Abstract
The peel of Citrus maxima (C. maxima) is the primary byproducts during the process of fruit or juice in food industries, and it was always considered as biomass waste for further treatments. In this study, the authors reported a simple and eco‐friendly method to synthesise gold nanoparticles (AuNPs) using C. maxima peel extract as reducing and capping agents. The synthesised AuNPs were characterised by UV–visible spectrum, X‐ray diffraction (XRD), transmission electron microscope (TEM) and Fourier‐transform infrared spectroscopy (FTIR). The UV–visible spectrum of the AuNPs colloid showed a characteristic peak at 540 nm. The peaks of XRD analysis at (2θ) 38.30°, 44.28°, 64.62°, 77.57° and 81.75° were assigned to (111), (200), (220), (311) and (222) planes of the face‐centered cubic (fcc) lattice of gold. The TEM images showed that AuNPs were nearly spherical in shape with the size of 8–25 nm. The FTIR spectrum revealed that some bioactive compounds capped the surface of synthesised AuNPs. The biosynthesised AuNPs performed strong catalytic activity in degradation of 4‐nitrophenol to 4‐aminophenol and good antibacterial activity against both gram negative (Escherichia coli) and gram positive (Staphylococcus aureus) bacterium. The synthesis procedure was proved simple, cost effective and environment friendly.
Inspec keywords: gold, nanoparticles, nanofabrication, X‐ray diffraction, ultraviolet spectra, visible spectra, transmission electron microscopy, Fourier transform infrared spectra, crystal structure, catalysis, antibacterial activity, nanobiotechnology
Other keywords: gold nanoparticles, Citrus maxima peel extract, UV–visible spectrum, X‐ray diffraction, transmission electron microscope, Fourier‐transform infrared spectroscopy, XRD analysis, faced centre cubic lattice, TEM images, catalytic activity, 4‐nitrophenol, 4‐aminophenol, antibacterial activity, gram negative bacterium, gram positive bacterium, Au
1 Introduction
Gold nanoparticles (AuNPs) have been proved with strong antibacterial [1] and catalytic activities [2]. They have been applied in many fields and attracted more and more attentions in recent years [3, 4, 5, 6, 7]. Many synthesis methods including some green and eco‐friendly methods for nanoparticles (NPs) have been developed [8, 9, 10, 11]. The biosynthesis methods using microorganism or extract of plants as reducing and capping agents are considered as nontoxic, simple, low‐cost and environment benign methods for the preparation of NPs [12, 13, 14]. Among these biosynthesis methods, phytobiosynthesis is regarded as the most promising route due to the fact that the natural available plants are usually inexpensive, and the process of synthesis is feasible for large‐scale productions [13, 14, 15].
The researchers in India can be regarded as the pioneers in this new field. Shankar et al. [16] used geranium leaf extract to reduce gold ions and stabilise the AuNPs more than ten years ago. To the best of our knowledge, this should be the first study about the biosynthesis of AuNPs using plant extract. Later on, the authors found the leaf of neem could also be applied for the preparation of AuNPs [17], and the reaction was more rapid compared with the previous study. The morphology of AuNPs was predominantly in planar and spherical shapes. Flavanones and terpenoids, which are abundant in the neem leaf acted as the reducing, stabiliser and capping agents for NPs without aggregation. Lemongrass was also used to synthesise AuNPs [18]. The shapes of AuNPs could be adjusted by the different fractions of lemongrass extract. Until now, the strategy of phytosynthesis has been greatly developed. Many plants have already been applied to synthesise AuNPs, such as Zingiber Officinale [19], Dioscore Bulbifera [20] and Barbated Skullcup [21]. In addition to metallic NPs, some metal–oxide NPs were also synthesised by natural plant extracts [14, 22, 23].
Citrus maxima (C. maxima) is one of the members of Rutaceae family and universally distributed in the southeast of Asia, especially in the south of the Yangtze River in China. The peel of C. maxima is the primary byproducts during the process of fruit or juice in food industries [24], and it was always considered as biomass waste for further treatments. It has been proved that a variety of flavonoids including narirutin, naringin, hesperidin, eriocitrin and neohesperidin were present in the peels and pulps [25]. These kinds of compounds are also known to possess antiviral, anticancer, anti‐inflammatory, antiallergenic and analgesic activities. Herein, the peel extract of C. maxima can potentially be used as reducing and capping agents for the preparation of AuNPs. Although the study about the biosynthesis of AuNPs using citrus flesh or pulps has been reported recently [26], there is no report to synthesise noble metal nanoparticles using C. maxima peels [27]. Compared with fruit flesh, C. maxima peels will be more cost effective. In addition, the growth of plants is usually influenced by regions and climates. How to logically obtain and economically store the biomass is another critical issue for the real applications and scaling‐up of phytobiosynthesis methods. C. maxima peels can be easily obtained, dried and stored for a long time. These features of C. maxima peels enable the proposed method more feasible and practical. To synthesise AuNPs using C. maxima peel not only provides us a facile biosynthesis method but also be valuable for the beneficial utilisation of agriculture waste.
The catalytic property of AuNPs is used in many fields, especially in removing contaminants in wastewater. Many researchers have reported that AuNPs synthesised by plant extract were used as catalysts for the degradation of 4‐nitrophenol (4‐NP) [28] and methylene blue [29]. The biosynthesised AuNPs showed much better catalytic activity than chemically synthesised AuNPs [30] without adding any toxic or harmful substances for their preparations. AuNPs are also applied as antibacterial agents. Some reports revealed that AuNPs synthesised via plant extract could kill or inhibit both gram negative and gram positive bacteria [31].
In this study, a new, simple and green biosynthesis method of AuNPs using peel extract of C. maxima was successfully developed. The formation of AuNPs was monitored by UV–visible spectra. The size and morphology of the obtained AuNPs were characterised by high‐resolution transmission electron microscopy (HRTEM) with X‐ray energy dispersive detector (EDS). Their crystalline and the chemical groups capped on the surface of the particles were further characterised by powder X‐ray diffraction (XRD) and Fourier‐transform infrared spectroscopy (FTIR). The catalytic and antibacterial activities of the obtained AuNPs were also investigated via the experiments of 4‐NP degradation and inhibition tests against both Escherichia coli (E. coli) and Staphylococcus aureus (S. auerus).
2 Materials and methods
2.1 Materials
C. maxima was purchased from a local market in Baoding, China. Chloroauric acid (HAuCl4 ·4H2 O) was purchased from Sinopharm Chemical Reagent Co. Ltd. A stock solution of HAuCl4 was prepared by dissolve 1.0 g HAuCl4 ·4H2 O in 24.28 ml distilled water. All glasses were cleaned with aqua regia solution and thoroughly rinsed with distilled water and dried in oven before use.
2.2 Preparation of the extract of C. maxima peel
The peel of C. maxima was dried in an oven at 50°C and ground to pieces. The extract was prepared by taking 1.0 g of peel into 100 ml deionised water and boiled for 10 min. The working extract was obtained by filtration the original extract through a microporous membrane and stored in a refrigerator at 4°C for use.
2.3 Synthesis of AuNPs using the extract of C. maxima peel
To biosynthesise AuNPs, 100 μl of chloroauric acid (100 mM) was added into 10 ml of C. maxima extract and shaken at room temperature. The colour of the mixture changed from light yellow to purple indicating the formation of AuNPs in the solution. The reaction mixture was then centrifuged at 16,000 rpm for 10 min and the pellet was washed with distilled water for three times to remove any possible impurities on the surface of the NPs.
The effect of HAuCl4 concentration was investigated by varying the volume of the HAuCl4 working solution. 50, 100, 150 and 200 μl of HAuCl4 (100 mM) were added into 10 ml of the peel extract, respectively. To study the effect of incubation temperature, the reaction mixtures containing 100 μl of HAuCl4 (100 mM) aqueous solution and 10 ml of the peel extract were placed in water bath at 40, 50, 60 and 70°C, respectively. The effect of pH was studied by adjusting the pH values of C. maxima peel extract with 0.1 M HCl and 0.1 M NaOH solutions. The pH values were adjusted to 3.0, 5.0, 7.0, 9.0 and 11.0. 100 μl of HAuCl4 aqueous solution were added to 10 ml of the above solution at different pH values. The formation of AuNPs was monitored by a UV–vis spectrometer. All of the experiments were carried out in triplicates.
2.4 Characterisation of the synthesised AuNPs
The formation of AuNPs was scanned by UV‐vis spectra (Pgenenal, China) from 300 to 800 nm at a resolution of 1 nm. The sizes and shapes of the AuNPs were measured by transmission electron microscope (Tecnai G2 F20 S‐TWIN, FEI/Netherland). Samples were prepared by placing a drop of solution sample onto a carbon‐coated Cu grid and evaporated in ambient condition. The microscope was also equipped with a genesis liquid nitrogen cooled energy‐dispersive X‐ray analysis (EDX) detector for detailed elemental analysis. X‐ray powder diffractometer (D8 ADVENCE, Bruker/Switzerland) with Cu‐Kα radiation (λ = 1.5418 Å) with a scan speed of 2°/min was used to analyse the crystalline structure of AuNPs.
Fourier‐transform infrared spectra (FTIR) were recorded using Magna‐IR 750 (America) in order to analyse the functional groups in C. maxima peel which are possibly involved in the synthesis of AuNPs. FTIR spectrophotometer was operated in the spectral range of 4000–400 cm–1 with resolution of 4 cm–1.
2.5 Catalytic activity of AuNPs
The degradation of 4‐NP to 4‐aminophenol (4‐AP) by NaBH4 was performed as a probe for the study of the catalytic activity of AuNPs [32]. In a standard quartz cell, 20 μl of 4‐NP (20 mM) solution and 400 μl of fresh NaBH4 (2.5 M) solution were mixed in 60 μl of the prepared AuNPs colloidal solution. The appropriate deionised water was added to keep its final volume constant (3 ml). The process of reaction was monitored by a UV–vis spectrometer. The different volumes of the AuNPs (20 and 40 μl) solution were added to measure the degradation rates.
2.6 Antibacterial activity of AuNPs
The extract of C. maxima peel, the biosynthesised AuNPs and gentamicin were used for the inhibition tests against both gram positive (S. aureus) and gram negative (E. coli) bacteria. The different concentrations of AuNPs were used to evaluate the antibacterial abilities. S. aureus and E. coli were cultured in nutrient broth at 37°C for 18 h. 100 μl of the bacterial suspensions were coated on the Luria‐Bertani (LB) solid medium, respectively. 10 min later, five Oxford cups were placed on the above LB solid medium. 50 μl of C. maxima peel extract, AuNPs (1.0, 1.5 and 2.0 mM) and gentamicin were added into the Oxford cups, respectively. The Petri dishes were incubated in the incubator at 37°C for 24 h. After culture, the zones of inhibition were observed and measured. Three replicates were tested for each bacteria strain.
3 Results and discussion
3.1 Biosynthesis of AuNPs
AuNPs always appears in ruddiness colour in aqueous solution due to excitation of surface plasmon vibrations [21]. The formation of AuNPs was confirmed by the colour change from pale yellow to purple after incubation for about 1 h. The synthesised AuNPs were initially investigated by UV–vis spectroscopy. The intensity and width of the surface plasmon resonance (SPR) peaks depend on the size, morphology, spatial orientation, optical constants of the particles and the embedding medium as well [33, 34]. Here, the reaction conditions such as concentration, reaction temperature and pH were investigated and discussed.
3.1.1 Effect of chloroauric acid concentration
Fig. 1 a shows the visual observation of AuNPs at various HAuCl4 concentrations after 2 h reaction. It was evident that the colours of the four samples were different, which indicated that the sizes or morphologies of the synthesised AuNPs were different. When the lower concentration of HAuCl4 was applied, the reduction rate was faster during the initial time span for the abundant presence of biomolecules in the mixture at the beginning of the reaction.
Fig. 1.
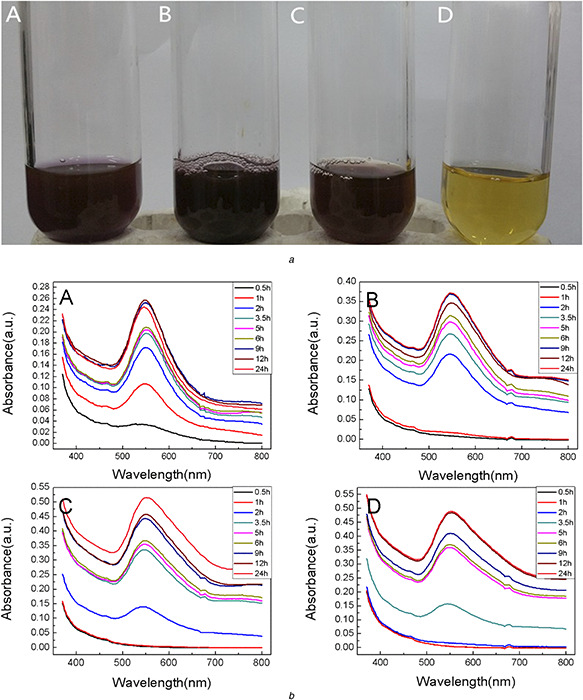
Effect of chloroauric acid concentration
(a) Visual observation of reaction mixture after 2 h with different chloroauric acid concentrations (A) 0.5 mM, (B) 1 mM, (C) 1.5 mM, (D) 2 mM, (b) UV–vis absorption spectrum of AuNPs at various time intervals with different concentrations ((A) 0.5 mM, (B) 1 mM, (C) 1.5 mM, (D) 2 mM)
It was also confirmed by UV–vis spectrum in Fig. 1 b (A). A characteristic absorption band at 540 nm was observed after 0.5 h reaction. With the reaction going on and more AuNPs being synthesised, the SPR band was lightly shifted from 540 to 554 nm when the elevated concentration of AuNPs was applied. The peak shift indicated that the sizes and shapes of the NPs were slightly changed compared with the initial colloid. The SPR bands were sharper and symmetric at the lower concentrations of HAuCl4, which indicated that the biosynthesised AuNPs were monodispersed in the solution [35]. The SPR bands appeared 2 h or longer after the extract were mixed with the higher concentrations of Au3+ solution (Fig. 1 b (B)– (D)). The prolonged appearance of AuNPs in solution was probably owing to the excessive Au3+ ions in the mixture. The abundant Au3+ ions were reduced to Au0 and produced a large number of opportunities for nucleation, which would take longer time for Au0 to grow into larger clusters [36]. After the mixture reacted for 24 h, the intensities of SPR bands at the higher concentrations of HAuCl4 solution were much stronger than that at the lower concentrations. The quantity of AuNPs formed at the higher concentrations of HAuCl4 was larger than that at the lower concentrations. An interesting phenomenon was observed that a weak absorption peak appeared at 680 nm. The peak lightly blue shifted and disappeared with the reaction. It might be due to the agglomeration of the formed AuNPs in solution or the particles in non‐spherical shapes [37]. The peaks lightly blue shifted and disappeared when the heterogeneous AuNPs became homogeneous and monodispersive.
3.1.2 Effect of reaction temperature
Fig. 2 a shows the UV–vis spectra of AuNPs at the different reaction temperatures. Temperature is one of the important parameters that influence the stability, activity and chemical characters of NPs [38]. In this study, a characteristic SPR band appeared after 5 min reaction when 70°C was employed.
Fig. 2.
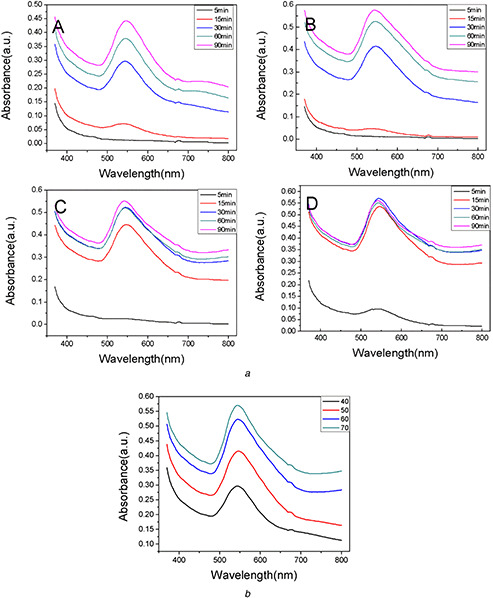
UV–vis spectra of AuNPs at different temperatures
(a) UV–vis spectra of biosynthesised AuNPs at different temperatures (A) 40°C, (B) 50°C, (C) 60°C, (D) 70°C, (b) UV–vis spectra of AuNPs at different temperatures
The colour of the mixture was purple. There was no colour changing or absorption band in 5 min at 40, 50 and 60°C. With the reaction time going on, the SPR bands gradually appeared in all of the four samples. The intensities of the absorption peaks increased, which indicated more AuNPs were synthesised in the solution. In Fig. 2 a (D), the maximum intensity of the peak appeared after 30 min reaction due to the exhaustive conversion of gold ions to AuNPs. Furthermore, the higher temperatures led to the higher reaction rates, which were confirmed by the UV–vis spectra shown in Fig. 2 b. The intensity of the peak increased with the temperature increasing during the same incubation time. Fig. 2 a and b also show that there was no significant influence of the temperature on the stability of AuNPs without any shifting and broadening of SPR band.
3.1.3 Effect of pH
pH plays a crucial role during the biosynthesis process of AuNPs. Fig. 3 shows the SPR bands of the AuNPs synthesised by the extract of C. maxima peel under various pH conditions (3.0, 5.0, 7.0, 9.0 and 11.0) at room temperature. An elevated pH value caused the intensity of the peaks increasing. The SPR band blue shifted gradually from 556 to 543 nm, which indicated that the AuNPs sizes decreased [39]. The conditions at higher pH could probably enhance the reaction rate and boost the homogeneous nucleation. On the contrary, the reaction rate was slow under the acidic conditions. It induced the heterogeneous nucleation and secondary nucleation of small AuNPs seeds [39], which gave rise to enlarge the sizes of AuNPs. Therefore, the sizes and shapes of AuNPs could be regulated by adjusting the pH of the reaction solution.
Fig. 3.
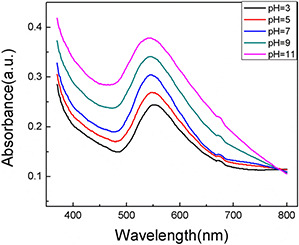
UV–vis spectra of AuNPs at different pH
3.1.4 Characterisation
TEM images and the particle size distribution histogram were shown in Fig. 4 a. It was observed that the prepared AuNPs were well dispersed, predominantly subspheroidal in shape with the size range from 8 to 25 nm (most of them were about 15 nm). HRTEM images (Fig. 4 b) showed the clear lattice fringes of 0.23 nm, indicating the growth of the AuNPs occurred preferentially on the (111) plane [29]. This was further confirmed by XRD patterns. Insert fast Fourier transform (FFT) image also showed a crystalline pattern of the synthesised AuNPs.
Fig. 4.
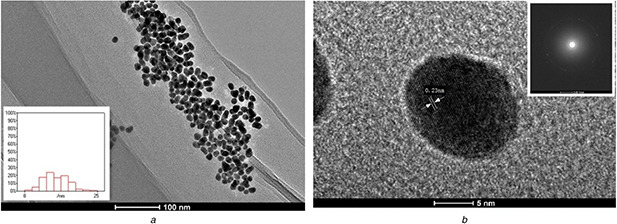
TEM and HRTEM micrograph of synthesised AuNPs
(a) TEM micrograph of synthesised AuNPs (inset shows size distribution histogram), and (b) HRTEM micrograph of synthesised AuNPs (inset correspond to FFT pattern)
The results of EDS analysis were shown in Fig. 5. A strong absorption peak was at 2.2 keV, which was a typical absorption peak of metallic Au [40]. The peaks at 8.4, 9.6 and 11.4 keV also showed the existence of elementary gold, which confirmed the composition of AuNPs.
Fig. 5.
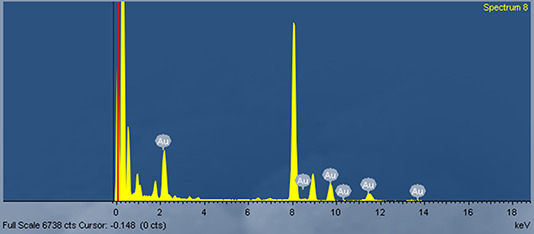
EDS analysis of synthesised AuNPs
The crystalline nature of the AgNPs was characterised by XRD. The XRD patterns were shown in Fig. 6. The diffraction peaks at (2θ) 38.30°, 44.28°, 64.62°, 77.57° and 81.75° were assigned to (111), (200), (220), (311) and (222) planes of the face‐centered cubic (fcc) lattice of gold (JCPDS no. 04‐0784) [41, 42]. The results demonstrated that the biosynthesised AuNPs were crystalline in nature. The size of AuNPs was calculated by Debye‐Scherrer's equation was about 11 nm, which was in agreement with the result of TEM analysis.
Fig. 6.
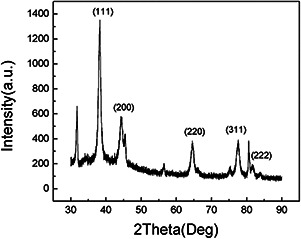
XRD pattern of the synthesised AuNPs
3.1.5 FTIR analysis
FTIR analysis was performed to identify the functional groups in the peel and on the surface of AuNPs which were probably active for the reduction of chloroauric acid and the stabilisation of the synthesised AuNPs (Fig. 7). The FTIR spectra of both C. maxima peel and AuNPs were recorded. The FTIR spectra of C. maxima peel powder illustrated that the characteristic peaks appeared at 3413, 2927, 1619, 1056 and 622 cm−1, respectively. The biosynthesised AuNPs also showed the characteristic peaks at 3411, 2925, 1619, 1051 and 622 cm−1. The similar FTIR spectra demonstrated that the biosynthesised AuNPs were capped with the molecules from the peel. The peak at 1619 cm−1 was contributed to the stretching vibrations of C=C. The peak at around 3411 cm−1 corresponded to the stretching vibrations of O–H of phenols and carboxylic acids. The strong peak at 2925 cm−1 was assigned to the symmetric and asymmetric C–H stretching vibrations of aliphatic acids [43]. Other peaks at 1051 and 622 cm−1 might be assigned to ν (C–O) and δ (C–H) bending vibrations [44]. It has been confirmed naringin and hesperidin were the principal active components in the C. maxima peel [45]. Here, we speculated that flavonoids such like naringin and hesperdin probably acted as reducing agent and stabiliser that adhere on the surface of AuNPs to prevent agglomerating during the biosynthesis process [46].
Fig. 7.
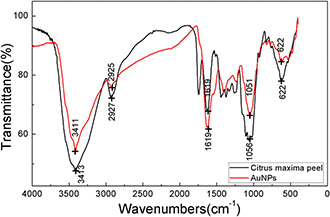
FTIR spectra of C. maxima peel and AuNPs
3.2 Catalytic activity of AuNPs
It is well known that the bulk gold is chemically inert, but AuNPs exhibit excellent chemical activities and catalytic properties owing to the changing of redox potential from positive value to negative value [47]. Especially, the AuNPs with the small size can show the remarkable catalytic activity due to the increasing of the surface volume ratio and the enhancing of surface energy.
4‐NP is a toxic water soluble compound which irritates the skin, eyes, and respiratory tract and inflammation [48]. Usually, 4‐NP was converted to 4‐AP to eliminate the toxicity of 4‐NP. Herein, the catalytic activity of AuNPs was investigated by degrading 4‐NP to 4‐AP in the presence of NaBH4. The process of degradation was monitored by UV–vis spectra. In the absence of NaBH4, 4‐NP showed a distinct peak at 320 nm. After addition of NaBH4, the absorption peak shifted to ∼400 nm because of the formation of 4‐nitrophenolate ions [49]. Without any catalyst, the peak from 4‐nitrophenolate ions remained unaltered for several days. However, when the biosynthesised AuNPs were added as catalysts, the intensity of the 4‐nitrophenolate peak at 400 nm immediately decreased and a new peak at 310 nm appeared for the formation of 4‐AP (Fig. 8 a). Around 30 min, the colour of the mixture changed from yellow to colourless, and the peak at 400 nm disappeared, which indicated the degradation reaction completed.
Fig. 8.
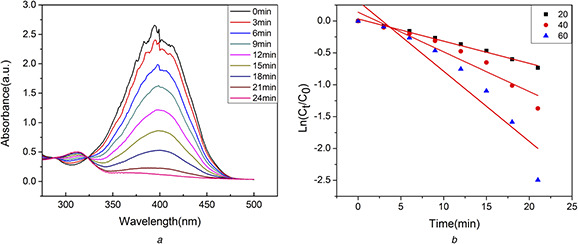
Biosynthesised AuNPs
(a) UV–vis spectra of mixture of 4‐NP and NaBH4 in the presence AuNPs (60 μl), (b) Logarithm of the ratio between its final concentration and the initial concentration of 4‐NP versus the corresponding time (min)
The Langmuir and Hinshelwood model can be used to describe the relationship between the rate of the degradation of 4‐NP in the presence of AuNPs with respect to time. Since the concentration of NaBH4 was large enough compared with the concentration of 4‐NP. Hence, the reaction rate (K a) was only assumed to be dependent of the concentration of the 4‐NP. The rate of equation can be simplified and integrated to be: −K at = ln(Ct /C 0) = ln(At /A 0); where Ct and At are the concentration and absorbance of reaction at any time, respectively, C 0 and A 0 are initial concentration and absorbance of reaction, respectively.
Plotting the ln(Ct /C 0) versus to the corresponding time (min) showed linear relationship in Fig. 8 b and the rate constants were shown in Table 1. Obviously, the rate of degradation increased with the amount of AuNPs increasing. Therefore, the AuNPs synthesised using the extract of C. maxima peel showed a strong catalytic activity in the degradation of 4‐NP, and it could be used for the treatment of organic wastewater.
Table 1.
The rate constants for the degradation of 4‐NP to 4‐AP
| Volume of Au nanoparticles solution, μL | Rate constant, min−1 |
|---|---|
| 20 | 0.03164 |
| 40 | 0.06224 |
| 60 | 0.10999 |
3.3 Antibacterial activity
AuNPs have also attracted great attentions as a new class of biomedical materials, since they can act as both antibacterial and antifungal agents which are capable of interacting with microorganisms to produce cellular damage [50]. Fig. 9 shows the antibacterial activity of AuNPs which was tested against both gram negative (E. coli) and gram positive (S. aureus) bacteria. From the zones of inhibition, it was observed that C. maxima peel extract did not have any inhibitory effect on the bacterium growth, while the biosynthesised AuNPs exhibited a strong antibacterial effect. The inhibitory effect slightly increased with the concentration of AuNPs increasing according to the inhibition zones. Therefore, the biosynthesised AuNPs in our study have the potential to be used as effective disinfectants with good antibacterial activities.
Fig. 9.
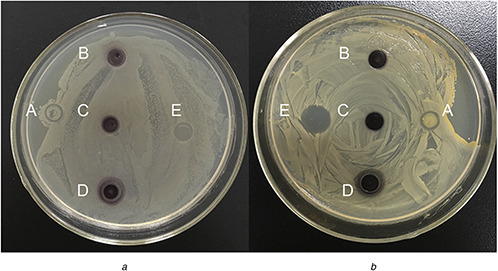
Antibacterial activities of the synthesised AuNPs against E. coli (A) and S. aureus (B)
(a) C. maxima peel extract, (b) 1 mM AuNPs, (c) 1.5 mM AuNPs, (d) 2 mM AuNPs, (e) Gentamicin
4 Conclusion
One green method of biosynthesis of AuNPs was developed in our study. The extract of C. maxima peel was applied to synthesise AuNPs. The synthesis procedure was proved simple, cost effective and environment friendly. The morphologies of the AuNPs were characterised by TEM, EDS, XRD and FTIR, and the NPs were predominantly subspheroidal with the size range from 8 to 25 nm. The as‐obtained AuNPs exhibited a strong catalytic activity in the degradation of 4‐NP and an antibacterial activity against both S. aureus (gram positive) and E. coli (gram negative) bacteria.
5 Acknowledgments
This work was kindly co‐funded by the National Natural Science Foundation of China (21277043, 21620102008), the Beijing Natural Science Foundation (8132038), and the Fundamental Research Funds for the Central Universities. The authors thank Ms. Weiping Cao in Plant Protection Institute, Hebei Academy of Agricultural and Forestry Sciences for her kindly help on their antibacterial experiments.
6 References
- 1. Das S.K. Das A.R. Guha A.K.: ‘Gold nanoparticles: microbial synthesis and application in water hygiene management’, Langmuir, 2009, 25, (14), pp. 8192 –8199 [DOI] [PubMed] [Google Scholar]
- 2. Marie‐Christine D. Didier A.: ‘Gold nanoparticles: assembly, supramolecular chemistry, quantum‐size‐related properties, and applications toward biology, catalysis, and nanotechnology’, Chem. Rev., 2004, 104, (1), pp. 293 –346 [DOI] [PubMed] [Google Scholar]
- 3. Jain P.K. Lee K.S. El‐Sayed I.H. et al.: ‘Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine’, J. Phys. Chem. B, 2006, 110, (110), pp. 7238 –7248 [DOI] [PubMed] [Google Scholar]
- 4. Tang H. Chen J. Nie L. et al.: ‘A label‐free electrochemical immunoassay for carcinoembryonic antigen (CAE) based on gold nanoparticles (AuNPs) and nonconductive polymer film’, Biosens. Bioelectron., 2007, 22, (6), pp. 1061 –1067 [DOI] [PubMed] [Google Scholar]
- 5. Tong L. Wei Q. Wei A. et al.: ‘Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects’, Photoch. Photobio., 2009, 85, (1), pp. 21 –32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boote E. Fent G. Kattumuri V. et al.: ‘Gold nanoparticle contrast in a phantom and juvenile swine: models for molecular imaging of human organs using X‐ray computed tomography’, Acad. Radiol., 2010, 17, (4), pp. 410 –417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kunjiappan S. Chowdhury R. Bhattacharjee C.: ‘A green chemistry approach for the synthesis and characterization of bioactive gold nanoparticles using Azolla Microphylla methanol extract’, Front. Mater. Sci., 2014, 8, (2), pp. 123 –135 [Google Scholar]
- 8. Dong C. Zhang X. Cai H.: ‘Green synthesis of monodisperse silver nanoparticles using hydroxy propyl methyl cellulose’, J. Alloy Compd., 2014, 583, (1), pp. 267 –271 [Google Scholar]
- 9. Khatami M. Pourseyedi S.: ‘ Phoenix Dactylifera (Date Palm) pit aqueous extract mediated novel route for synthesis high stable silver nanoparticles with high antifungal and antibacterial activity’, IET Nanobiotechnol., 2015, 9, (4), pp. 184 –190 [DOI] [PubMed] [Google Scholar]
- 10. Kumar B. Smita K. Cumbal L.: ‘Biofabrication of nanogold from the flower extracts of Lantana Camara ’, IET Nanobiotechnol., 2016, 10, (3), pp. 154 –157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohanta Y.K. Singdevsachan S.K. Parida U.K. et al.: ‘Green synthesis and antimicrobial activity of silver nanoparticles using wild medicinal mushroom Ganoderma Applanatum (Pers.) Pat. from Similipal Biosphere Reserve, Odisha, India’, IET Nanobiotechnol., 2015, 9, (6), pp. 184 –189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soltani N.M. Khatami M. Shahidi Bonjar G.H.: ‘Extracellular synthesis gold nanotriangles using biomass of Streptomyces microflavus ‘, IET Nanobiotechnol., 2016, 10, (1), pp. 33 –38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nazeruddin G.M. Prasad N.R. Waghmare S.R. et al.: ‘Extracellular biosynthesis of silver nanoparticle using Azadirachta Indica leaf extract and its anti‐microbial activity’, J. Alloy Compd., 2014, 583, (583), pp. 272 –277 [Google Scholar]
- 14. Sharma J.K. Akhtar M.S. Ameen S. et al.: ‘Green synthesis of CuO nanoparticles with leaf extract of Calotropis Gigantea and its dye‐sensitized solar cells applications’, J. Alloy Compd., 2015, 632, pp. 321 –325 [Google Scholar]
- 15. Mohapatra B. Kuriakose S. Mohapatra S.: ‘Rapid green synthesis of silver nanoparticles and nanorods using Piper Nigrum extract’, J. Alloy Compd., 2015, 637, (3), pp. 119 –126 [Google Scholar]
- 16. Shankar S.S. Ahmad A. Pasricha R. et al.: ‘Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes’, J. Mater. Chem., 2003, 13, (7), pp. 1822 –1826 [Google Scholar]
- 17. Shankar S.S. Rai A. Ahmad A. et al.: ‘Rapid synthesis of Au, Ag, and bimetallic Au core‐Ag shell nanoparticles using Neem (Azadirachta Indica) leaf broth’, J. Colloid Interf. Sci., 2004, 275, (2), pp. 496 –502 [DOI] [PubMed] [Google Scholar]
- 18. Shankar S.S. Rai A. Ankamwar B. et al.: ‘Biological synthesis of triangular gold nanoprisms’, Nat. Mater., 2004, 3, (7), pp. 482 –488 [DOI] [PubMed] [Google Scholar]
- 19. Kumar K.P. Paul W. Sharma C.P.: ‘Green synthesis of gold nanoparticles with Zingiber Officinale extract: characterization and blood compatibility’, Process Biochem., 2011, 46, (10), pp. 2007 –2013 [Google Scholar]
- 20. Ghosh S. Patil S. Ahire M. et al.: ‘Synthesis of gold nanoanisotrops using Dioscorea Bulbifera tuber extract’, J. Nanomater., 2011, 2011, (44), pp. 18083 –18088 [Google Scholar]
- 21. Wang Y. He X. Wang K. et al.: ‘ Barbated Skullcup herb extract‐mediated biosynthesis of gold nanoparticles and its primary application in electrochemistry’, Colloid Surf. B, 2009, 73, (1), pp. 75 –79 [DOI] [PubMed] [Google Scholar]
- 22. Thema F.T. Beukes P. Gurib‐Fakim A. et al.: ‘Green synthesis of monteponite CdO nanoparticles by Agathosma Betulina natural extract’, J. Alloy Compd., 2015, 646, pp. 1043 –1048 [Google Scholar]
- 23. Diallo A. Ngom B.D. Park E. et al.: ‘Green synthesis of ZnO nanoparticles by Aspalathus Linearis: structural & optical properties’, J. Alloy Compd., 2015, 646, pp. 425 –430 [Google Scholar]
- 24. Sood S. Arora B. Bansal S. et al.: ‘Antioxidant, anti‐inflammatory and analgesic potential of the Citrus Decumana L. peel extract’, Inflammopharmacology, 2009, 17, (5), pp. 267 –274 [DOI] [PubMed] [Google Scholar]
- 25. Xi W. Fang B. Zhao Q. et al.: ‘Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus Grandis Osbeck.) varieties’, Food Chem., 2014, 161, (11), pp. 230 –238 [DOI] [PubMed] [Google Scholar]
- 26. Yu J. Xu D. Guan H.N. et al.: ‘Facile one‐step green synthesis of gold nanoparticles using Citrus maxima aqueous extracts and its catalytic activity’, Mater. Lett., 2015, 166, pp. 110 –112 [Google Scholar]
- 27. Wei Y. Fang Z. Zheng L. et al.: ‘Green synthesis of Fe nanoparticles using Citrus maxima peels aqueous extracts’, Mater. Lett., 2016, 185, pp. 384 –386 [Google Scholar]
- 28. Zayed M.F. Eisa W.H.: ‘ Phoenix Dactylifera L. leaf extract phytosynthesized gold nanoparticles; controlled synthesis and catalytic activity’, Spectrochim. Acta A, 2013, 121C, (1), pp. 238 –244 [DOI] [PubMed] [Google Scholar]
- 29. Paul B. Bhuyan B. Purkayastha D.D. et al.: ‘Green synthesis of gold nanoparticles using Pogestemon Benghalensis (B) O. Ktz. leaf extract and studies of their photocatalytic activity in degradation of methylene blue’, Mater. Lett., 2015, 148, (12), pp. 37 –40 [Google Scholar]
- 30. Das S.K. Dickinson C. Lafir F. et al.: ‘Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopusoryzae protein extract’, Green Chem., 2012, 14, pp. 1322 –1334 [Google Scholar]
- 31. Mubarakali D. Thajuddin N. Jeganathan K. et al.: ‘Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens’, Colloid Surf. B, 2011, 85, (2), pp. 360 –365 [DOI] [PubMed] [Google Scholar]
- 32. Tamuly C. Hazarika M. Bordoloi M.: ‘Biosynthesis of Au nanoparticles by Gymnocladus Assamicus and its catalytic activity’, Mater. Lett., 2013, 108, (5), pp. 276 –279 [Google Scholar]
- 33. Amendola V. Meneghetti M.: ‘Size evaluation of gold nanoparticles by UV−vis spectroscopy’, J. Phys. Chem. C, 2009, 113, (11), pp. 4277 –4285 [Google Scholar]
- 34. Li D. He Q. Li J.: ‘Smart core/shell nanocomposites: intelligent polymers modified gold nanoparticles’, Adv. Colloid Interf. Sci., 2009, 149, (1‐2), pp. 28 –38 [DOI] [PubMed] [Google Scholar]
- 35. Srinath B.S. Rai R.V.: ‘Biosynthesis of gold nanoparticles using extracellular molecules produced by Enterobacter Aerogenes and their catalytic study’, J. Clust. Sci., 2015, 26, (5), pp. 1483 –1494 [Google Scholar]
- 36. Basavegowda N. Idhayadhulla A. Yong R.L.: ‘Phyto‐synthesis of gold nanoparticles using fruit extract of Hovenia Dulcis and their biological activities’, Ind. Crop. Prod., 2014, 52, (52), pp. 745 –751 [Google Scholar]
- 37. Shipway A.N. Lahav M. Rachel Gabai A. et al.: ‘Investigations into the electrostatically induced aggregation of Au nanoparticles’, Langmuir, 2000, 16, (23), pp. 8789 –8795 [Google Scholar]
- 38. Link S. El‐Sayed M.A.: ‘Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles’, J. Phys. Chem. B, 1999, 103, (21), pp. 4212 –4217 [Google Scholar]
- 39. Zhan G. Huang J. Lin L. et al.: ‘Synthesis of gold nanoparticles by Cacumen Platycladi leaf extract and its simulated solution: toward the plant‐mediated biosynthetic mechanism’, J. Nanoparticle Res., 2011, 13, (10), pp. 4957 –4968 [Google Scholar]
- 40. Dauthal P. Mukhopadhyay M.: ‘In‐vitro free radical scavenging activity of biosynthesized gold and silver nanoparticles using Prunus Armeniaca (Apricot) fruit extract’, J. Nanoparticle Res., 2013, 15, (1), pp. 1 –11 [Google Scholar]
- 41. Emmanuel R. Karuppiah C. Chen S.M. et al.: ‘Green synthesis of gold nanoparticles for trace level detection of a hazardous pollutant (Nitrobenzene) causing methemoglobinaemia’, J. Hazard. Mater., 2014, 279, pp. 117 –124 [DOI] [PubMed] [Google Scholar]
- 42. Muthuvel A. Adavallan K. Balamurugan K. et al.: ‘Biosynthesis of gold nanoparticles using Solanum Nigrum leaf extract and screening their free radical scavenging and antibacterial properties’, Biomed. Prevent. Nutr., 2014, 4, (2), pp. 325 –332 [Google Scholar]
- 43. Li F.T. Yang H. Zhao Y. et al.: ‘Novel modified pectin for heavy metal sdsorption’, Chin. Chem. Lett., 2007, 18, (3), pp. 325 –328 [Google Scholar]
- 44. Williams D.H. Fleming I.: ‘Spectroscopic methods in organic chemistry’ (McGraw‐Hill, 1980) [Google Scholar]
- 45. Wu T. Guan Y. Ye J.: ‘Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection’, Food Chem., 2007, 100, (4), pp. 1573 –1579 [Google Scholar]
- 46. Park Y. Hong Y.N. Weyers A. et al.: ‘Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles’, Chem. Inf., 2012, 5, (6), pp. 69 –78 [DOI] [PubMed] [Google Scholar]
- 47. Hvolbæk B. Janssens T.V.W. Clausen B.S. et al.: ‘Catalytic activity of Au nanoparticles’, Nano Today, 2007, 2, (4), pp. 14 –18 [Google Scholar]
- 48. Gu S. Wunder S. Lu Y. et al.: ‘Kinetic analysis of the catalytic reduction of 4‐Nitrophenol by metallic nanoparticles’, J. Phys. Chem. C, 2014, 118, (32), pp. 18618 –18625 [Google Scholar]
- 49. Muniyappan N. Nagarajan N.S.: ‘Green synthesis of silver nanoparticles with Dalbergia Spinosa leaves and their applications in biological and catalytic activities’, Process Biochem., 2014, 49, (6), pp. 1054 –1061 [Google Scholar]
- 50. Hernández‐Sierra J.F. Ruiz F. Pena D.C.C. et al.: ‘The antimicrobial sensitivity of streptococcus mutans to nanoparticles of silver, zinc oxide, and gold’, Nanomed. Nanotechnol. Biol. Med., 2008, 4, (3), pp. 237 –240 [DOI] [PubMed] [Google Scholar]


