size of AgNPs was 50 nm and occasional agglomeration of the AgNPs has been observed.
Fig. 3.

TEM micrograph (50 nm bar scale) of AgNPs showing spherical‐shaped particles with varied sizes synthesised using different concentrations (S1–S4) of Stevia rebaudiana leaf extract
3.4 Germination%
The data presented in Tables 1 and 2 clearly shows that S3 and S4 samples promoting germination of peanut, rice and maize compared with the bulk and control. Whereas, samples S1 and S2 show less growth compared with bulk samples in monocots like rice and maize and within the AgNPs the germination percentage exhibited size dependent variation in germination percentages (Table 1). In peanut with all the AgNPs treatment (S1–S4) recorded higher (100%) germination. In maize, S3 and S4 samples recorded (100%) higher germination percentages compared with S1 and S2, but less than bulk (60%) and control (60%) samples. In rice, the S3 and S4 recorded higher percent of germination (100%) compared with bulk (25%) and control (75%).
Table 1.
Germination percentages in ground nut, maize and rice under different treatments
| Treatments | Germination percentage | ||
|---|---|---|---|
| Ground nut | Rice | maize | |
| S1 | 100 ± 0.5 | 20 ± 0.3 | 30 ± 0.4 |
| S2 | 100 ± 0.5 | 20 ± 0.6 | 10 ± 0.5 |
| S3 | 100 ± 0.2 | 100 ± 0.2 | 40 ± 0.2 |
| S4 | 100 ± 0.3 | 100 ± 0.4 | 40 ± 0.3 |
| B | 81.8 ± 0.4 | 20 ± 0.8 | 60 ± 0.2 |
| control | 90 ± 0.2 | 75 ± 0.8 | 60 ± 0.4 |
Each value is the ± SE of three replications.
Table 2.
Average root and shoot lengths in ground nut, maize and rice under different size treatments
| Treatments | Ground nut | Maize | Rice | |||
|---|---|---|---|---|---|---|
| At 24th day average length, cm | Average length, cm | Average length, cm | ||||
| Root | Shoot | Root | Shoot | Root | Shoot | |
| S1 | 7.1 ± 0.2 | 25.5 ± 0.4 | 5.06 ± 0.2 | 8.6 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 |
| S2 | 8.4 ± 0.4 | 23.5 ± 0.2 | 5.0 ± 0.2 | 8.5 ± 0.2 | 3.04 ± 0.2 | 2.18 ± 0.6 |
| S3 | 7.5 ± 0.5 | 22.8 ± 0.7 | 6.05 ± 0.4 | 8.88 ± 0.4 | 6.08 ± 0.7 | 3.02 ± 0.4 |
| S4 | 5.4 ± 0.3 | 19.2 ± 0.5 | 7.3 ± 0.4 | 8.95 ± 0.7 | 3.68 ± 0.4 | 2.76 ± 0.5 |
| B | 4.2 ± 0.7 | 18.0 ± 0.4 | 3.2 ± 0.3 | 3.16 ± 0.6 | 0.2 ± 0.4 | 0.2 ± 0.6 |
| control | 5.2 ± 0.2 | 17.9 ± 0.2 | 3.86 ± 0.2 | 5.56 ± 0.5 | 3.8 ± 0.2 | 2.88 ± 0.4 |
a Each value is the ±SE of 3 replications.
3.5 Root and shoot lengths
Similar to that of germination percentage AgNPs samples S3 and S4 enumerated the growth promoting function with respect to shoot and root lengths in rice and maize seedlings measured during 12th day in rice, maize and peanut measured during 24th day after germination. The images depicted in Fig. 4 a average values (Table 2) of root and shoot lengths in three crops peanut, maize and rice clearly explain the size dependent effects of AgNPs on the tested seeds. The average root and shoot length values indicates that phytogenic AgNPs promotes the growth of root and shoot of peanut, maize and rice except with the sample S1 and rice in comparison with bulk silver (bulk) and control. Whereas, in case of bulk sample retarted growth of root and shoot was observed in comparison with control shows the phytotoxic nature of bulk silver during germination of the plants similar to other heavy metals such as, cadmium [52], copper [53], lead [54] and arsenic [55] as reported earlier. Several reports also available in the literature on silver to ascertain their effect on seed germination, biomass reduction [34] and other parameters in plants such as, Daphnia magna [31], rose plants [56], snap dragon [57], Cucurbita pepo (54) on other nutrients of plants [58] also in human cells [59].
Fig. 4.

Average values of root and shoot lengths
(a) Size dependent effects of AgNPs on the growth of peanut seedlings (after 24 days of germination), (b) Size dependent effects on antifungal activity of AgNPs synthesised using different concentrations of (S1–S4) Stevia rebaudiana leaf extract against A. niger, (c) Control
3.6 Chlorophyll content
The photosynthetic pigments Chls a and b are the essential to plants for proper photosynthetic light phase reactions, during the growth and development of plants (62) and the same was estimated in the foliage of seedlings.
3.6.1 Rice
The total chlorophyll content recorded in rice with the application of phytogenic AgNPs (38.3% – S1, 49.7% – S2, 20.6% – S3, 46.8% – S4 and 16.85% – bulk silver) is less compared with control (Fig. 5). A similar trend was followed when compared with the bulk silver (25.8% – S1, 39.5% – S2 and 4.5% – S3) except 36.04% increment on application of S4 (98.9 nm). The concentrations of Chls a and b values have given changes in the compositions of important photosynthetic pigments. The Chl a/b values (Supplementary data) recorded with the application of phytogenic AgNPs are higher (53.8% – S2, 135.3% – S3, 66.1% – S4 and 123% – bulk silver) compared with control except 23% less in S1.
Fig. 5.
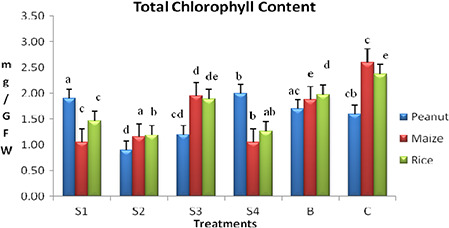
Total chlorophyll content in peanut, maize and rice leaf with the application of different sizes of phytogenic AgNPs
The photoprotection mechanism of carotenoids in rice with the application of differently sized phytogenic AgNPs was clear with results indicating 25% (S1), 83.35% (S2), 45.8% (S1) increase and 25% (S3), 85% (bulk silver) decrease in carotenoid content compared with control. Thus there is an increased in photoprotection mechanism to resist the low total chlorophyll content in rice due to bulk and AgNPs treatments.
3.6.2 Maize
The chlorophyll content of maize plants treated with four different sizes of phytogenic AgNPs was given in Fig. 6. The low chlorophyll content (59.6, 55.7, 25 and 59.6% in S1–S4, respectively) compared with control in all treatments, indicates inhibitory effects of phytogenic AgNPs in photosynthesis process. However, phytogenic AgNPs with hydrodynamic diameter 68.6 nm (S3) promoted photosynthesis process by recording 4% higher chlorophyll content compared to bulk silver.
Fig. 6.
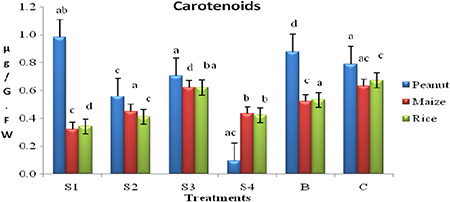
Carotenoid content in peanut, maize and rice leaf with the application of different sizes of phytogenic AgNPs
The Chl a/b ratio was recorded higher (135.1, 117.9, 16.2 and 58.9% in S1–S4, respectively) when compared with control and compared with bulk silver, higher values of 61.4% in S1, 49.12% in S2 and 8.77% in S4 were recorded except that 20.4% less in S3 (68.6 nm) (Supplementary data). The above results clearly explain the size dependent effects of phytogenic AgNPs in promoting the Chls a and b synthesis in maize.
Carotenoid, a photopigment content also influenced by the size of the phytogenic AgNPs. Total carotenoid content (Fig. 6) was recorded 50, 33.3 and 33.3% less in S1, S2 and S4, respectively, and 16% less in bulk silver compared with control. Similarly, when compared with bulk silver 40, 15 and 20% less recorded in S1, S2 and S4, respectively, and incontrast 20% higher carotenoid content was recorded in S3. The experimental results could be attributed to the higher photodamage in relatively smaller size phytogenic AgNPs (S3 – 68.6 nm) and less photodamage in bigger AgNPs (S1, S2 and S4).
3.6.3 Peanut
Total chlorophyll content in peanut was investigated to be higher in S1 (11.7%) and S4 (17.6%) compared with bulk silver (Fig. 5). This explains the toxic role of bulk silver on total chlorophyll synthesis process. However, in the case of S2 and S3 the chlorophyll contents were found to be 47 and 29.45% less in comparison with bulk samples (Fig. 6). The possible reason for this change in chlorophyll content among the chemically similar AgNPs treatments is due to change in physico‐chemical properties such as size and zeta potential of samples.
Chl a/b ratio similar to total chlorophyll content Chl a/b ratio in S1, S2 and S3, are 6, 62.5 and 31.25 higher and 6% less in S4 respectively, with control (Supplementary data). Compared with bulk silver 41.6, 116, 75 and 25% higher Chla/b ratio in S1–S4, respectively, evidences the growth promotive role of phytogenic AgNPs in the synthesis of Chl a and b pigments individually.
Carotenoid content (Fig. 6) indicates the photo‐protection mechanism in plants (62). In this study, the increase in carotenoid contents in S1, S4 and bulk treatments represent higher damage to Chl a, whereas in S2 and S3 samples low carotenoid content of 0.557 and 0.703 µg/g FW (leaf), respectively, was recorded compared with bulk and control samples. In S2 and S3, the photodamage is minimal and in S1, S4 and bulk samples it is high, this may be due to development of photo‐protection mechanism. Increase in photo‐protection mechanism leads to increase in carotenoid content represents higher absorption of light and subsequent damaging function of electron generation in photosynthesis process.
As a whole from the results of total chlorophyll content (Fig. 5), carotenoid content (Fig. 6) it is clear that each plant species differs in its response physically and biochemically to different sizes of applied phytogenic AgNPs. Similar type of results was not reported earlier in case of staple food crops with the application of phytogenic AgNPs and in depth studies at molecular level are in progress to reveal the exact reasons for the obtained results.
Decrease in Chl a/b ratio represents the increase in Chl b pigment content which is an undesired character. The increase in Chl b pigment content causes photodamage [60, 61]. The above mentioned phenomenon of photodamage is corrected by the increase in the carotenoid content. Thus among the four AgNPs samples S2, S3 promotes Chl a synthesis and Chl b is promoted by S1 and S4.
Similar results of chlorophyll pigment damage were earlier reported by treatment with heavy metals like, lead [61, 62] in plants, and also in Chlorella vulgaris a cyanobacteria by zinc toxicity [63, 64]. Thus in this study the Chl a, pigment damage is clearly observed in the (Supplementary data) bulk and with different sizes of phytogenic AgNPs.
3.6.4 Protein
An important biomolecule required for the functioning of any living cell is a protein, which plays a vital role in performing all the metabolomic functions. Similar to total chlorophyll content, Chl a/b ratio and carotenoid content, differential total soluble protein content was recorded in all the three crop plants with the applied phytogenic AgNPs.
The protein content per gram leaf was found higher in rice seedlings was recorded as increased to 14% – S1, 165.9% – S2, 251% – S3 and 173% – S4 and decrement of 24.8% in bulk silver was recorded in comparison with control (Fig. 7). Similarly, in maize fresh leaf protein quantity increased to 4% – S1, 39% – S2, 433% – S3 and 45.9% – S4 and decreased to 57.6% in bulk silver in comparison with control (Supplementary data Fig. 8). In peanut, the protein content was recorded as increased to74% – S1, 19% – S2 and 23% – S3, decreased 70% – S4 and 22.8% – bulk silver treatments when compared with control (Fig. 7). Therefore, significant enhancement in leaf protein content in peanut, maize and rice has been observed with the application of different size phytogenic AgNPs.
Fig. 7.
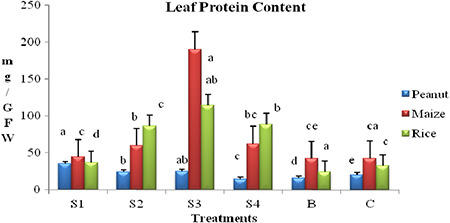
Total protein content in peanut, maize and rice leaf with the application of different sizes of phytogenic AgNPs
Fig. 8.
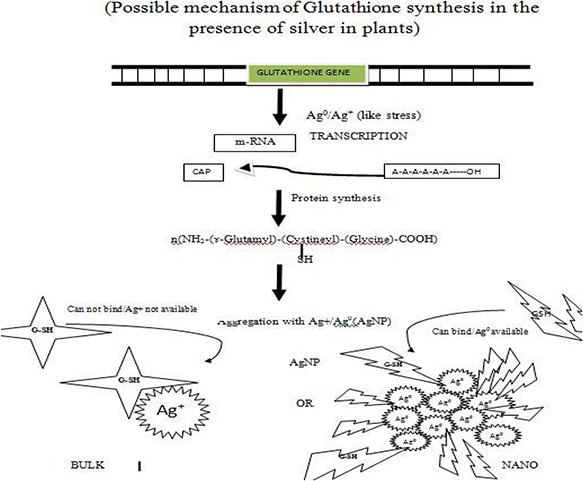
Schematic representation of glutathione molecular gene switching model involving thiol group binding with the AgNPs
Thus from the above results of germination percentage and chlorophyll content and protein content biochemically the bulk form of the silver has inhibited the growth of seedlings. Similar results were reported about bulk silver effect on protein content by Hong et al. [65]. Though there are much nutrients available in the pod the bulk silver treated plant show stunted growth in comparison with control. However, in case of phytogenic AgNPs S1 and S4 the nanoscale silver has promoted the seedling growth. In a study, it has been reported that treating of fennel (Foeniculum vulgare Mill) seeds to 60 ppm of nano TiO2 significantly improved germination percentage [66]. A similar trend was followed by shoot dry weight and germination rate [66]. The change in behaviour of nanoparticles in biological systems with respect to size was reported in mammalian cells uptake, using gold nanoparticles [67, 68] change in antimicrobial activity of phytogenic AgNPs, interactions of AgNPs with chitin like biomolecules [63]. In the present investigation, the enhancement of growth and biochemical parameters with the application of phytogenic AgNPs in peanut, maize and rice crop plants may be attributed to the stimulated production of glutathione in the presence of silver [69]. Glutathione, a tri‐peptide well known for removal of seed dormancy and transport of amino acids. The possible mechanism of enhancing the glutathione production in the presence of silver is depicted in Fig. 8.
Similar results showing the changes in physical properties of gold nanoparticles with respect to size and different refractive indices were reported by Veronica 2004 [70] including change in bioimaging properties of gold nanoparticles.
3.7 Antifungal efficacy of Stevia rebaudiana leaf‐extract mediated AgNPs
AgNPs obtained from Stevia leaf extract have very strong inhibitory action against Aspergillus niger (Fungal sp.), the higher concentrations of (S1) AgNPs and streptomycin showed significant antifungal effect Table 3 compared with other concentrations (S2–S4). The inhibitory action of the microbes may be attributed to the loss of replication ability of DNA upon treatment with the silver ion. The higher antifungal activity of smaller‐sized nanoparticles could be due to the large surface area‐to‐volume ratio and the enhanced surface activity. Further, the fact is that nanoparticles are more abrasive in nature than bulk AgNO3, thus contributing to the greater mechanical damage to the cell membrane resulting in enhanced fungal effect shown in Fig. 4 b.
Table 3.
In vitro antifungal studies against Aspergillus niger fungi isolated from ground nut seeds using Stevia rebaudiana plant leaves extract mediated AgNPs
| Compound | Clear zone diameter |
|---|---|
| S1 | 2.5 ± 0.4 |
| S2 | 0.2 ± 0.2 |
| S3 | 0.2 ± 0.3 |
| S4 | 0.5 ± 0.3 |
| streptomycin | 3.5 ± 0.4 |
a Each value is the ±SE of three replications.
4 Conclusion
In the applications of nanoscale materials, size plays an important role in revealing their potentiality in different fields including biology. With the change in concentration of the plant extract, synthesis of varied sizes of AgNPs was achieved. As the material exhibits size dependent properties at nanoscale, their behaviour in a biological system like plant is being interesting. Size dependent and species specific triggering of the biochemical processes in plants by the phytogenic AgNPs has been observed. Growth promoting properties have been observed with all the sizes and crops tested, which was not the same case with the bulk silver. Therefore, it is evident that at nanoscale, the size of particles determines the behaviour of materials in different cellular machinery (plants and microbes). So one can alter the size of the nanoscale materials to achieve the prominent output applications in these respective fields.
5 Acknowledgment
The authors thank Acharya N G Ranga Agricultural University for research facilities provided at Institute of Frontier Technology, Regional Agricultural Research Station, Tirupati to carry out this research work.
6 References
- 1. Klaus‐Joerger T. Joerger R. Olsson E. et al.: ‘Bacteria as workers in the living factory: metal‐accumulating bacteria and their potential for materials science’, TRENDS Biotechnol., 2001, 19, (1), pp. 15 –20 [DOI] [PubMed] [Google Scholar]
- 2. Klaus T. Joerger R. Olsson E. et al.: ‘Silver‐based crystalline nanoparticles, microbially fabricated’, Proc. Natl. Acad. Sci., 1999, 96, (24), pp. 13611 –13614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joerger R. Klaus T. Granqvist C.G.: ‘Biologically produced silver–carbon composite materials for optically functional thin‐film coatings’, Adv. Mater., 2000, 12, (6), pp. 407 –409 [Google Scholar]
- 4. Nair B. Pradeep T.: ‘Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains’, Crystal Growth Des., 2002, 2, (4), pp. 293 –298 [Google Scholar]
- 5. Mukherjee P. Ahmad A. Mandal D. et al.: ‘Fungus‐mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis’, Nano Lett., 2001, 1, (10), pp. 515 –519 [Google Scholar]
- 6. Ahmad A. Mukherjee P. Senapathi S. et al.: ‘Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum ’, Colloids Surf. B, Biointerfaces, 2003, 28, (4), pp. 313 –318 [Google Scholar]
- 7. Durán N. Marcato D. Alves L. et al.: ‘Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains’, J. Nanobiotechnol., 2005, 3, (8), pp. 1 –7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gade A. Ingle A. Whiteley C. et al.: ‘Mycogenic metal nanoparticles: progress and applications’, Biotechnol. Lett., 2010, 32, (5), pp. 593 –600 [DOI] [PubMed] [Google Scholar]
- 9. Shankar S.S. Ahmad A. Renu P. et al.: ‘Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes’, J. Mater. Chem., 2003, 13, (7), pp. 1822 –1826 [Google Scholar]
- 10. Shankar S.S. Rai A. Ahmad A. et al.: ‘Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using neem (Azadirachta indica) leaf broth’, J. Coll. Interface Sci., 2004, 275, (2), pp. 496 –502 [DOI] [PubMed] [Google Scholar]
- 11. Shankar S.S. Akhilesh R. Ahmad A. et al.: ‘Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared‐absorbing optical coatings’, Chem. Mater., 2005, 17, (3), pp. 566 –572 [Google Scholar]
- 12. Chandran S.P. Chaudhary M. Pasricha R. et al.: ‘Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract’, Biotechnol. Progr., 2006, 22, (2), pp. 577 –583 [DOI] [PubMed] [Google Scholar]
- 13. Song J.Y. Kwon E.‐Y. Kim B.S.: ‘Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract’, Bioprocess Biosyst. Eng., 2010, 33, (1), pp. 159 –164 [DOI] [PubMed] [Google Scholar]
- 14. Prasad T.N.V.K.V. Elumalai E.K.: ‘Marine algae mediated synthesis of silver nanoparticles using Scaberia agardhü Greville’, J. Biol. Sci., 2013, 13, (6), p. 566 [Google Scholar]
- 15. Brayner R. et al.: ‘Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme‐mediated route’, J. Nanosci. Nanotechnol., 2007, 7, (8), pp. 2696 –2708 [DOI] [PubMed] [Google Scholar]
- 16. Prasad T.N.V.K.V. Elumalai E.K.: ‘Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antifungal activity’, Asian Pacific J. Trop. Biomed., 2011, 1, (6), pp. 439 –442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohanpuria P. Rana N.K. Yadav S.K.: ‘Biosynthesis of nanoparticles: technological concepts and future applications’, J. Nanoparticle Res., 2008, 10, (3), pp. 507 –517 [Google Scholar]
- 18. Song J.Y. Kim B.S.: ‘Biological synthesis of bimetallic Au/Ag nanoparticles using Persimmon (Diopyros kaki) leaf extract’, Korean J. Chem. Eng., 2008, 25, (4), pp. 808 –811 [Google Scholar]
- 19. Philip D.: ‘Honey mediated green synthesis of silver nanoparticles’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2010, 75, (3), pp. 1078 –1081 [DOI] [PubMed] [Google Scholar]
- 20. Sathishkumar M. Sneha K. Yun Y.‐S.: ‘Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity’, Bioresour. Technol., 2010, 101, (20), pp. 7958 –7965 [DOI] [PubMed] [Google Scholar]
- 21. Valodkar M. Bhadoria A. Pohnekar J. et al.: ‘Morphology and antibacterial activity of carbohydrate‐stabilized silver nanoparticles’, Carbohydr. Res., 2010, 345, (12), pp. 1767 –1773 [DOI] [PubMed] [Google Scholar]
- 22. Moraru C.I. Panchapakesan C.P. Huang Q. et al.: ‘Nanotechnology: a new frontier in food science’, Food Technol., 2003, 57, (12), pp. 24 –29 [Google Scholar]
- 23. Lee K.J. Nallathamby P.D. Browning L.M. et al.: ‘In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos’, ACS Nano, 2007, 1, (2), pp. 133 –143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen H. Yada R.: ‘Nanotechnologies in agriculture: new tools for sustainable development’, Trends Food Sci. Technol., 2011, 22, (11), pp. 585 –594 [Google Scholar]
- 25. Nanda A. Saravanan M.: ‘Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE’, Nanomed., Nanotechnol. Biol. Med., 2009, 5, (4), pp. 452 –456 [DOI] [PubMed] [Google Scholar]
- 26. Chae Y.J. Pham C.H. Lee J. et al.: ‘Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes)’, Aquat. Toxicol., 2009, 94, (4), pp. 320 –327 [DOI] [PubMed] [Google Scholar]
- 27. Wise J.P. Goodale B.C. Wise S.S. et al.: ‘Silver nanospheres are cytotoxic and genotoxic to fish cells’, Aquat. Toxicol., 2010, 97, (1), pp. 34 –41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Griffitt R.J. Luo J. Gao J. et al.: ‘Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms’, Environ. Toxicol. Chem., 2008, 27, (9), pp. 1972 –1978 [DOI] [PubMed] [Google Scholar]
- 29. Gaiser B.K. Fernandes T.F. Jepson M. et al.: ‘Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments’, Environ. Health, 2009, 8, (Suppl 1), p. S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao C.‐M. Wang W.‐X.: ‘Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna ’, Environ. Toxicol. Chem., 2011, 30, (4), pp. 885 –892 [DOI] [PubMed] [Google Scholar]
- 31. Miao A.‐J. Zhiping L. Chi Shou C. et al.: ‘Intracellular uptake: a possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica ’, PLoS One, 2010, 5, (12), p. e15196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barrena R. Casals E. Colon J. et al.: ‘Evaluation of the ecotoxicity of model nanoparticles’, Chemosphere, 2009, 75, (7), pp. 850 –857 [DOI] [PubMed] [Google Scholar]
- 33. Kumari M. Mukherjee A. Chandrasekaran N.: ‘Genotoxicity of silver nanoparticles in Allium cepa ’, Sci. Total Environ., 2009, 407, (19), pp. 5243 –5246 [DOI] [PubMed] [Google Scholar]
- 34. Stampoulis D. Sinha S.K. White J.C.: ‘Assay‐dependent phytotoxicity of nanoparticles to plants’, Environm. Sci. Technol., 2009, 43, (24), pp. 9473 –9479 [DOI] [PubMed] [Google Scholar]
- 35. Yin L. Cheng Y. Espinasse B. et al.: ‘More than the ions: the effects of silver nanoparticles on Lolium multiflorum ’, Environm. Sci. Technol., 2011, 45, (6), pp. 2360 –2367. [DOI] [PubMed] [Google Scholar]
- 36. Soejarto D.D. Douglas Kinghorn A. Farnsworth N.R.: ‘Potential sweetening agents of plant origin. III. Organoleptic evaluation of Stevia leaf herbarium samples for sweetness’, J. Natl. Prod., 1982, 45, (5), pp. 590 –599 [DOI] [PubMed] [Google Scholar]
- 37. Martelli A. Frattini C. Chialva F.: ‘Unusual essential oils with aromatic properties – I. Volatile components of Stevia rebaudiana bertoni ’, Flavour Fragrance J., 1985, 1, (1), pp. 3 –7 [Google Scholar]
- 38. Rajbhandari A. Roberts M.F.: ‘The flavonoids of Stevia rebaudiana ’, J. Natl. Prod., 1983, 46, (2), pp. 194 –195. [Google Scholar]
- 39. Sholichin M. Yamasaki K. Miyama R. et al.: ‘Labdane‐type diterpenes from Stevia rebaudiana ’, Phytochemistry, 1980, 19, (2), pp. 326 –327 [Google Scholar]
- 40. Oshima Y. Saito J.‐I. Hikino H.: ‘Sterebins A, B, C and D, bisnorditerpenoids of steviarebaudiana leaves’, Tetrahedron, 1986, 42, (23), pp. 6443 –6446 [Google Scholar]
- 41. Oshima Y. Saito J.‐I. Hikino H.: ‘Sterebins E, F, G and H, diterpenoids of Stevia rebaudiana leaves’, Phytochemistry, 1988, 27, (2), pp. 624 –626 [Google Scholar]
- 42. Kinghorn A.D. Soejarto D.D.: ‘Current status of stevioside as a sweetening agent for human use’, in Wagner H. Hikino H. Farnsworth N.R.): (eds. ‘Economic and medicinal plant research’ (Academic Press, New York, 1985), vol. 1, p. 1 [Google Scholar]
- 43. Brandle J.E. Starratt A.N. Gijzen M.: ‘ Stevia rebaudiana: its agricultural, biological, and chemical properties’, Can. J. Plant Sci., 1998, 78, (4), pp. 527 –536 [Google Scholar]
- 44. Starratt A.N. Kirby C.W. Pocs R. et al.: ‘Rebaudioside F, a diterpene glycoside from Stevia rebaudiana ’, Phytochemistry, 2002, 59, (4), pp. 367 –370 [DOI] [PubMed] [Google Scholar]
- 45. Varshney R. Bhadauria S. Gaur M.S.: ‘Biogenic synthesis of silver nanocubes and nanorods using sundried Stevia rebaudiana leaves’, Adv. Mat. Lett, 2010, 1, (3), pp. 232 –237 [Google Scholar]
- 46. Maguire J.D.: ‘Speed of germination—aid in selection and evaluation for seedling emergence and vigor’, Crop Sci., 1962, 2, (2), pp. 176 –177 [Google Scholar]
- 47. Lowry O.H. Rosebrough N.J. Farr A.L. et al.: ‘Protein measurement with the Folin phenol reagent’, J. biol. Chem., 1951, 193, (1), pp. 265 –275 [PubMed] [Google Scholar]
- 48. Cushing B.L. Kolesnichenko V.L. O'Connor C.J.: ‘Recent advances in the liquid‐phase syntheses of inorganic nanoparticles’, Chem. Rev., 2004, 104, (9), pp. 3893 –3946 [DOI] [PubMed] [Google Scholar]
- 49. Burda C. Chen X. Narayan R. et al.: ‘Chemistry and properties of nanocrystals of different shapes’, Chem. Rev., 2005, 105, p. 1025 [DOI] [PubMed] [Google Scholar]
- 50. Daniel M.‐C. Astruc D.: ‘Gold nanoparticles: assembly, supramolecular chemistry, quantum‐size‐related properties, and applications toward biology, catalysis, and nanotechnology’, Chem. Rev., 2004, 104, (1), pp. 293 –346 [DOI] [PubMed] [Google Scholar]
- 51. Prasad T.N.V.K.V. Kambala S.R.V. Naidu R.: ‘A critical review on biogenic silver nanoparticles and their antimicrobial activity’, Curr. Nanosci., 2011, 7, (4), pp. 531 –544 [Google Scholar]
- 52. Zhu Y.L. Pilon‐Smits A.H. Tarun S. et al.: ‘Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ‐glutamylcysteine synthetase’, Plant Physiol., 1999, 121, (4), pp. 1169 –1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Musante C. White J.C.: ‘Toxicity of silver and copper to Cucurbita pepo: differential effects of nano and bulk‐size particles’, Environ. Toxicol., 2012, 27, (9), pp. 510 –517 [DOI] [PubMed] [Google Scholar]
- 54. Berti W.R. Cunningham S.D.: ‘Phytostabilization of metals’ ‘Phytoremediation of toxic metals: using plants to clean‐up the environment’ in Raskins I. Ensley B.D. (Eds.), (John Wiley & Sons, Inc, New York, 2000), pp. 71 –88 [Google Scholar]
- 55. Dhankher O.P. Li Y. Rosen B.P. et al.: ‘Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γ‐glutamylcysteine synthetase expression’, Nat. Biotechnol., 2002, 20, (11), pp. 1140 –1145 [DOI] [PubMed] [Google Scholar]
- 56. Ohkawa K. Kasahara Y. Suh J.‐N.: ‘Mobility and effects on vase life of silver‐containing compounds in cut rose flowers’, HortScience, 1999, 34, (1), pp. 112 –113 [Google Scholar]
- 57. Ichimura K. Yoshioka S. Yumoto‐Shimizu H.: ‘Effects of silver thiosulfate complex (STS), sucrose and combined pulse treatments on the vase life of cut snapdragon flowers’, Environ. Control Biol., 2008, 46, (3), pp. 155 –162 [Google Scholar]
- 58. Koontz H.V. Berle K.L.: ‘Silver uptake, distribution, and effect on calcium, phosphorus, and sulfur uptake’, Plant Physiol., 1980, 65, (2), pp. 336 –339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. AshaRani P.V. Low Kah Mun G. Hande M.P. et al.: ‘Cytotoxicity and genotoxicity of silver nanoparticles in human cells’, ACS Nano, 2008, 3, (2), pp. 279 –290. 279 [DOI] [PubMed] [Google Scholar]
- 60. Oxborough K.: ‘Imaging of chlorophyll a fluorescence: theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance’, J. Exp. Bot., 2004, 55, (400), pp. 1195 –1205 [DOI] [PubMed] [Google Scholar]
- 61. Sinha S.K. Srivastava H.S. Mishra S.N.: ‘Effect of lead on nitrate reductase activity and nitrate assimilation in pea leaves’, Acta Soc. Botanicorum Poloniae, 1988, 57, (4), pp. 457 –463 [Google Scholar]
- 62. Foy C.D. Chaney R.L. White M.C.: ‘The physiology of metal toxicity in plants’, Annu. Rev. Plant Physiol., 1978, 29, (1), pp. 511 –566 [Google Scholar]
- 63. Rai L.C. Kumar A.: ‘Effects of certain environmental factors on the toxicity of zinc to Chlorella vulgaris ’, Microbios Lett., 1980, 13, pp. 79 –84. [Google Scholar]
- 64. Plekhanov S.E. Chemeris Y.K.: ‘Early toxic effects of zinc, cobalt, and cadmium on photosynthetic activity of the green alga Chlorella pyrenoidosa Chick S‐39’, Biol. Bull. Russ. Acad. Sci., 2003, 30, (5), pp. 506 –511 [PubMed] [Google Scholar]
- 65. Hong F. Zhou J. Liu C. et al.: ‘Effect of nano‐TiO2 on photochemical reaction of chloroplasts of spinach’, Biol. Trace Element Res., 2005, 105, (1–3), pp. 269 –279 [DOI] [PubMed] [Google Scholar]
- 66. Feizi H. Kamali M. Jafari L. et al.: ‘Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill)’, Chemosphere, 2013, 91, (4), pp. 506 –511 [DOI] [PubMed] [Google Scholar]
- 67. Chithrani B.D. Ghazani A.A. Chan W.C.W.: ‘Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells’, Nano Lett., 2006, 6, (4), pp. 662 –668 [DOI] [PubMed] [Google Scholar]
- 68. Chithrani B.D. Chan W.C.W.: ‘Elucidating the mechanism of cellular uptake and removal of protein‐coated gold nanoparticles of different sizes and shapes’, Nano Lett., 2007, 7, (6), pp. 1542 –1550 [DOI] [PubMed] [Google Scholar]
- 69. Singh B.K.: ‘Complexation behaviour of glutathione with metal ions’, Asian J. Chem., 2005, 17, (1), pp. 1 –32 [Google Scholar]
- 70. Armendariz V. et al.: ‘Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology’, J. Nanoparticle Res., 2004, 6, (4), pp. 377 –382 [Google Scholar]


