Abstract
A facile and green process to synthesise cuttlebone supported palladium nanoparticles (Pd NPs/cuttlebone) is reported using Conium maculatum leaf extract and in the absence of chemical solvents and hazardous materials. The antioxidant content of the C. maculatum leaf extract played a significant role in converting Pd2+ ions to Pd NPs. Various techniques were used for the characterisation of the Pd NPs/cuttlebone such as field‐emission scanning electron microscopy, X‐ray diffraction, energy dispersive X‐ray spectroscopy, Fourier transform infrared and ultraviolet–visible spectroscopy. This Pd NPs/cuttlebone showed excellent catalytic activity in the reduction of 2,4‐dinitrophenylhydrazine to 2,4‐diaminophenylhydrazine by sodium borohydride as the source of hydrogen at ambient condition. The catalyst could be separated and recycled up to five cycles with no loss of its activity.
Inspec keywords: catalysis, catalysts, chemical engineering, palladium, nanoparticles, field emission electron microscopy, scanning electron microscopy, X‐ray diffraction, X‐ray chemical analysis, sodium compounds, ultraviolet spectroscopy, visible spectroscopy
Other keywords: catalytic reduction; 2,4‐dinitrophenylhydrazine; cuttlebone; Conium maculatum leaf extract; green process; palladium nanoparticles; antioxidant content; field‐emission scanning electron microscopy; X‐ray diffraction; energy dispersive X‐ray spectroscopy; Fourier transform infrared; ultraviolet–visible spectroscopy; 2,4‐diaminophenylhydrazine; sodium borohydride
1 Introduction
The influential removal of poisonous organic dyes and nitroaromatic compounds is an important challenge of researchers during the past few years, because they are common organic pollutants in industrial wastewater. These contaminants are chemically and biologically stable; therefore, their degradation by natural degradation processes is difficult [1, 2, 3, 4]. Among them, nitroaromatic compounds are usually toxic, posing serious risks to the environment, particularly when released into natural water. Therefore, the development of renewable technologies for the removal of such contaminant compounds is highly desirable.
Current removal of nitroaromatic compounds is through nanocatalytic reduction process in the presence of sodium borohydride (NaBH4) as the reducing agent and their transformation to valuable aromatic amines [5, 6, 7]. However, due to the high surface energy of nanoparticles (NPs), their agglomeration is inevitable [8, 9, 10]. Also, isolation of nanocatalyst from the reaction medium is very difficult. To overcome these limitations, magnetite [11], titanium dioxide [12], zeolite [13], graphene oxide [14] and eggshell [15] have been used widely as support for the immobilisation of the metal NPs (MNPs).
There are a variety of chemical and physical methods that can be used to synthesis the NPs. However, some limitations are associated with these strategies such as harsh reaction conditions, the use of non‐environmentally friendly dangerous chemicals and reagents and formation of toxic by‐products limit their medical utilisation [16]. Therefore, an increasing requirement to find the new simple, green, environmentally benign and biological procedures for the preparation of MNPs and their application for the reduction of nitroaromatic compounds.
The green synthesis of the MNPs using plant extracts is one of the most attractive aspects of nanotechnology and can be used as a valuable and economic alternative for the large‐scale production of the MNPs [11, 12, 13, 14, 15]. Plant‐mediated biological synthesis of the MNPs caused to a suitable progression over the synthetic procedures for its safety, mild reaction conditions, high yield, simplicity and low cost. However, there have been only a few reports on the immobilisation of the MNPs on the various supports by using plant extracts as reducing and stabilising agents [11, 12, 13, 14, 15]. During our previous works, we considerably biosynthesised the MNPs in aqueous solutions using plant extracts [11, 12, 13, 14, 15]. This motivated us to explore the possible bioreduction of metal ions into MNPs.
The annual or biennial herb of Conium maculatum is the most poisonous species of the Apiaceae family used as traditional medicines by both Greek and Arabian physicians for the cure of indolent tumours, swellings and pains of the joints (Fig. 1) [17, 18, 19, 20, 21, 22]. In fact, the biological activities of the plant extract such as anti‐inflammation, anticancer, antioxidant and antimicrobial were demonstrated by many researches. The secondary active metabolites of the plant are alkaloids, phenolic antioxidants (glycosides and aglycones), steroids and tannins.
Fig. 1.

C. maculatum image
In this paper, we demonstrate an environmentally friendly, simple, economic and non‐toxic method to produce Pd NPs/cuttlebone by the antioxidant reducing C. maculatum leaf extract without the application of NPs stabilising surfactants. The cuttlebone supported Pd NPs demonstrated the high catalytic activity and stability toward the reduction of 2,4‐dinitrophenylhydrazine (2,4‐DNPH) to 2,4‐diaminophenylhydrazine (2,4‐DAPH) with NaBH4 at ambient condition. To improve our study, for the first time we report the use of green synthesised Pd NPs/cuttlebone for the reduction of 2,4‐DNPH with NaBH4.
2 Experimental results
2.1 Instruments and reagents
PdCl2, 2,4‐DNPH and NaBH4 were obtained from Sigma‐Aldrich company (USA) and used without further purification. The leaves of Conium maculatum was collected from the Salwat Abad area (Province of Kurdistan, Iran). Cuttlebone was obtained from Iran. The analytical instrumentations used for this research were Fourier transform infrared (FT‐IR) (potassium bromide) spectrometer (Perkin‐Elmer 781 model). X‐ray diffraction (XRD) with a Philips powder diffractometer type PW 1373 goniometer and equipped with a graphite monochromator crystal and the X‐ray wavelength of 1.5405 A°, 2θ range (10–90) diffraction patterns and scanning speed of 2°/min. Scanning electron microscopy (SEM) (Cam scan MV2300) equipped with Energy Dispersive X‐ray Spectroscopy‐S3700N (EDS), and finally ultraviolet–visible (UV–vis) double‐beam spectrophotometer (Hitachi, U‐2900).
2.2 Preparation of C. maculatum leaf extract
The leaf of the plant was dried in shadow and powdered. About 50 g of its powder was mixed in 300 ml distillated water and well shacked at 80°C for 30 min to produce the extract. The extract centrifuged in 7000 rpm and then filtered and used to further stages of the experiment.
2.3 Biosynthesis of the Pd NPs
About 15 ml extract of the plant and 50 ml of 0.003 M solution of PdCl2 were mixed at 80°C to produce Pd(0) around 7 min and then centrifuged at 7000 rpm for 45 min for completely separation. The formation of palladium (Pd) NPs was monitored by UV–vis and FT‐IR techniques for more convenience. The synthesised Pd NPs were kept under argon noble gas to more conservation.
2.4 Biosynthesis of the Pd NPs/cuttlebone by using C. maculatum leaf extract
In experimental procedure, 50 ml of the plant extract was added dropwise into 1.0 g cuttlebone and 0.18 g PdCl2 at 80°C under continuous stirring. About ∼2 h later, the synthesised product by C. maculatum leaf extract was centrifuged and then washed several times, then dried under vacuum at 80°C for 1 h to obtain dried Pd NPs/cuttlebone.
2.5 2,4‐DNPH catalytic reduction in the presence of the Pd NPs/cuttlebone
In experimental procedure, 25 ml of the 2,4‐DNPH aqueous solution (0.076 mM) and 7.0 mg of the Pd NPs/cuttlebone were mixed under stirring for 1 min. In the next step, 25 ml of fresh NaBH4 aqueous solution (7.93 mM) was added to the previous mixture under stirring at ambient conditions. The yellow colour of the solution gradually disappeared, while formation of 2,4‐DAPH was checked by UV–vis spectroscopy. After each cycle of reduction experiment, the recycling of the catalyst for latter cycles was done by simple centrifugation, washing and drying.
3 Results and discussion
Through this paper we investigate the antioxidant potential of the C. maculatum leaf extract using ferric reduction of antioxidant power (FRAP) method and then use the plant extract to green synthesis of the Pd NPs and their immobilisation on cuttlebone as a natural support for biosynthesis of the Pd NPs/cuttlebone as a heterogeneous catalyst. The synthesised catalyst has been comprehensively characterised by various techniques such as field‐emission SEM (FESEM), EDS, XRD and FT‐IR. For more convenience, the Pd NPs was first identified by FT‐IR and UV–vis techniques.
3.1 Characterisation of C. maculatum extract
According to Fig. 2, the specified bonds at λ max 275 nm (bond I) and 238 nm (bond II) are concerned to the π →π * transitions of cinnamoyl and benzoyl phenolic systems, respectively. Therefore, this paper strongly supports the results obtained by literatures about the plant antioxidant phenolic contents [23].
Fig. 2.

UV–vis finger printing of the C. maculatum extract
In Fig. 3 a, peaks appeared at 3315, 1630, 1472 and 1300–1100 cm−1 are assigned to the OH, carbonyl group (C=O), aromatic C=C and C–OH vibrations, respectively. These peaks are related to the phenolic functional groups as the major plant phyto‐constituents.
Fig. 3.

FT‐IR spectrum of
(a) P lant extract, (b) Pd NPs
3.2 Reduction of Pd2+ ions to Pd(0)
The antioxidant capacity of the plant aqueous medium was studied by the FRAP assay according to the Benzie and Strain method with no modifications [24] based on the reduction of ferric tripyridyl–triazine (TPTZ) complex to the ferrous TPTZ (Fe(II)‐TPTZ) at low pH and 593 nm using the standard curve. The obtained results of this paper confirmed the potent antioxidant characteristic of the C. maculatum aqueous extract and its good potential to biosynthesis the nanostructures.
The reduction of the Pd2+ ions was clearly observed when the plant extract was added into PdCl2 solution heated at 80°C for 7 min. Changing the colour following the SPR phenomenon showed the biosynthesis of the Pd NPs. The phenolic antioxidants of the extract were involved in this green process according to the below mechanism (Fig. 4).
Fig. 4.

Mechanism for green synthesis of the Pd NPs using C. maculatum aqueous extract
The appearance of absorbance maximum in 261 nm followed by changing the solution colour (Fig. 5) demonstrated the SPR process and reduction of the Pd2+ to Pdo and then formation of Pd NPs. Furthermore, the NPs are almost quite stable even for 14 days.
Fig. 5.

UV–vis spectrum of the Pd NPs synthesised by C. maculatum extract between 7 min and 14 days
Furthermore, the FT‐IR of obtained Pd NPs shows peaks at 3415, 1628, 1485 and 1305–1150 cm−1 assigned to the OH, carbonyl (C=O), aromatic C=C and C–OH vibrations, respectively (Fig. 3 b). These signals confirm the adsorption and interaction of the PdCl2 with plant phytochemicals.
3.3 Characterisation of the Pd NPs/cuttlebone
FT‐IR signals of the cuttlebone were given in Fig. 6. FT‐IR spectrum of cuttlebone shows peaks at 1084 and about 1400–1500 cm−1 correspond to the symmetric and asymmetric stretching vibrations of the carbonate ion, respectively, which are diagnostic of aragonite (a meta‐stable form of calcium carbonate) [25, 26]. The peaks at 708 and 856 cm−1 are attributed to the split in‐plane and out‐of‐plane bending vibrations of the in the free CO3 −2 ion. Also, the peaks at about 3315–3450 cm−1 correspond to the OH and NH stretching vibrations, which are related to absorption bands of β ‐chitin segment of cuttlebone. Furthermore, signals identified at ∼2850–2977 cm−1 due to the symmetric stretching modes of CH, CH3 and asymmetric stretching vibrations of CH2.
Fig. 6.

FT‐IR spectrum of the cuttlebone
For the investigation of crystalline phase and purity of the synthesised Pd NPs/cuttlebone powder, XRD measurements were carried out. Fig. 7 displays the XRD pattern of the Pd NPs/cuttlebone. The obtained results indicate the immobilisation of the Pd NPs on cuttlebone surface. The diffraction peaks at 2θ values of 40.4°, 46.7°, 68.5°, 82.1° and 86.9° corresponding to, respectively, Pd (111), (200), (220), (311) and (222) (JCPDS No. 5‐0681) crystallinity of nanocomposite.
Fig. 7.
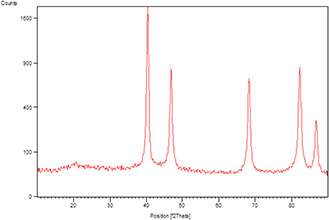
XRD pattern of the Pd NPs/cuttlebone
The surface morphology of the Pd NPs/cuttlebone was studied by FESEM micrographs as shown in Fig. 8. The FESEM images display spherical morphology of the Pd NPs. Furthermore, the minimum and maximum size ranges of the Pd NPs have been found as 16, 28 and 42 nm. Combined with XRD and SEM results of the Pd NPs/cuttlebone, the successful fabrication and immobilisation of Pd NPs on cuttlebone surface is depicted.
Fig. 8.

FESEM images of the Pd NPs/cuttlebone
EDS analysis was employed to determine the chemical composition of the synthesised Pd NPs/cuttlebone (Fig. 9). The EDS spectra showed well‐defined peaks of Ca, O and Pd, thereby confirming the successful anchoring of the Pd NPs on the cuttlebone surface.
Fig. 9.

EDS spectrum of the Pd NPs/cuttlebone
3.4 Catalytic activity of the Pd NPs/cuttlebone for reduction of the 2,4‐DNPH
After demonstrating the Pd NPs/cuttlebone catalyst, its catalytic activity studied on the reduction of the 2,4‐DNPH using NaBH4 as reductant at room temperature in aqueous medium (Fig. 10). The reaction was monitored by UV–vis spectroscopy. The characteristic absorption of the 2,4‐DNPH at about 353 nm was selected as monitoring the catalytic reduction process (Fig. 11). The result shows that 2,4‐DNPH solution is stable without Pd NPs/cuttlebone. According to the UV–vis analysis, the absorption peak of the 2,4‐DNPH at about 353 nm remains unchanged in the absence of the Pd NPs/cuttlebone. The presence of the Pd NPs/cuttlebone in aqueous solution containing the 2,4‐DNPH and NaBH4 could result in more obvious reduction of the 2,4‐DNPH, but no product was detected in the absence of NaBH4. According to Fig. 11, when Pd NPs/cuttlebone added to the 2,4‐DNPH and NaBH4 mixture, the intensity of signal around 353 nm gradually replaced by a signal at 290 nm related to the reduction of the 2,4‐DNPH–2,4‐DAPH. We can see that the solution colour have shifted from yellow to white gradually after adding catalyst. The catalytic reduction of the 2,4‐DNPH–2,4‐DAPH is completed within 4 min, indicating the superior catalytic performance of the Pd NPs/cuttlebone. After 4 min, the whole absorbance peak of the 2,4‐DNPH at about 353 nm disappeared with transparency of the solution colour expressing the conversion of the 2,4‐DNPH–2,4‐DAPH. The 2,4‐DNPH solution can be decolourised into colourless at the end of reaction.
Fig. 10.

Reduction of 2,4‐DNPH on the surface of the Pd NPs/cuttlebone
Fig. 11.

UV–vis spectra during reduction of the 2,4‐DNPH catalysed by Pd NPs/cuttlebone
To check the effect of the cuttlebone as a natural support, the reaction was also carried out using cuttlebone as catalyst without loading of Pd NPs, but no reduction reaction was observed. Hence, cuttlebone reduced the Pd NPs agglomeration, simplify the recyclability of catalyst and enhance its synergic effect during the reaction process. The catalytic reduction of the 2,4‐DNPH–2,4‐DAPH is an electron transfer (ET) process. The catalytic reduction of the 2,4‐DNPH–2,4‐DAPH is of two consequent steps: (i) diffusion and adsorption of the 2,4‐DNPH onto the surface of the catalyst via π –π stacking interactions and (ii) the ET from the BH4 − donor to the acceptor 2,4‐DNPH using the Pd NPs. The green synthesised nanocomposite can efficiently catalyse the reaction by simplifying the electron transformation from BH4 − to 4‐NP on the catalyst surface, leading to the reduction of the 2,4‐DNPH. The 2,4‐DNPH will be reduced to the 2,4‐DAPH and finally the product desorbed from the catalyst surface.
3.5 Catalyst recyclability
The recyclability of the Pd NPs/cuttlebone was investigated in the reduction of the 2,4‐DNPH. In fact, the separation of catalyst from the reaction mixture and using for the next cycles was simply possible by its centrifugation and washing successively with water and ethanol and then drying. No significant decrease in catalytic activity after five runs was observed, indicating that the Pd NPs/cuttlebone has relatively high stability during catalytic reduction of the 2,4‐DNPH. Also, the reduction time of whole 2,4‐DNPH was almost same up to the fifth cycle.
4 Conclusions
During this paper, for the first time, we presented the reduction of the 2,4‐DNPH using green synthesised Pd NPs/cuttlebone as an efficient nanocatalyst. Furthermore, the application of cuttlebone showed a great tendency to adsorption of Pd NPs on its surface which promote the catalyst surface area and follow the synergic effect and efficiency of the reaction. Moreover, the first time application of C. maculatum as antioxidant source and a novel plant in catalyst researches lead to the phytochemical adsorption on the surface of NPs and substrate that increasingly enhance stabilisation of nanocatalyst and green synthesis of Pd NPs/cuttlebone to show the excellent catalytic applicability for the reduction of the 2,4‐DNPH using NaBH4 as reductant at ambient conditions. The Pd NPs/cuttlebone could be recycled and simply recovered five times without loss of catalytic activity. The synthesis of the Pd NPs by this method is rapid, convenient, facile, less time consuming and environmentally safe and this protocol can be used for the synthesis of metal and other metal oxide NPs. The great advantage of our method is using cuttlebone as a natural support and C. maculatum leaf extract as a reducing and stabilising agent which minimises the interest of using poisonous and dangerous reagents, solvents and other chemical compounds for the nanofabrication process.
5 Acknowledgments
We gratefully acknowledge the Iranian Nano Council and the University of Qom for the support of this work.
6 References
- 1. Dai R. Chen J. Lin J. et al.: ‘Reduction of nitro phenols using nitroreductase from E. coli in the presence of NADH’, J. Hazard. Mater., 2009, 170, (1), p. 141 [DOI] [PubMed] [Google Scholar]
- 2. Spain J.C.: ‘Biodegradation of nitroaromatic compounds’, Annu. Rev. Microbiol., 1995, 49, (1), pp. 523 –555 [DOI] [PubMed] [Google Scholar]
- 3. Mittal A. Teotia M. Soni R.K. et al.: ‘Applications of egg shell and egg shell membrane as adsorbents: a review’, J. Mol. Liq., 2016, 223, pp. 376 –387 [Google Scholar]
- 4. Mittal J. Mittal A., Feather H.: ‘A remarkable adsorbent for dye removal’, in Sharma S.K. (Ed.): ‘Green chemistry for dyes removal from wastewater’ (Scrivener Publishing LLC, USA, 2015), pp. 409 –457, Chapter 11 [Google Scholar]
- 5. Naik B. Prasad V.S. Ghosh N.N.: ‘Preparation of Ag nanoparticle loaded mesoporous γ‐alumina catalyst and its catalytic activity for reduction of 4‐nitrophenol’, Powder Technol., 2012, 232, pp. 1 –6 [Google Scholar]
- 6. Naik B. Hazra S. Muktesh P. et al.: ‘A facile method for preparation of Ag nanoparticle loaded MCM‐41 and study of its catalytic activity for reduction of 4‐nitrophenol’, Sci. Adv. Mater., 2011, 3, (6), pp. 1025 –1030 [Google Scholar]
- 7. Saha S. Pal A. Kundu S. et al.: ‘Photochemical green synthesis of calcium‐alginate‐stabilized Ag and Au nanoparticles and their catalytic application to 4‐nitrophenol reduction’, Langmuir, 2010, 26, (4), pp. 2885 –2893 [DOI] [PubMed] [Google Scholar]
- 8. Kundu S.: ‘A new route for the formation of Au nanowires and application of shape‐selective Au nanoparticles in SERS studies’, J. Mater. Chem., 2013, C1, (4), pp. 831 –842 [Google Scholar]
- 9. Kundu S. Wang K. Huitinkand D. et al.: ‘Photoinduced formation of electrically conductive thin palladium nanowires on DNA scaffolds’, Langmuir, 2009, 25, (17), pp. 10146 –10152 [DOI] [PubMed] [Google Scholar]
- 10. Mandal M. Kundu S. Ghosh S.K. et al.: ‘Micelle‐mediated UV‐photoactivation route for the evolution of Pd core‐Au shell and Pd core‐Ag shell bimetallics from photogenerated Pd nanoparticles’, J. Photochem. Photobiol. A Chem., 2004, 167, (1), pp. 17 –22 [Google Scholar]
- 11. Sajjadi M. Nasrollahzadeh M. Sajadi S.M.: ‘Green synthesis of Ag/Fe 3 O 4 nanocomposite using Euphorbia peplus Linn leaf extract and evaluation of its catalytic activity’, J. Colloid Interface Sci., 2017, 497, pp. 1 –3 [DOI] [PubMed] [Google Scholar]
- 12. Rostami‐Vartooni A. Nasrollahzadeh M. Salavati‐Niasari M. et al.: ‘Photocatalytic degradation of azo dyes by titanium dioxide supported silver nanoparticles prepared by a green method using Carpobrotus acinaciformis extract’, J. Alloys Compd., 2016, 689, pp. 15 –20 [Google Scholar]
- 13. Nasrollahzadeh M. Sajadi S.M. Maham M. et al.: ‘In situ green synthesis of Cu nanoparticles supported on natural Natrolite zeolite for the reduction of 4‐nitrophenol, congo red and methylene blue’, IET Nanobiotechnol., 2017, 11, (5), pp. 538 –545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nasrollahzadeh M. Atarod M. Jaleh B. et al.: ‘In situ green synthesis of Ag nanoparticles on graphene oxide/TiO 2 nanocomposite and their catalytic activity for the reduction of 4‐nitrophenol, congo red and methylene blue’, Ceram. Int., 2016, 42, (7), pp. 8587 –8596 [Google Scholar]
- 15. Nasrollahzadeh M. Sajadi S.M. Hatamifard A.: ‘Waste chicken eggshell as a natural valuable resource and environmentally benign support for biosynthesis of catalytically active Cu/eggshell, Fe 3 O 4/eggshell and Cu/Fe 3 O 4/eggshell nanocomposites’, Appl. Catal. B Environ., 2016, 191, pp. 209 –27 [Google Scholar]
- 16. Molnar A.: ‘Efficient, selective, and recyclable palladium catalysts in carbon‐carbon coupling reactions’, Chem. Rev., 2011, 111, (3), pp. 2251 –2320 [DOI] [PubMed] [Google Scholar]
- 17. Reecha M. Bikramjit S. Suresh K.: J. Pharm. Biomed. Sci., 2010, 1, p. 18 [Google Scholar]
- 18. Binev R. Mitev J. Miteva T.: ‘Intoxication with Poison Hemlock (Conium maculatum L.) in calves’, Trakia J. Sci., 2007, 5, pp. 40 –50 [Google Scholar]
- 19. Salma Begum B. Mastan M.: ‘'Phytochemical Screening, Chromatographic analysis of Chloroform extract of Conium maculatum’, Int. Res. J. Biol. Sci., 2015, 4, (3), pp. 27 –29 [Google Scholar]
- 20. Perez R.M.: ‘Anti‐inflammatory activity of compounds isolated from plants’, Sci. World J., 2001, 1, pp. 713 –784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Radulovic N.S. Dordevic N.D.: ‘Steroids from poison hemlock (Conium maculatum L.): a GC‐MS analysis’, J. Serb. Chem. Soc., 2011, 76, (11), pp. 1471 –1483 [Google Scholar]
- 22. Mehrabi R. Bagheri G. Alipour F.: ‘Anticancer effects of Anbuta and Vinoblastin on breast cancer cell line ZR 75‐1’, J. Entomol. Zool. Stud., 2015, 3, (2), pp. 208 –210 [Google Scholar]
- 23. Bhat S.V. Naga Sampagi B.A. Sivakumar M.: ‘Chemistry of natural products’ (Narosa Publishing House, New Delhi, 2005), p. 585 [Google Scholar]
- 24. Benzie I.F.F. Strain J.J.: Anal. Biochem., 1996, 239, p. 70 [DOI] [PubMed] [Google Scholar]
- 25. Florek M. Fornal E. Romero P. et al.: ‘Complementary microstructural and chemical analyses of Sepia officinalis endoskeleton’, Mater. Sci. Eng. C, 2009, 29, (4), pp. 1220 –1226 [Google Scholar]
- 26. Adler H.H. Kerr P.F.: ‘Infrared study of aragonite and calcite’, Am. Mineral., 1962, 47, pp. 700 –717 [Google Scholar]


