Abstract
In present investigation, copper oxide (CuO) nanostructures have been prepared via green chemistry. Olea europaea leaf extract act as strong chelating agent for tailoring physical as well as bio‐medical characteristics of CuO at the nano‐size. Physical characterisation such as scanning electron microscope analysis depicts the formation of homogenised spherical shape nanoparticles (NPs) with average size of 42 nm. X‐ray diffraction and Fourier transform infrared spectroscopy further confirmed the crystalline pure phase and monoclinic structure. High performance liquid chromatography (HPLC) testing is performed to evaluate the relative concentration of bioactive molecules in the O. europaea leaf extract. From HPLC results capping action of organic molecules around CuO‐NPs is hypothesised. The antimicrobial potency of biosynthesised CuO‐NPs have been evaluated using colony forming unit (CFU) counting assay and disc diffusion method which shows a significant zone of inhibition against bacterial and fungal strains may be highly potential for future antimicrobial pharmaceutics. Furthermore, reduction of various precursors by plant extract will reduce environmental impact over chemical synthesis.
Inspec keywords: copper compounds, antibacterial activity, biochemistry, nanoparticles, nanomedicine, biomedical materials, chromatography
Other keywords: CuO, size 42 nm, chemical synthesis, antimicrobial pharmaceutics, bacterial strain, fungal strain, disc diffusion method, colony forming unit counting assay, biosynthesised CuO‐NP, bioactive molecules, high‐performance liquid chromatography testing, monoclinic structure, crystalline pure phase, Fourier transform infrared spectroscopy, X‐ray diffraction, homogenised spherical shape nanoparticles, scanning electron microscope analysis, CuO biomedical characteristics, chelating agent, green fabricated CuO nanobullets, green chemistry, copper oxide nanostructures, antimicrobial potential, Olea europaea leaf extract
1 Introduction
Copper oxide (CuO) being member of copper family compounds exhibit diverse range of characteristics. In meticulous, CuO in bulk format with exclusive set of properties such as non‐toxicity, chemically stable, electrochemically active, copious accessibility of constituents, and little fabrication cost have been extensively exploited for miscellaneous applications such as solar cells, gas sensors, high temperature superconductors, catalysis, field emissions etc. [1, 2, 3, 4, 5, 6, 7]. In spite of these properties, nanoparticles (NPs) of CuO espouse novel attributes. CuO in number of cases acts like a semiconductor of n‐type. The monoclinic crystal structure is present in Cu2 O having 12.1–15.1 eV band gap energy while on the other side cuprous oxide (II–VI semiconductor) exhibit ∼2 eV band gap energy [8]. In CuO/Cu2 O molecular equilibrium comprises of electronic and ionic conduction mechanism and thus, during the process CuO and Cu2 O layers are made simultaneously via oxidation and reduction, respectively [9]. Dissociation and recombination of oxygen molecules and ions are the regulatory steps during the redox reactions [10].
Due to the fascinating properties of CuO‐NPs, several methods are in use for their synthesis, including hydrothermal [11], spin‐coating [1], electro‐deposition [12], sol–gel [13], solvothermal [14], thermal oxidation [15], sonochemical etc. [16]. Moreover, considerable attentions have been attempted to achieve the assembly of CuO nanostructures with various morphologies to improve their performance in the presently existing applications. In the case of fabrication of CuO‐NPs, using various methodologies, a combination of CuO, Cu2 O, Cu3 O4, and metallic copper exist in the oxidation product that can upset the properties of the product as an oxygen carrier [17]. Calcination and sintering at elevated temperatures (<1053°C under air) helps in the reduction of other compounds than CuO but a single phase homogeneous oxide product has been yet difficult to obtain [18]. In the midst of various natural surfactant materials used for NPs synthesis, plants seem to be the finest candidates, and NPs produced by plants are more stable, are of various sizes and shapes, and the rate of fabrication is faster than in the case of microorganisms [19].
Now a day, suppression of microbial infections has been the subject of universal concern in healthcare systems. This demands the development of effective nano materials having higher antimicrobial properties. In this perspective, it is found that inorganic NPs exhibit greater potential due to their excellent catalytic, optical and antibacterial characteristics [20]. Silver nanomaterials are also extensively studied as a antibacterial agent against a variety of bacteria [21] but their cytotoxicity for normal cells restrict their uses as an antibacterial agent [22].
Olea europaea belongs to the family Oleaceae and is native to tropical and warm temperate regions of the world [23]. Olive leaves have the highest percentage of bioactive compounds (e.g. oleuropein content in olive oil ranges between 0.005 and 0.12% and that in alperujo reaches up to 0.87%, while that in olive leaves ranges between 1 and 14%) with extensive pharmaceutical applications [24, 25].
We here describe the green synthesis of pure phase CuO‐NPs through O. europaea leaf extract as an effective chelating agent without utilising any acid or base standard chemicals. Moreover, we also have investigated the structure, antibacterial and antifungal properties of synthesised CuO‐NPs.
2 Experimental
2.1 Collection and processing of plant material
Authenticated O. europaea leaves were procured from the Barani Agricultural Research Institute (BARI), Chakwal, categorise as centre of excellence for Olive research by the government of Pakistan. Taxonomic identification of the selected plant was made by expert botanist Ghulam Kibria at BARI. The fresh leaves were washed with deionised H2 O and then were air dried under shade at room temperature to avoid photo‐dissociation. For extract preparation 20 g of dried leaves were finely grinded, powder was added in 200 ml of deionised H2 O and placed this mixture was placed undisturbed for 3 hours under ambient conditions. In the next step mixture were placed in shaking incubator for 2 hours at 50°C supported with 60 rpm. Finally, the obtained extraction was filtered using Whatman No. 1 filter paper and the filtrate was collected and stored at room temperature for further usage.
2.2 Fabrication of CuO‐NPs through O. europaea leaf extract
For green synthesis of CuO‐NPs, 7.98 g of copper acetate monohydrate (Sigma‐Aldrich) was added in 200 ml of O. europaea leaf extract and put at Scilogex Magnetic Hotplate Stirrer adjusted to 60°C at ∼1500 rpm for ∼2 hours. Appearance of brown colour indicate completion of the reaction, thereafter mixture was repeatedly loaded in a GR BioTek centrifuge at ∼8000 rpm for 10 min, brown pellets were isolated and collected. Washing with deionised H2 O was done thrice to get rid of any uncoordinated biological compounds. The obtained product was then dried using Memmert hot air oven at 60°C for ∼6 h and further annealed, to achieve desired crystallinity, in high temperature Gallenkamp furnace at 400°C for ∼2 h. Finally, the fabricated CuO‐NPs were subjected to characterisations and for antimicrobial assay.
2.3 Characterisation of CuO‐NPs
2.3.1 Scanning electron microscopy (SEM)
CuO‐NPs size and nanostructure were calculated by JOEL‐JSM‐6490LA SEM operating at 20 kV, CuO‐NPs were suspended in deionised water at a concentration of 1 mg/ml and then sonicated using a sonicator bath until the sample forms a homogenous suspension. For size measurement, this sonicated stock solution of CuO‐NPs (1 mg/ml) was diluted 20 times. After this one drop of the sonicated aqueous solution was taken on a glass plate and allowed to dry. Furthermore, the sample was gold coated and images were taken.
2.3.2 X‐ray diffraction (XRD) (crystallographic structure)
To inspect the crystallographic configuration of green fabricated CuO‐NPs, XRD analysis was carried out using PANalytical X'Pert3 Powder with nickel monochromator in the range of 2θ from 20° to 80° using Cu Kα radiation of wavelength 1.5406 Å. XRD pattern was observed at an operating voltage of 40 kV with 30 mA current provided at room temperature. To calculate the crystallite size of the prepared sample, Scherer's equation [D = 0.9λ /β cosθ] is employed, where D is the average crystalline domain size perpendicular to the reflecting planes, λ is the X‐ray wavelength (1.5406 A°), ß is the angular full width at half maximum in radians, and θ is the diffraction angle (2 Theta (degree) is the measured angle of diffraction in degrees) or Bragg's angle.
2.3.3 FTIR spectroscopy
Fourier transform infrared spectroscopy (FTIR) analysis has been utilised to observe the presence of vibrational modes on the surface of the prepared sample. FTIR spectroscopy for synthesized sample were performed using KBr pellet methodology by SHIMADZU FTIR in the wave number ranged 400–4000 cm−1.
2.4 High performance liquid chromatography (HPLC) analysis
We have followed the procedure of Brenes et al. [26] with slight modifications. For the quantification of bio‐molecules, 5 g of O. europaea L. leaf powder was weighted and 30 ml of 60% aqueous methanol was added using blender. This mixture was centrifuged for 5 min at 3000 rpm and filtered thrice. The final solution was filtered using a 0.22 µm micro‐filter prior to being injected to HPLC (Agilent Technologies, Ireland) analysis. Acetonitrile and acidified water (0.5% acetic acid solution) were used as the mobile phases A and B, respectively. The column temperature was set at 25°C along with flow rate of 1 ml/min. Elution time (to ) of unretained peak and retention time (t R) – determines the sample identity were graphed and examined later on.
2.5 Antibacterial assay of CuO‐NPs
Antibacterial assay was performed against four bacterial strains, i.e. Gram positive (G+ve) bacterial strains such as Staphylococcus aureus (ATCC 6633), Micrococcus luteus (ATCC 10240) and Gram negative (G−ve) bacterial strains include Salmonella typhimurium (ATCC 14028) and E. coli (ATCC 15224). Disc diffusion assay [27] was adopted with slight modification. On each disc 5 μl of 4 mg/ml of sample were poured. Discs were placed on the agar plate containing inoculated test bacterial strain. Deionised H2 O was used as negative control while Roxithromycin solution (2 mg/ml) was used as positive control. Finally, zone of inhibition (ZOI) was measured after 24 hours.
2.6 Antifungal assay of CuO‐NPs
Antifungal assay was analyzed against Aspergillus fumagatus (FCBP‐66), Mucor specie (FCBP‐0300), Aspergillus niger (FCBP‐0198), and Aspergillus flavis (FCBP‐0064). Disc diffusion method was used for antifungal activity investigation. Sabouraud dextrose agar (Sigma‐Aldrich), pH 5.7) was autoclaved and poured in petri plates under sterile conditions. Fungal lawns were prepared by inoculating spores on the surface of the media after that discs loaded with 5 μl of 4 mg/ml sample and were placed on the surface of petri plate. Deionised H2 O was used as negative control while Clotrimazole solution (05μg/disc) was used as positive control. The plates were incubated at 25°C for 1 day and ZOI was measured, respectively.
2.7 Colony forming unit (CFU) counting assay
To inspect the time dependent antibacterial potential of green synthesised CuO‐NPs, we have conducted the CFU counting test following our recently reported method [28] with modifications. The assay was carried out in sample tubes, each having 2 ml of autoclaved liquid nutrient broth. Test tubes were inoculated with bacterial strains, i.e. G+ve bacterial strains such as S. aureus (ATCC 6633), M. luteus (ATCC 10240) and G−ve bacterial strains include Salmonella typhimurium (ATCC 14028) and E. coli (ATCC 15224), respectively. 20 µg/5 µl of CuO‐NPs was added in each of the bacterial inoculated tubes. In this experiment Cefixime was used as positive control while deionised water acts as negative control. Positive and negative control test was performed on E. coli (ATCC 15224).
2.8 Statistical analysis
All the results are the mean of at least three individual experiments presented with standard deviation. The statistical analysis of the results were carried out through Student's t tests by using SPSS software package and considered significant at <0.05 p values.
3 Results and discussions
3.1 Biosynthesis process (visual observation)
It is the well‐established fact that O. europaea aqueous extract contains variety of bio active molecules [24, 25] that might be effectively involved as chelating agent in green synthesis of CuO‐NPs, upon addition of copper acetate monohydrate. As shown in the schematic diagram (Fig. 1) that appearance of brown colour upon reaction completion predicts the successful formation of CuO‐NPs, further conferred by SEM, XRD and FTIR characterisations.
Fig. 1.
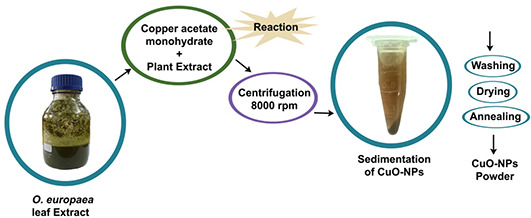
Schematic diagram of CuO‐NPs synthesis using O. europaea leaf extract
3.2 SEM analysis
It is obvious from an SEM micrograph (Fig. 2) that CuO‐NPs are highly homogenous and symmetrical in morphology with spherical in shape, the findings clearly represents the mean particle size of 42 nm. Resemblance in morphological shapes with uniform average grain size of less than 50 nm was also reported previously [29].
Fig. 2.

SEM images of CuO‐NPs synthesised using plant extract
3.3 XRD (crystallographic structure) analysis
Fig. 3 shows typical XRD profile of green fabricated CuO‐NPs. It is manifest that CuO‐NPs possess single‐phase CuO with monoclinic structure without any additional peaks, presence of several broad Bragg peaks corresponds to (110), (111), (−202), (020), (202), (−113), (−311), (113), (311) and (004) orientations and are precisely well indexed according to JCPDS‐05‐0661. The present experimental results confirmed that CuO‐NPs possess a crystalline structure were found to be in agreement with the reported diffraction patterns of CuO‐NPs prepared by Das et al. [30]. Using Debye–Scherrer approximation and from the broadening of the peaks the approximate particle size of CuO‐NPs found near to 42 nm as assisted by SEM studies.
Fig. 3.
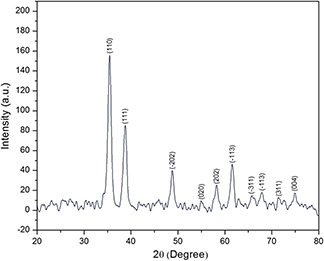
XRD spectrum green synthesised CuO‐NPs
3.4 Fourier transform infrared spectroscopy analysis
The FTIR spectrum (Fig. 4 a) of CuO‐NPs clearly predicts broad absorption peaks between 2800 and 4000 cm−1 mainly ascribed to OH– and C−O groups present on the surface of the CuO crystals nanostructure. The characteristic peaks at 592, 607 and 675 cm−1 corresponds to the Cu‐O stretching prints which indicates the formation of monoclinic CuO‐NPs. Similar Cu‐O stretching band frequencies in the range between 500 to 700 cm−1 are also reported by Padil and Černík [31]. Fig. 4 b represents the presence of active organic compounds from O. europaea aqueous extract involved in reduction of Cu(CO2 CH3)2 · H2 O into CuO‐NPs [24, 25, 28]. The spectrum ranging between 1350 to 1550 cm−1 corresponds to presence of bio‐molecules with nitro‐group.
Fig. 4.
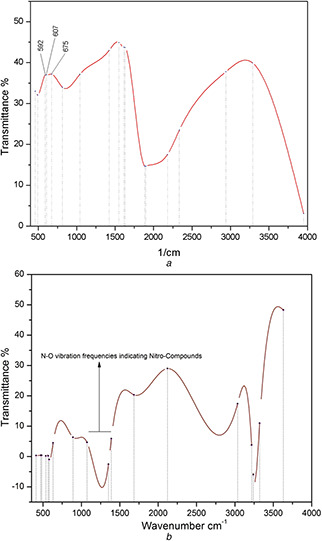
FTIR spectrum analysis of
(a) CuO‐NPs, (b) O. europaea aqueous extract
3.5 HPLC examinations
O. europaea L. leaf extract was screened to find the types and quantity of bioactive molecules involved in a possible reduction of copper acetate monohydrate to CuO‐NPs. HPLC profiles of phenolic compounds are graphed in (Fig. 5). Several peaks corresponding to different poly‐phenols which were identified from their retention times and UV–Vis spectra, as rutin (t R = 6.7) and verbascoside (oleuropeosides) (t R = 7.9), luteolin‐7‐O‐glucoside (flavones) (t R = 8.9), apigenin‐7‐O‐glucoside (t R = 16.2) oleuropein (t R = 23.6) and oleuroside (t R = 33.8). All these bioactive compounds have previously been reported to occur in O. europaea leaf extract [32, 33]. Our HPLC results shows that olive leaf extracts have maximum concentration of oleuropein as compared with other phenolic compounds. These findings are well indexed to previously reported studies [34].
Fig. 5.
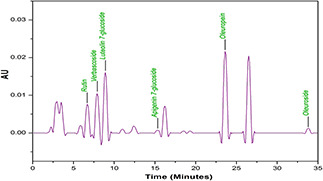
HPLC spectrum analysis of O. europaea leaf extract
HPLC and FTIR examinations depict the relative abundance of organic compounds in olive leaf extract. As oleuropein is the richest bioactive compounds in O. europaea aqueous extract which lysed into more polarisable and highly reactive hydroxytyrosol (C8 H10 O3) [28]. Hence, it can be hypothesised that this molecule may part in the redox mechanism in tuning and capping of CuO‐NPs as shown in Fig. 6.
Fig. 6.
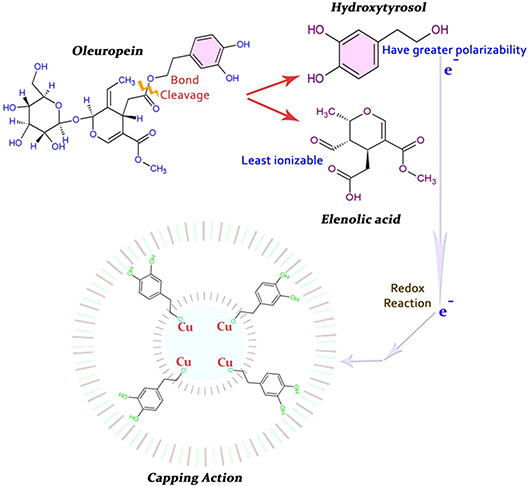
Hypothesis of capping mechanism of CuO‐NPs
3.6 Antibacterial analysis of CuO‐NPs
The antibacterial assay has been evaluated against (G+ve) and (G−ve) bacterial strains using 5 μl of 4 mg/ml loaded concentration of CuO‐NPs sample on discs. Fig. 7 shows measurements in the size of ZOI around CuO‐NPs poured disc. From the results of XRD and SEM the average CuO‐NPs size is found to be 42 nm. Higher surface area due to smaller crystal size results in greater antibacterial activity [35]. Significant susceptibility, in terms of ZOI, towards S. aureus (G+ve) strain was recorded, maximum of 10.8 ± 0.5 mm. While on the other hand mild to low activity of 8.5 ± 0.4 mm was observed against E. coli, a (G−ve) bacteria that might be due to the unique cell membrane structure that help bacterium to resist against antimicrobial agents. Besides all this, other features such as the size and rate at which NPs diffuses also play a crucial role against various bacterial strains [36]. Bioactive compounds from the O. europaea green extract also contributed fair antibacterial activity (Fig. 5) ranges from 1.2 ± 0.5 mm to 4.1 ± 0.6 mm (ZOI). This observed effect was probably due to presence of efficient oleuropein, found in highest concentration in O. europaea leafs with well‐known antimicrobial potential [24, 25]. Exact bactericidal mechanisms of CuO‐NPs were not well understood, but some studies had been carried out in order to explain the machinery of bactericidal action which NPs play. Gram‐positive bacteria S. aureus are more resistant to silver NPs in comparison with the Gram‐negative bacteria E. coli [37] and this fact enlightened that this might be due to the cell wall thickness or due to the peptidoglycan layer that might avert, to some degree, the action of NPs, on the other hand, established that some types of E. coli were unaffected as compared with S. aureus to silver NPs [38]. Hence, the bactericidal potential of NPs is does not rely only on the membrane’s structure. Work in this regard needs more exploration in future.
Fig. 7.
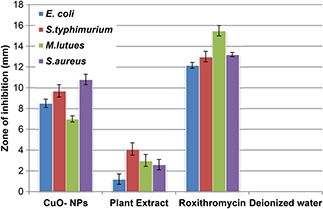
Antibacterial analysis of CuO‐NPs, plant extract, roxithromycin (positive control) and deionised water (negative control) taking their mean values with ±STDV
3.7 Antifungal studies of CuO‐NPs
Promising antifungal properties of green synthesised CuO‐NPs have been recorded as shown in Fig. 8, ZOI from 6.35 ± 0.3 mm to 9.2 ± 0.4 mm corresponds mild to moderate antifungal activity. The exact mechanism of CuO‐NPs antifungal activity is not well understood but as CuO slowly oxidise into cupric ion which is then available in the biological system and able to produce lethal hydroxyl free radicals when immediate vicinity of the lipid membrane. These free radicals generate oxidative deterioration of lipids, which form cell membrane [39] which in fact cause outflow of K+ ions and other substances from the cell [40], which ultimately leads toward the disturbance of major biochemical reactions which takes place inside the cells. In our results enhanced antifungal activity of CuO‐NPs is observed, which is far better than the reported values [41].
Fig. 8.
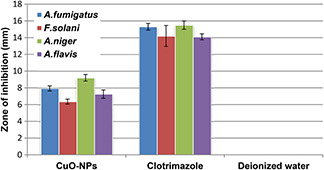
Antifungal analysis of CuO‐NPs, Clotrimazole (positive control) and deionised water (negative control) with taking their mean values and ±STDV
3.8 Study of CFU counting assay
Fig. 9 depicts the bacterial growth progression during CFU test. It is quite obvious that addition of CuO‐NPs declines the bacterial growth with time. Maximum reduction in growth was observed in G+ve strain S. aureus while all of the other strains reflects relatively small decline in growth rate with time. Maximum surface area due to extremely small size (Fig. 2), CuO‐NPs successfully inhibits the bacterial growth persists for a long time in small dosage. This prominent antibacterial behaviour of CuO‐NPs were may be due to the generation of ROS within host biosynthetic machinery, defects in membrane permeability, intolerance in biodegradation of metallic nano‐structures and probably degeneration of bacterial vital proteins [42, 28].
Fig. 9.
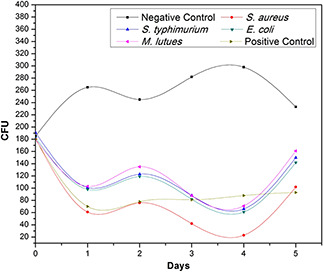
CFU counting assay of green synthesised CuO‐NPs against G+ve and G−ve bacterial strains
4 Conclusion
The present green method for the synthesis of CuO‐NPs is low cost, easy‐going and environmentally friendly. From SEM and XRD observations, it is found that the green synthesised CuO‐NPs exhibit spherical pure phase structure with an average grain size of 40 nm. Intensified peaks between 590 to 680 cm−1 in the FTIR spectrum depicts CuO‐NPs formation with monoclinic structure. The synthesised CuO‐NPs rendered significant ZOI against all of the bacterial as well as fungal strains examined, could be future for biomedical applications. Our study proposed that mechanism of antimicrobial response of CuO‐NPs should be further investigated.
5 References
- 1. Erdoğan İ.Y. Güllü Ö.: ‘Optical and structural properties of CuO nanofilm: its diode application’, J. Alloys Compd, 2010, 492, pp. 378 –383 [Google Scholar]
- 2. Mukherjee N. Show B. Maji S.K. et al.: ‘CuO nano‐whiskers: electrodeposition, Raman analysis, photoluminescence study and photocatalytic activity’, Mater. Lett., 2011, 65, pp. 3248 –3250 [Google Scholar]
- 3. Wijesundera R.P.: ‘Fabrication of the CuO/Cu2 O heterojunction using an electrodeposition technique for solar cell applications’, Semicond. Sci. Technol., 2010, 25, p. 045015 [Google Scholar]
- 4. Yang C. Su X. Xiao F. et al.: ‘Gas sensing properties of CuO nanorods synthesized by a microwave‐assisted hydrothermal method’, Sens. Actuators B Chem., 2011, 158, pp. 299 –303 [Google Scholar]
- 5. MacDonald A.H.: ‘Superconductivity: copper oxides get charged up’, Nature, 2001, 414, pp. 409 –410 [DOI] [PubMed] [Google Scholar]
- 6. Hu Y. Huang X. Wang K. et al.: ‘Kirkendall‐effect‐based growth of dendrite‐shaped CuO hollow micro/nanostructures for lithium‐ion battery anodes’, J. Solid State Chem., 2010, 183, pp. 662 –667 [Google Scholar]
- 7. Hsieh C.T. Chen J.M. Lin H.H. et al.: ‘Field emission from various CuO nanostructures’, Appl. Phys. Lett., 2003, 83, pp. 3383 –3385 [Google Scholar]
- 8. Salavati‐Niasari M. Davar F.: ‘Synthesis of copper and copper (I) oxide nanoparticles by thermal decomposition of a new precursor’, Mater. Lett., 2009, 63, pp. 441 –443 [Google Scholar]
- 9. Li J. Wang S.Q. Mayer J.W. et al.: ‘Oxygen‐diffusion‐induced phase boundary migration in copper oxide thin films’, Phys. Rev. B, 1989, 39, p. 12367 [DOI] [PubMed] [Google Scholar]
- 10. Grzesik Z. Migdalska M.: ‘On the mechanism of Cu2 O oxidation at high temperatures’, Defect Diffus. Forum, 2009, 289, pp. 429 –436 [Google Scholar]
- 11. Cheng Z. Xu J. Zhong H. et al.: ‘Hydrogen peroxide‐assisted hydrothermal synthesis of hierarchical CuO flower‐like nanostructures’, Mater. Lett., 2011, 65, pp. 2047 –2050 [Google Scholar]
- 12. Yang J. Jiang L.C. Zhang W.D. et al.: ‘A highly sensitive non‐enzymatic glucose sensor based on a simple two‐step electrodeposition of cupric oxide (CuO) nanoparticles onto multi‐walled carbon nanotube arrays’, Talanta, 2010, 82, pp. 25 –33 [DOI] [PubMed] [Google Scholar]
- 13. Punnoose A. Magnone H. Seehra M.S. et al.: ‘Bulk to nanoscale magnetism and exchange bias in CuO nanoparticles’, Phys. Rev. B, 2001, 64, p. 174420 [Google Scholar]
- 14. Aslani A. Oroojpour V.: ‘CO gas sensing of CuO nanostructures, synthesized by an assisted solvothermal wet chemical route’, Physica B Condens. Matter, 2011, 406, pp. 144 –149 [Google Scholar]
- 15. Vila M. Diaz‐Guerra C. Piqueras J.: ‘Optical and magnetic properties of CuO nanowires grown by thermal oxidation’, J. Phys. D Appl. Phys., 2010, 43, p. 135403 [Google Scholar]
- 16. Ranjbar‐Karimi R. Bazmandegan‐Shamili A. Aslani A. et al.: ‘Sonochemical synthesis, characterization and thermal and optical analysis of CuO nanoparticles’, Phys. B Condens. Matter, 2010, 405, pp. 3096 –3100 [Google Scholar]
- 17. Vaseem M. Umar A. Hahn Y.B. et al.: ‘Flower‐shaped CuO nanostructures: structural, photocatalytic and XANES studies’, Catal. Commun., 2008, 10, pp. 11 –16 [Google Scholar]
- 18. Li Y. Yang X.Y. Rooke J. et al.: ‘Ultralong Cu (OH)2 and CuO nanowire bundles: PEG200‐directed crystal growth for enhanced photocatalytic performance’, J. Colloid and Interface Sci., 2010, 348, pp. 303 –312 [DOI] [PubMed] [Google Scholar]
- 19. Iravani S.: ‘Green synthesis of metal nanoparticles using plants’, Green Chem., 2011, 13, pp. 2638 –2650 [Google Scholar]
- 20. Jan T. Iqbal J. Ismail M. et al.: ‘Synthesis, physical properties and antibacterial activity of metal oxides nanostructures’, Mater. Sci. Semicond. Process., 2014, 21, pp. 154 –160 [Google Scholar]
- 21. Monteiro D.R. Gorup L.F. Takamiya A.S. et al.: ‘The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver’, Int. J. Antimicrob. Agents, 2009, 34, pp. 103 –110 [DOI] [PubMed] [Google Scholar]
- 22. Tranquada J.M. Sternlieb B.J. Axe J.D. et al.: ‘Evidence for stripe correlations of spins and holes in copper oxide superconductors’, Nature, 1995, 375, pp. 561 –563 [Google Scholar]
- 23. Soni M.G. Burdock G.A. Christian M.S. et al.: ‘Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods’, Food Chem. Toxicol., 2006, 44, pp. 903 –915 [DOI] [PubMed] [Google Scholar]
- 24. Japón‐Luján R. Luque‐Rodríguez J.M. De Castro M.L.: ‘Dynamic ultrasound‐assisted extraction of oleuropein and related biophenols from olive leaves’, J. Chromatogr. A, 2006, 1108, pp. 76 –82 [DOI] [PubMed] [Google Scholar]
- 25. Omar S.H.: ‘Oleuropein in olive and its pharmacological effects’, Sci. Pharm., 2010, 78, pp. 133 –154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brenes M. Rejano L. Garcia P. et al.: ‘Biochemical changes in phenolic compounds during Spanish‐style green olive processing’, J. Agric. Food Chem., 1995, 43, pp. 2702 –2706 [Google Scholar]
- 27. Thovhogi N. Diallo A. Gurib‐Fakim A. et al.: ‘Nanoparticles green synthesis by Hibiscus Sabdariffa flower extract: Main physical properties’, J. Alloys Compd, 2015, 647, pp. 392 –396 [Google Scholar]
- 28. Maqbool Q. Nazar M. Naz S. et al.: ‘Antimicrobial potential of green synthesized CeO2 nanoparticles from Olea europaea leaf extract’, Int. J. Nanomed., 2016, 11, pp. 5015 –5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akhavan O. Ghaderi E.: ‘Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts’, Surf. Coat. Technol., 2010, 205, pp. 219 –223 [Google Scholar]
- 30. Das D. Nath B.C. Phukon P. et al.: ‘Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles’, Colloids Surfaces B Biointerfaces, 2013, 101, pp. 430 –433 [DOI] [PubMed] [Google Scholar]
- 31. Padil V.V.T. Černík M.: ‘Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application’, Int. J. Nanomed., 2013, 8, pp. 889 –898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atiok E. Bayçin D. Bayraktar O. et al.: ‘Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibrion’, Sep. Purif. Technol., 2008, 62, pp. 342 –348 [Google Scholar]
- 33. Briante R. Patumi M. Terenziani S. et al.: ‘ Olea europaea L. leaf extract and derivatives: antioxidant properties’, J. Agric. Food Chem., 2002, 50, pp. 4934 –4940 [DOI] [PubMed] [Google Scholar]
- 34. Benavente‐Garcıa O. Castillo J. Lorente J. et al.: ‘Antioxidant activity of phenolics extracted from Olea europaea L. leaves’, Food Chem., 2000, 68, pp. 457 –462 [Google Scholar]
- 35. Hameed A.S.H. Karthikeyan C. Sasikumar S. et al.: ‘Impact of alkaline metal ions Mg2+, Ca2+, Sr2+ and Ba2+ on the structural, optical, thermal and antibacterial properties of ZnO nanoparticles prepared by the co‐precipitation method’, J. Mater. Chem. B, 2013, 1, pp. 5950 –5962 [DOI] [PubMed] [Google Scholar]
- 36. Azam A. Ahmed A.S. Oves M. et al.: ‘Size‐dependent antimicrobial properties of CuO nanoparticles against Gram‐positive and‐negative bacterial strains’, Int. J. Nanomedicine, 2012, 7, pp. 3527 –3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim J.S. Kuk E. Yu K.N. et al.: ‘Antimicrobial effects of silver nanoparticles’, Nanomedicine: Nanotechnol. Biol. Med., 2007, 3, pp. 95 –101 [DOI] [PubMed] [Google Scholar]
- 38. Ruparelia J.P. Chatterjee A.K. Duttagupta S.P. et al.: ‘Strain specificity in antimicrobial activity of silver and copper nanoparticles’, Acta biomater., 2008, 4, pp. 707 –716 [DOI] [PubMed] [Google Scholar]
- 39. Howlett N.G. Avery S.V.: ‘Induction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation’, Appl. Environ. Microbiol., 1997, 63, pp. 2971 –2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lippert H. Brinkmeyer R. Mülhaupt T. et al.: ‘Antimicrobial activity in sub‐Arctic marine invertebrates’, Polar Biol., 2003, 26, pp. 591 –600 [Google Scholar]
- 41. Wei Y. Chen S. Kowalczyk B. et al.: ‘Synthesis of stable, low‐dispersity copper nanoparticles and nanorods and their antifungal and catalytic properties’, J. Phys. Chem. C, 2010, 114, pp. 15612 –15616 [Google Scholar]
- 42. Dhineshbabu N.R. Rajendran V.: ‘Antibacterial activity of hybrid chitosan–cupric oxide nanoparticles on cotton fabric’, IET Nanobiotechnol., 2016, 10, pp. 13 –19 [DOI] [PMC free article] [PubMed] [Google Scholar]


