Abstract
This study was organised to check the effect of silver nanoparticles and silver nitrate on rice growth against biotic stress. Silver nanoparticles were synthesised by using plant extract as reducing agent, followed by characterisation through UV Vis spectroscopy, XRD, EDS and SEM. Aspergillus application significantly reduced rice plant fresh mass (0.9%), dry mass (0.21%), root length (2.3%), shoot length (5.2%) and root number (1%) in comparison to control. Similarly, leaf area, leaf fresh mass, dry mass and leaf number were also reduced by 23.1, 0.02, 0.11 and 0.9%, respectively. AgNPs and AgNO3 treatments increased the root length (16.2 & 12.8%), shoot length (21 & 20%), root number (8.1 & 6.8%), plant fresh weight (6.4 & 5%) and plant dry weight (4.6 & 3.5%) in 75mg/l treatment of AgNPs and AgNO3 respectively. Similarly, AgNPs and AgNO3 treatment (75 mg/l concentrations) reflected remarkable increase in leaf area (58.8 & 57.2 %), leaf number (4.3 & 3.7 %), leaf fresh weight (1.7 & 1.4 %) and leaf dry weight (0.9 & 0.8 %). Overall AgNPs showed more significant results as compared to AgNO3. The quantity of aflatoxins ranged from 3.1 to 7.7 μg/kg against tolerable limit (4 µg/kg). Overall AgNPs and AgNO3 treatments showed significant results and it could be considered as a strategy for aflatoxin management in rice plants.
Inspec keywords: crops, scanning electron microscopy, nanoparticles, X‐ray diffraction, toxicology, agricultural pollution, agricultural safety, silver compounds, ultraviolet spectroscopy
Other keywords: biotic stress, silver nanoparticles, AgNPs, silver nitrate, super kernel rice, Kala Shah Kaku research centre, UV–Vis spectroscopy, X‐ray diffraction, energy‐dispersive X‐ray spectroscopy, rice plant fresh mass, root number, leaf area, leaf fresh mass, dry mass, leaf number, root length, leaf fresh weight, leaf dry weight, examined rice plants, rice growth, aflatoxins, scanning electron microscopy, Aspergillus application, AgNO3
1 Introduction
The vitality of agriculture cannot be denied, specially for the developing countries, as it is inevitable for existence of life on earth. It is also good and cheap source of raw material and supply food, feeds, and fibres. Rice (Oryza sativa L.) is one of the major food crop for ∼65% of the world's population, particularly in Asia, and supplying ∼20% of the calories consumed worldwide [1]. Rapidly increasing population of the world is an alarming situation which certainly raises pressure on our agricultural land. It is stated that for the fulfilment of the demand of increasing world's population, it is very necessary to raise the annual cereal productions from ∼2.6 to 3.0 billion t by 2050. Keeping in view the world's growing population and the increasing rice consumption, rice constantly remains as one of the major chosen research crop.
Among various biotic stresses, mycotoxin contamination caused by saprophytic fungi, especially Aspergillus, Penicillium, and Fusarium, at both pre‐ and post‐harvest stage adversely affect the quality of rice grains [2]. Aflatoxins considered to be the major toxins have been reported in Asia [3]. Climate of most of Pakistani cultivatable area is hot and humid, so foods of Pakistan have high proclivity to be susceptible to aflatoxin contamination, and the situation is becoming alarming due to unaesthetic farming practices in agronomy, processers, and inadequate storage facilities [4]. The high‐level contamination of aflatoxins in Pakistani rice and chillies has resulted in decreased export from Pakistan. Therefore, it is distress situation for economic aspects because Pakistani exports are significantly dependent on these crop [5]. It has been reported that 70% of rice samples contaminate due to aflatoxins by mean concentration of 4.9 μg/kg, which is greater than the standard [6]. Taken up of these aflatoxins infected food for prolonged period might cause health risks, i.e. hepatitis B and C, impaired function of male reproductive organs, testicular degeneration, inflammation, immune disorders, possibility of stroke, and bronchitis [7].
Several approaches such as physical (cleaning, heating, irradiation, adsorption), chemical (chemical compounds, ozonisation), cultural, and biological (applying bacteria, yeast, and non‐toxigenic Aspergillus strains) have been proposed to reduce the risks of aflatoxin (B1, B2, G1, G2) contamination in different crops [8, 9]. These techniques are not feasible in application, because of their expensive nature. So, it is the need of the hour to look forward for accessible, workable, and convenient techniques which can overcome all these infirmities.
Currently, nanotechnology is the rising and prominent science field of 21st century, which might cause worthy affects on globe's economy, industry, and mankind by serving nanocarriers, nanosensors, nanorods, nanodrugs, nanoparticles, nanotubes etc [10]. Nanoparticles might work according to their size, broad surface ratio, and remarkable optical aspects, which exhibit peculiar biological, chemical, and physical properties [11]. Various agricultural aspects of crop production in state of stress conditions might be raised and improved by utilising nanotechnology [12]. Among different nanoparticles, silver nanoparticles (AgNPs) are widely utilised due to their diverse chemical and physical properties [13]. Furthermore, plant‐mediated AgNPs might increase dry weight and leaf area [14]. The efficiency of AgNPs is examined by their size, chemical composition, reactivity, surface covering, and significantly their effective dosage [15]. It has been reported that AgNPs improve seed germination and plant growth, i.e. increased leaf area, root length, and shoot length of Phaseolus vulgaris, Brassica juncea, and Zeya mays [16, 17].
Furthermore, recent studies revealed some adverse and beneficial effects of AgNPs and silver nitrate (AgNO3) on plant growth and development, but their affects are still unknown in rice plants under biotic stress (Aspergillus). Hence, the present research was put through to negotiate (i) impact of AgNPs and AgNO3 on morphological characters of rice plants, (ii) whether AgNPs and silver nitrate are effective in control of aflatoxins level in rice (O. sativa L).
2 Materials and methods
2.1 Experimental
Silver nitrate (AgNO3) was taken from Merck (Germany). Fresh leaves of Moringa oleifera were obtained from mother plant present in PMAS Arid Agriculture University Rawalpindi, Pakistan. The leaves were detached from shoots, followed by washing with tap and distilled water to remove all dust material. The washed leaves were dried off by scattering on blotting paper at room temperature. About 30 g of leaves was taken to boil in 200 ml of distilled water until formation of dark brown colour extract. This extract was filtered and filtrate was stored at 4°C in refrigerator for further use. The protocol of Hussain et al. [18] was considered for green synthesis of AgNPs. For this purpose, preparation of 1 mM solution of AgNO3 was done by mixing 0.17 g AgNO3 in 1 l distilled water. After boiling the solution for 10 min, plant extract with quantity of 10–20 ml was poured gradually until colour of solution becomes brown (indication for AgNPs synthesis). The solution was centrifuged at 14,000 rpm for 10 min. AgNPs were taken in the form of pallet after removing supernatant. The obtained pellet (AgNPs) was centrifuged again at 14,000 rpm for 10 min in distilled water to wash away remaining of AgNO3 salt. At the end, pellet (AgNPs) was treated for further characterisation process. The whole process was done under sterile environment.
AgNPs were differentiated through UV–Vis spectrophotometer, X‐ray diffraction (XRD) analysis, energy dispersive X‐ray spectroscopy (EDS), and scanning electron microscopy (SEM). UV–Vis spectrum was taken by analysis of sample in spectrophotometer. XRD was done on Rigaku D/max‐2200 PC diffractometer worked at 40 kV/40 mA, using CuKa1 radiation at 1.54 Å wavelength with an angle of 05°–55° on 2θ scale. EDS was done to check the elemental analysis of AgNPs. SEM was applied to check the amorphous nature of AgNPs, in the Institute of Space and Technology (IST), Islamabad. The Debye–Scherrer equation was used for analysis of AgNPs size and shape [19]
where k is the shape factor, λ the X‐ray wavelength, θ the Bragg's angle, and β the full width in radians at half maximum.
2.2 Plant material, site description, and growth conditions
Rice seeds of variety super kernel were taken from Rice Research Institute Kala Shah Kaku, Pakistan. The surface sterilisation of healthy seeds of rice was done in 10% sodium hypochlorite solution for 10 min. Then seeds were thoroughly washed with distilled water to remove all traces of sterilising agent [20]. The field experiment was planted for the duration of 3 months from 24 July 2017 to 24 October 2017 at the experimental area near Chak Qazi Bhera, District Sargodha, Pakistan. The irrigation of plants was ensured for whole experiment to avoid drought stress and plants were not treated with any fertiliser. The weeds were eradicated from time to time by hand.
2.3 Application of AgNPs, silver nitrate, and Aspergillus innoculum
The stock solutions of AgNO3 and AgNPs, i.e. 25, 50, 75, and 100 mg/l, were prepared and stored in refrigerator. The volume, i.e. 16.7, 33.3, 50, and 66.7 ml, of AgNO3 and AgNPs from each stock solution was given separately to rice plants at heading stage through soil. Aspergillus innoculum was applied to rice plants after 10 days of application of AgNPs. Controls were the plants without AgNPs, AgNO3, and Aspergillus treatments and with Aspergillus treatment only.
2.4 Determination of morphological parameters
During the course of growth, three rice plants were taken from each treatment randomly for analysing morphological parameters such as plant fresh and dry weight, root and shoot length, root number, leaf fresh and dry weight leaf number, and leaf area. The roots of each plant were separated from shoots. Plant fresh biomass was measured in grams and samples were placed in oven at 65°C (12 h) for obtaining plant dry weight. Root and shoot length was measured in centimetres through 1 m tape. Root and leaf number was counted manually by separating roots and leaves from each sample plant. Leaf area was computed with the help of leaf area meter (CID, CI‐202) and then leaves were placed in oven at 65°C (12 h) for taking dry weight.
2.5 Experimental design, data collection, and statistical analysis
Experiment was established two times with three replicates. The mean standard deviation (±SD) was calculated from obtained data. The experimental layout was analysis of variance (one way). Statistical analysis was done through SPSS software package for Windows (SPSS 16.1). The comparison of taken values was correlated with control. In the case of statistical difference (p = 0.05), the mean values were further illustrated by Duncan's multiple range test.
3 Results and discussion
3.1 AgNPs synthesis and characterisation
The AgNPs synthesis takes place by reduction in silver salt (AgNO3) using leaf extract of M. oleifera, followed by characterisation through UV–Vis spectrophotometer, XRD, EDS, and SEM. Green synthesised AgNPs are more suitable when compared with physically and chemically synthesised ones. AgNPs were formed through reduction reaction of plant extract with AgNO3. Rauwel et al. [21] illustrated that compounds of plants are involved in synthesis of AgNPs through coating of silver ion. This plant‐mediated capping reduced the toxicity of AgNPs when inoculated to plants. Similar reports were given by Yasur and Rani [22]. Likewise, Shah et al. [23] also proposed that plants ingredients are directly involved in coating metal ions for the formation of stable metal nanoparticles.
Techniques such as UV–Vis spectroscopy, XRD, EDS, and SEM were applied to characterise diverse properties of green synthesised AgNPs as single technique is not sufficient for AgNPs elaboration (Figs. 1, 2, 3, 4). The presence of AgNPs was checked through UV–visible spectrophotometer at the absorption of 300–450 nm (Fig. 1). The variation in peak is due to adverse shapes and sizes of colloidal AgNPs [24]. In the present work, the Debye–Scherrer equation was used to evaluate size of AgNPs, which was 34 nm. Rahman et al. [25] also affirmed similar results. The results of SEM depicted nebulous‐shaped AgNPs due to their colloidal properties (Fig. 4). The size‐dependent effects of AgNPs were also found in Arabidopsis root growth [26]. These results are consistent with that reported for ZnONPs and TiO2 NPs, where the larger sized NPs caused more cell damage than the smaller ones [27].
Fig. 1.
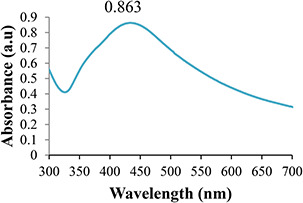
UV–visible spectrum of AgNPs
Fig. 2.
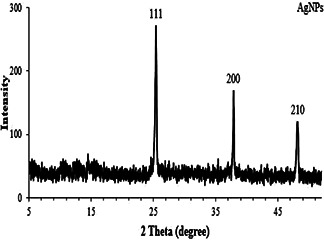
XRD pattern of the synthesised AgNPs
Fig. 3.

EDX spectrum of the synthesised AgNPs
Fig. 4.
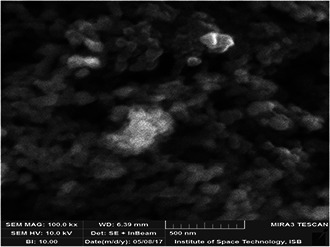
SEM micrograph of green synthesised AgNPs
3.2 Effect of AgNPs and AgNO3 on growth attributes of rice under biotic stress
Different concentrations of silver nitrate (AgNO3) and AgNPs (25, 50, 75, and 100 mg/l) were applied to check their effect on rice plants growth against Aspergillus (biotic stress). The results of silver salt and AgNPs‐related treatments on morphological performance of rice in response to application of Aspergillus flavus have been provided in Figs. 5 and 6. The impact of AgNO3 and AgNPs on morphological growth against Aspergillus application was estimated in terms of plant fresh mass, dry mass, root shoot length, leaf fresh and dry mass, leaf number, and leaf area (Figs. 5 and 6). Aspergillus application acts as biotic stress on rice plants and significantly reduced plant fresh mass (0.9%), dry mass (0.21%), root length (2.3%), shoot length (5.2%), and root number (1%) in comparison to control (Figs. 5 a –e).
Fig. 5.
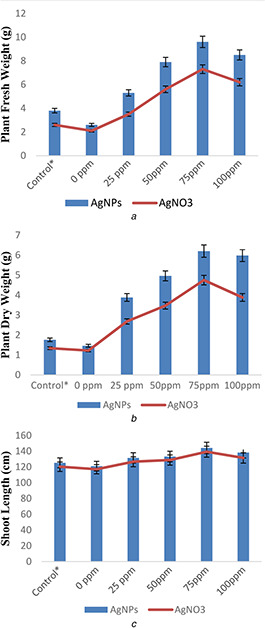
Effect of AgNPs and silver salt (AgNO3) on
(a) Plant fresh weight, (b) Dry weight, (c) Shoot length
Fig. 6.
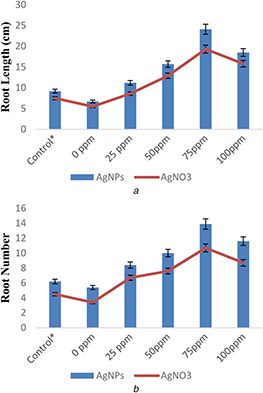
Effect of AgNPs and silver salt (AgNO3) on
(a) Root length, (b) Root number of rice plants under biotic (A. flavus) stress
Similarly, leaf area, leaf fresh mass, dry mass, and leaf number were also reduced by 23.1, 0.02, 0.11, and 0.9%, respectively, under the same exposures of biotic stress in comparison to control (Figs. 6 a –d). However, AgNPs and AgNO3 treatments with different concentrations showed significant results on rice plants growth when compared with control under biotic stress. These also inhibit Aspergillus growth in rice plants and protect rice (O. sativa L) plants against biotic stress by improving plant root and shoot length, fresh and dry weight, leaf area, leaf number, leaf fresh weight, and leaf dry weight when compared with control. A significant increase in root length (16.2 and 12.8%), shoot length (21 and 20%), root number (8.1 and 6.8%), plant fresh weight (6.4 and 5%), and plant dry weight (4.6 and 3.5%) were recorded in 75 mg/l AgNPs and AgNO3, respectively, when compared with control. However, the effects of AgNPs were more pronounced on growth of rice plants when compared with AgNO3 under biotic stress (Figs. 5 and 6).
Growth of plants is inhibited by fungi and it is directly associated with production of some secondary metabolites such as aflatoxins in plants. Owing to this, many growth aspects become suppressed because of changes at cellular level [28]. In the present study, growth of rice plants was enhanced due to AgNPs and AgNO3 treatment when compared with control. There is very less data available regarding AgNPs treatments on plants under adverse conditions [29, 30, 31]. The current results regarding pronounced growth of rice plants in response to AgNPs treatment are in accordance with findings of Iqbal et al. [30] as they observed increase in root and shoot length and leaf area in wheat plants due to AgNPs. The same results were also obtained by Thuesombat et al. [32], Hao et al. [33], and Ndeh et al. [31] in rice plants in response to silver, iron, and gold nanoparticles. This growth‐stimulating effect of NPs varies from plant to plant [34]. According to Syu et al. [35], AgNPs promote shoot growth by inhibiting ethylene production and translocation in shoots as it is growth suppressor of plants shoot.
The reduction of 23% in leaf area, 0.9% in leaf number, 0.02% in leaf fresh weight, and 0.11% in leaf dry weight was occurred under biotic stress when compared with control (Figs. 7 and 8). However, AgNPs and AgNO3 treatment (75 mg/l concentrations) reflected remarkable results in leaf area (58.8 and 57.2%), leaf number (4.3 and 3.7%), leaf fresh weight (1.7 and 1.4%), and leaf dry weight (0.9 and 0.8%) when compared with control under biotic stress. More growth was observed in AgNPs‐treated rice plants when compared with AgNO3 ‐treated plants (Figs. 6 a –d). Thus, from the present results, it can be stated that AgNPs efficiently increase growth of rice plants under biotic stress. Previously, Mishra et al. [36] illustrated more wheat growth in terms of increased fresh and dry biomass due to TiO2 NPs treatment under drought conditions. Similarly, Mengmeng et al. [37] reported enhanced growth of peanut plants treated with AgNPs.
Fig. 7.
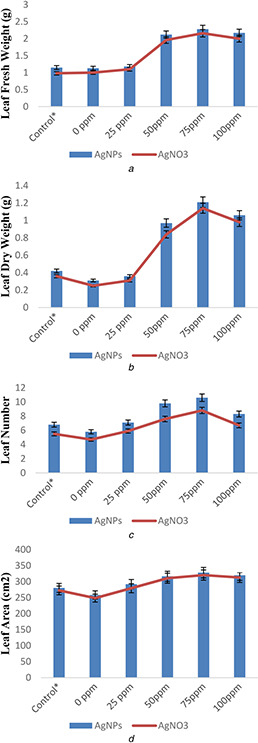
Effect of AgNPs and silver salt (AgNO3) on
(a) Leaf fresh weight, (b) Leaf dry weight, (c) Leaf number, (d) Leaf area of rice plants under biotic (A. flavus) stress
Fig. 8.

Growth of rice plants in response to
(a) Control (untreated) (b) 0 mg/l (c) 25 mg/l (d) 50 mg/l (e) 75 mg/l (f) 100 mg/l concentrations of silver nanoparticles under biotic stress
Likewise, Raliya and Tarafdar [38] reported ZnONPs‐mediated improvement in biomass, root and shoot growth, and root area of Cyamopsis tetragonoloba. Fuping et al. [27] stated that metallic nanoparticles improve growth of plants by changing levels of microRNAs expression which are directly responsible for growth attributes and metabolic processes in plants. Recently, Iqbal et al. [30] illustrated growth‐enhancing effect of AgNPs on wheat plants under heat stress.
3.3 Estimation of aflatoxins in rice plants treated with AgNPs and AgNO3
The presence and amount of aflatoxins was determined in rice plants as shown in Table 1. The quantity of aflatoxins was in the range of 3.1–7.7 μg/kg in examined rice plants. As shown in Table 1, the incidence rate of aflatoxins was high in untreated rice plants when compared with plants treated with AgNPs and silver nitrate (Table 1).
Table 1.
Analysis of aflatoxins in rice plants in response to AgNPs and AgNO3 treatment against Aspergillus application
| Treatments | Aflatoxins, μg/kg | |
|---|---|---|
| R 1 | R 2 | |
| Control (without AgNPs/AgNO3 and Aspergillus) | 7.7 ± 0.1a | 7.7 ± 0.1a |
| 0 mg/l (without AgNPs/AgNO3 + with Aspergillus) | 8.3 ± 0.0b | 8.4 ± 0.1a |
| 25 mg/l (AgNPs/AgNO3 + Aspergillus) | 5.9 ± 0.3c | 6.2 ± 0.0b |
| 50 mg/l (AgNPs/AgNO3 + Aspergillus) | 4.2 ± 0.2d | 4.8 ± 0.4e |
| 75 mg/l (AgNPs/AgNO3 + Aspergillus) | 3.5 ± 0.1a | 3.9 ± 0.3c |
| 100 mg/l (AgNPs/AgNO3 + Aspergillus) | 3.1 ± 0.0b | 3.4 ± 0.2d |
R 1, concentrations of aflatoxins in response to AgNPs treatments.
R 2, concentrations of aflatoxins in response to AgNO3 treatments.
a–e The direct depiction of the statistical analysis done using statistic software. The data present are the statistical significance level in the form of superscripts.
The aflatoxin level was decreased in rice plants with gradual increase in dose. Overall reduction in incidence of aflatoxins was recorded in rice plants treated with AgNPs when compared with silver nitrate and control. A list of researchers [39, 40, 41, 42] detected the presence of aflatoxins in various collected samples of medicinal plants by use of adverse analytical techniques. Similarly, Nisa et al. [43] and Mukhtar et al. [44] quantified level of aflatoxins in different rice samples. According to Cotty and Jaime‐Garcia [45], incidence level of aflatoxins vary in plants when treated with A. flavus and Aspergillus parasiticusis, individually or in combined form. Reddy et al. [28] and Fakruddin et al. [46] stated that aflatoxins presence due to fungal species may cause severe risks for health of organisms mostly to human beings.
3.5 Evaluation of silver contents in rice plants
The plant samples were assessed to check the presence or absence of silver contents as deposited form. The plants were macerated with acetone and extract was taken after filtration. The filtrate was diluted and analysed through method given by Iqbal et al. [30]. The results manifested in Table 2 showing varying quantity of residual Ag content in plant tissues. The presence of residual Ag may be toxic which is due to their size and coating material [47]. In the present investigation, the natural compounds of plant extract coated the Ag to form particle‐like structure. Nanoparticles have attracted immense attention currently with respect to their toxicity aspects [48, 49], but possible reasons regarding NP toxicity are to be revealed due to variations in size, shape, and encapsulation of NPs. Various techniques such as surface‐enhanced Raman scattering and inductively coupled plasma agents are used for estimation of Ag ion effects in plants [50]. The increase in Ag accumulation was found when the plants were treated with higher concentration of AgNPs. This is similar to the evidence found in other crop plants [41]. Similarly, Gebreselassie et al. [51] detected Ag contents both on surface and in internal tissues of plants.
Table 2.
EDS showing the residual Ag in treated rice plant tissues
| Treatments | Elements (weight) | |||
|---|---|---|---|---|
| O K | S K | Cl K | Ag L | |
| control | 93.7 | 1.1 | 5.2 | 0 |
| 0 mg/l | 87.3 | 9.4 | 5.3 | 0 |
| 25 mg/l | 84.9 | 0.59 | 0.74 | 13.77 |
| 50 mg/l | 82.2 | 0.53 | 0.69 | 16.60 |
| 75 mg/l | 79.5 | 0.41 | 0.56 | 19.53 |
| 100 mg/l | 76.8 | 0.37 | 0.51 | 22.32 |
4 Conclusion
In this study, the effect of AgNPs and AgNO3 on rice plants growth under biotic stress was illustrated. AgNPs and AgNO3 concentrations showed obvious effect on growth of rice plants. Higher concentrations of AgNPs and AgNO3 also inhibited the level of aflatoxins significantly, while lower dose failed to do so. It seemed that AgNPs and AgNO3 treatments had different bio‐effect mainly due to their different dose level. To our best understanding, it was the first time to report the possible role of AgNPs and AgNO3 on growth of rice plants against biotic stress. Thus, the studied mechanism needs to be further investigated at physiological, biochemical, and proteome level.
5 References
- 1. Ghadirnezhad R. Fallah A.: ‘Temperature effect on yield and yield components of different rice cultivars in flowering stage’, Int. J. Agro., 2014, 14, pp. 1 –4 [Google Scholar]
- 2. CAST : ‘Mycotoxins: risks in plant, animal, and human systems’ (Council for Agricultural Science and Technology (CAST; ), Ames, IA, USA, 2003) [Google Scholar]
- 3. Bhat R. Rai R.V. Karim A.A.: ‘Mycotoxins in food and feed: present status and future concerns’, Compr. Rev. Food Sci. Food Saf., 2010, 9, pp. 57 –81 [DOI] [PubMed] [Google Scholar]
- 4. Mobeen A.K.: ‘Aflatoxins B1 and B2 contamination of peanut and peanut products and subsequent microwave detoxification’, J. Pharm. Nutr. Sci., 2011, 1, pp. 1 –31 [Google Scholar]
- 5. Hussain A.: ‘Studies on contamination level of aflatoxins in Pakistani rice’, Chem. Soc. Pak., 2011, 33, pp. 1 –4 [Google Scholar]
- 6. Akhtar S.: ‘Food safety challenges – a Pakistan's perspective’, Crit. Rev. Food Sci. Nut., 2014, 55, pp. 219 –226 [DOI] [PubMed] [Google Scholar]
- 7. Jiang Y.: ‘Aflatoxin‐related immune dysfunction in health and in human immunodeficiency virus disease’, Clin. Dev. Immunol., 2008, 10, pp. 79 –83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dorner J.W.: ‘Management and prevention of mycotoxins in peanuts’, Food Add. Cont., 2008, 25, pp. 203 –208 [DOI] [PubMed] [Google Scholar]
- 9. Moradi M. Hokmabadi H.: ‘Control of mycotoxin bioactives in nuts: farm to fork’, in Tokusoglu O. Hal C. III (Eds.): ‘Fruit and cereal bioactives sources, chemistry, and applications’ (CRC Press, Turkey, 2011), pp. 291 –315 [Google Scholar]
- 10. Aziz N. Faraz M. Pandey R. et al.: ‘Facile algae‐derived route to biogenic silver nanoparticles: synthesis, antibacterial, and photocatalytic properties’, Langmuir, 2015, 31, pp. 11605 –11612 [DOI] [PubMed] [Google Scholar]
- 11. Ditta A.: ‘How helpful is nanotechnology in agriculture?’, Adv. Nat. Sci. Nanosci. Nanotechnol., 2012, 3, pp. 2 –33 [Google Scholar]
- 12. Prasad R. Kumar V. Prasad K.S.: ‘Nanotechnology in sustainable agriculture: present concerns and future aspects’, Afr. J. Biotechnol., 2015, 13, pp. 705 –713 [Google Scholar]
- 13. Hussain M. Raja N.I. Iqbal M. et al.: ‘Applications of plant flavonoids in the green synthesis of colloidal silver nanoparticles and impacts on human health’, Iran. J. Sci. Technol. Trans. Sci., 2017, 10.1007/s40995-017-0431-6 [DOI] [Google Scholar]
- 14. Ngo Q.B. Dao T.H. Nguyen H.C. et al.: ‘Effects of nanocrystalline powders (Fe, Co and Cu) on the germination, growth, crop yield and product quality of soybean (Vietnamese species DT‐51)’, Adv. Nat. Sci. Nanosci. Nanotechnol., 2014, 5, pp. 15 –23 [Google Scholar]
- 15. Khodakovskaya M.V. Silva K.D. Biris A.S. et al.: ‘Carbon nanotubes induce growth enhancement of tobacco cells’, ACS Nano., 2012, 6, pp. 2128 –2135 [DOI] [PubMed] [Google Scholar]
- 16. Salama H.M.H.: ‘Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.)’, Int. Res. J. Biotechnol., 2012, 3, pp. 190 –197 [Google Scholar]
- 17. Sharma P. Jha A.B. Dubey R.S. et al.: ‘Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions’, J. Bot., 2012, 12, pp. 134 –136 [Google Scholar]
- 18. Hussain M. Raja N.I. Mashwani Z.R. et al.: ‘In vitro germination and biochemical profiling of Citrus reticulata in response to green synthesized zinc and copper nanoparticles’, IET Nanobiotechnol., 2017, 11, (7), pp. 790 –796 [Google Scholar]
- 19. Arokiyaraj S. Arasu M.V. Vincent S.: ‘Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum and its antibacterial and cytotoxic effects: an in vitro study’, Int. J. Nanomed., 2014, 9, pp. 379 –388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iqbal M. Asif S. Ilyas N. et al.: ‘Effect of plant derived smoke on germination and post germination expression of wheat (triticumaestivum L.)’, Am. J. Plant. Sci., 2016, 7, pp. 806 –813 [Google Scholar]
- 21. Rauwel P. Kuunal S. Ferdov S. et al.: ‘A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM’, Adv. Mater. Sci. Eng., 2015, 15, pp. 1 –9 [Google Scholar]
- 22. Yasur J. Rani P.U.: ‘Environmental effects of nanosilver: impact on castor seed germination, seedling growth, and plant physiology’, Environ. Sci. Pollut. Res. Int., 2013, 20, pp. 8636 –8648 [DOI] [PubMed] [Google Scholar]
- 23. Shah M. Fawcett D. Sharma S. et al.: ‘Green synthesis of metallic nanoparticles via biological entities’, Materials, 2015, 8, pp. 7278 –7308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iravani S.: ‘Green synthesis of metal nanoparticles using plants’, Green Chem., 2011, 13, pp. 2638 –2650 [Google Scholar]
- 25. Rahman L.U. Qureshi R. Yasinzai M.M. et al.: ‘Synthesis and spectroscopic characterization of Ag–Cu alloy nanoparticles prepared in various ratios’, Compt. Rendus Chim., 2012, 15, pp. 533 –538 [Google Scholar]
- 26. Geisler‐Lee J. Wang Q. Yao Y. et al.: ‘Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana ’, Nanotoxicology, 2013, 7, pp. 323 –337 [DOI] [PubMed] [Google Scholar]
- 27. Fuping J. Pan J. Zhu S. et al.: ‘Physiological and biochemical effects of Ag nanoparticles on wheat (Triticum aestivum L.)’, Fresen. Environ. Bull., 2017, 26, (1a), pp. 1084 –1090 [Google Scholar]
- 28. Reddy K.R.N. Abbas H.K. Abel C.A. et al.: ‘Mycotoxin contamination of commercially important agricultural commodities’, Toxin Rev., 2009, 28, pp. 154 –168 [Google Scholar]
- 29. Krishnaraj C. Jagan E.G. Ramachandran R. et al.: ‘Effect of biologically synthesized silver nanoparticles on Bacopamonnieri (Linn.) Wettst.plant growth metabolism’, Process Biochem., 2012, 47, pp. 651 –658 [Google Scholar]
- 30. Iqbal M. Raja N.I. Mashwani Z.R. et al.: Effect of silver nanoparticles on growth of wheat under heat stress’, Iran. J. Sci. Technol. Trans. Sci., 2017, 10.1007/s40995-017-0417-4 [DOI] [Google Scholar]
- 31. Ndeh N.T. Maensiri S. Maensiri D.: ‘The effect of green synthesized gold nanoparticles on rice germination and roots’, Adv. Nat. Sci. Nanosci. Nanotechnol., 2017, 8, pp. 35 –38 [Google Scholar]
- 32. Thuesombat P. Hannongbua S. Akasit S. et al.: ‘Effect of silver nanoparticles on rice (oryzasativa L. cv. KDML 105) seed germination and seedling growth’, Ecotoxicol. Environ. Saf., 2014, 104, pp. 302 –309 [DOI] [PubMed] [Google Scholar]
- 33. Hao Y. Zhang Z. Rui Y. et al.: ‘Effect of different nanoparticles on seed germination and seedling growth in rice’. 2nd Annual Int. Conf. on Advanced Material Engineering, Wuhan, Hubei, China, 2016, p. 233 [Google Scholar]
- 34. Jie Y. Jiang F. Ma C. et al.: ‘Alteration of crop yield and quality of wheat upon exposure to silver nanoparticles in a life cycle study’, J. Agri. Food Chem., 2018, 66, (11), pp. 2589 –2597 [DOI] [PubMed] [Google Scholar]
- 35. Syu Y.Y. Hung J.H. Chen J.C. et al.: ‘Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression’, Plant Physiol. Biochem., 2014, 83, pp. 57 –64 [DOI] [PubMed] [Google Scholar]
- 36. Mishra V. Mishra R.K. Dikshit A. et al.: ‘Interactions of nanoparticles with plants: an emerging prospective in the agriculture industry’, in Ahmad P. Rasool S. (Eds.): ‘Emerging technologies and management of crop stress tolerance’, vol. 1, Biol. Tech., 2014, pp. 159 –180 [Google Scholar]
- 37. Mengmeng R. Ma C. Tang X. et al.: ‘Phytotoxicity of silver nanoparticles to peanut (Arachis hypogaea L.): physiological responses and food safety’, ACS Sustain. Chem. Eng., 2017, 5, (8), pp. 6557 –6567 [Google Scholar]
- 38. Raliya R. Tarafdar J.C.: ‘Zno nanoparticle biosynthesis and its effect on phosphorous mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsistetragonoloba L.)’, Agric. Res., 2013, 2, pp. 48 –57 [Google Scholar]
- 39. Romagnoli B. Menna V. Gruppioni N. et al.: ‘Aflatoxins in spices, aromatic herbs, herb‐teas and medicinal plants marketed in Italy’, Food Control, 2007, 18, pp. 697 –701 [Google Scholar]
- 40. Santos L. Marín S. Sanchis V. et al.: ‘Screening of mycotoxin multi‐contamination in medicinal and aromatic herbs sampled in Spain’, J. Sci. Food Agric., 2009, 89, pp. 1802 –1807 [Google Scholar]
- 41. Lee S.D. Yu I.S. Jung K. et al.: ‘Incidence and level of aflatoxins contamination in medicinal plants in Korea’, Mycobiology, 2014, 42, pp. 339 –345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell K. Ferreira Cavalcante A.L. Galvin‐King P. et al.: ‘Evaluation of an alternative spectroscopic approach for aflatoxin analysis: comparative analysis of food and feed samples with UPLC‐MS/MS’, Sens. Actutator B‐Chem., 2017, 239, pp. 1087 –1097 [Google Scholar]
- 43. Nisa A. Zahra N. Hina S.: ‘Detection of aflatoxins in rice samples’, Bangla. J. Sci. Ind. Res., 2014, 49, pp. 189 –194 [Google Scholar]
- 44. Mukhtar H. Farooq Z. Manzoor M.: ‘Determination of aflatoxins in super kernel rice types consumed indifferent regions of Punjab, Pakistan’, J. Animal Plant Sci., 2016, 26, pp. 542 –548 [Google Scholar]
- 45. Cotty P.J. Jaime‐Garcia R.: ‘Influences of climate on aflatoxin producing fungi and aflatoxin contamination’, Int. J. Food Microbiol., 2007, 119, pp. 109 –115 [DOI] [PubMed] [Google Scholar]
- 46. Fakruddin M. Chowdhury A. Nur‐Hossain M. et al.: ‘Characterization of aflatoxin producing Aspergillus flavus from food and feed samples’, Springer Plus., 2015, 4, p. 159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chichiricco G. Poma A.: ‘Penetration and toxicity of nanomaterials in higher plants’, Nanomaterials, 2015, 5, pp. 851 –873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aslani F. Bagheri S. Julkapli N.M. et al.: ‘Effects of engineered nonmaterial's on plants growth: an overview’, Sci. World J., 2014, 6, pp. 22 –28 [Google Scholar]
- 49. Yang F. Hong F. You W. et al.: ‘Influence of nano‐anatase TiO2 on the nitrogen metabolism of growing spinach’, Biol. Trace Elem. Res., 2006, 110, pp. 179 –190 [DOI] [PubMed] [Google Scholar]
- 50. Wang X. Yang X. Chen S. et al.: ‘Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis’, Front. Plant Sci., 2016, 6, pp. 1243 –1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gebreselassie R. Dereje A. Solomon H.: ‘On farm pre harvest agronomic management practices of Aspergillus infection on groundnut in Abergelle, Tigray’, J. Plant Pathol. Microbiol., 2014, 5, p. 228 [Google Scholar]


