Abstract
In this present study, a hybrid Chi‐Fe3 O4 was prepared, characterised and evaluated for its antibacterial and antibiofilm potential against Staphylococcus aureus and Staphylococcus marcescens bacterial pathogens. Intense peak around 260 nm in the ultraviolet–visible spectrum specify the formation of magnetite nanoparticles. Spherical‐shaped particles with less agglomeration and particle size distribution of 3.78–46.40 nm were observed using transmission electron microscopy analysis and strong interaction of chitosan with the surface of magnetite nanoparticles was studied using field emission scanning microscopy (FESEM). X‐ray diffraction analysis exhibited the polycrystalline and spinel structure configuration of the nanocomposite. Presence of Fe and O, C and Cl elements were confirmed using energy dispersive X‐ray microanalysis. Fourier transform infrared spectroscopic analysis showed the reduction and formation of Chi‐Fe3 O4 nanocomposite. The antibacterial activity by deformation of the bacterial cell walls on treatment with Chi‐Fe3 O4 nanocomposite and its interaction was visualised using FESEM and the antibiofilm activity was determined using antibiofilm assay. In conclusion, this present study shows the green synthesis of Chi‐Fe3 O4 nanocomposite and evaluation of its antibacterial and antibiofilm potential, proving its significance in medical and biological applications
Inspec keywords: visible spectra, particle size, magnetic particles, nanocomposites, nanoparticles, X‐ray diffraction, nanofabrication, transmission electron microscopy, X‐ray chemical analysis, nanomagnetics, microorganisms, antibacterial activity, iron compounds, ultraviolet spectra, biomedical materials, field emission scanning electron microscopy, Fourier transform infrared spectra, filled polymers, crystal growth from solution, polymer structure
Other keywords: potential antibacterial material, antibiofilm potential, magnetite nanoparticles, solvothermal‐assisted green synthesis, hybrid Chi‐Fe3 O4 nanocomposites, staphylococcus aureus, staphylococcus marcescens, bacterial pathogens, ultraviolet–visible spectrum, spherical‐shaped particles, particle size, transmission electron microscopy, FESEM, field emission scanning electron microscopy, X‐ray diffraction, spinel structure, polycrystalline structure, energy dispersive X‐ray microanalysis, Fourier transform infrared spectroscopic analysis, deformation, bacterial cell walls, Fe3 O4
1 Introduction
Magnetite nanoparticles (NPs) have revealed superior impact due to its magnetic property, biocompatibility and size in different areas of biology, medicine and physics [1, 2, 3]. The major hindrance in the use of these NPs is their high surface energy and solid dipole–dipole contact between them related with the high surface area to volume ratio, that creates a core uncertainty that leads to the aggregation of the particles [4, 5]. There are several methods to synthesise nanostructured magnetic materials of which co‐precipitation method [6], sol–gel method [7], thermal decomposition of organometallic compounds [8], hydrothermal synthesis [9] etc. are used more frequently. [10]. Although some of the physical and chemical methods have shown the synthesis of uniformly sized NPs a major drawback would be lack of stability and high agglomeration of the particles resulting with a different physical property and toxic chemicals used for the synthesis. In contrast, the biological method of NP synthesis using leaf extracts are ecologically feasible and are found extremely stable with very less agglomeration [11, 12, 13, 14]. The biological extract used for the synthesis plays a major role in influencing the size of the NPs. In comparison, lower concentration of the extract resulted in an average size <50 nm and higher concentration resulted in an increased size >70 nm [15]. At the same time, the higher concentration of extract also resulted with a smaller size of the NPs [16], these correlates that the amount of phytochemicals, functional groups and metabolites majorly influence the size regardless of the concentration of the extract [17].
On the other hand, the solvothermal method is well known for synthesising rich mesoporous Fe3 O4 NPs with increased stability due to the use of stabiliser and surfactants [18]. In this study, we adopted to use a solvothermal assisted green synthesis using floral extracts for its efficient biological application. Talinum portulacifolium, a medicinal flora of the family Portulacaceae, majorly found in the regions of Asia and Africa. The secondary metabolites screening of T. portulacifolium leaves extract exhibited the presence of active compounds substantially considered for its anti‐diabetic and antioxidant property [19, 20].
Numerous stabiliser and surfactants are being used to increase the stability of Fe3 O4 NPs, this generates several external modifications on the surface of the particles [21, 22]. Polymers of the biological source are exceedingly preferred as a better solution for stabilising the surface charge of the NPs. A linear polysaccharide of deacetylated beta‐1, 4‐D‐glucosamine, commonly known as Chitosan, a chitin derivative that has been widely used in the area of medicine for its remarkable biological and chemical properties like biodegradability, biocompatibility, hydrogelation, anti‐microbial property and polycationic nature [23, 24, 25, 26, 27, 28]. Plentiful studies have reported the use of chitosan and magnetic NPs in the form of hybrids, composites etc. that are highly potential and efficient in biological applications. [29, 30, 31] Encapsulation of the NP using the polymeric chains of chitosan on holds the stable anionic magnetic core with a cation surface that is functionally improved. Manorjan et al. (2015) also showed the modulation of the surface charge potential of iron oxide NP by encapsulation with chitosan. This helps in the improved interaction of the polymer‐coated NPs with the surface of the bacteria with relatively high ROS production [28] and its biofilm resulting in better antibacterial activity [32, 33]. Also, recent works show that NPs with an average size of less <50 nm are more effective due to its ability to permeate and disrupt the bacterial cell wall [15, 33].
Nowadays, a major global health concern is the growth and increased tolerance of bacteria against several potential antibiotics [34, 35, 36] through the development of bacterial slime and biofilms, thus improving the bacterial resistance [37, 38, 39]. The progressing field of nanotechnology and the expansion of various nanomaterials are being considered to address these research problems [40, 41]. The present study shows the development of a magnetite‐chitosan nanocomposite by solvothermal assisted green synthesis method and its characterisation using different modern instrumentation. To the best of our knowledge, this is the first report on the solvothermal assisted green synthesis of hybrid chitosan‐magnetite nanocomposite using T. portulacifolium leaf extract. The main objective of the study is to develop a stable surface charge modified magnetite‐chitosan nanocomposite and evaluation of its enhanced properties, surface interactions and its biological applications.
2 Materials and methods
2.1 Chemicals
Iron (II) chloride tetrahydrate (FeCl2 ·4H2 O), iron (III) chloride hexahydrate (FeCl3 ·6H2 O), ethylene glycol and chitosan (C56 H103 N9 O39) (low‐molecular weight, degree of deacetylation 70–90%), were procured from HiMedia, India. All the reagents were prepared using deionised water from Merck Millipore Laboratory water purification system, Germany. The clinical isolated Gram‐negative bacteria Staphylococcus marcescens and Staphylococcus aureus were obtained from the Aravind eye hospital, Coimbatore, Tamilnadu, India.
2.2 Microbial cultures
The procured bacterial strains S. marcescens and S. aureus were serial diluted and were swabbed on an agar plates of Mueller Hinton agar (MHA) medium to acquire the desired colony forming units (CFUs) (1.5 × 108 CFU/mL).
2.3 Synthesis of Fe3 O4 NPs
The plant extract is prepared by boiling 5 g of (dried and powdered) leaf in 100 mL distilled water 80°C for 30 min. FeCl2 ·4H2 O and FeCl3 ·6H2 O solution was prepared at the ratio of 1:2 M and mixed with 100 mL of plant extract, to which 60 mL of ethylene glycol solution was added. The resulting solution was kept at 80°C for 10 min under continuous stirring on a magnetic stirrer, during which the solution was observed for the development of yellowish colour. To this solution, 10 mL of 1.0 M freshly prepared NaOH solution was added dropwise at a rate of 3 mL/min, permitting constant magnetite precipitation under continuous stirring and the mixture was adjusted to pH 11.
A colour change from yellow solution to a black coloured solution was observed after 30 min, after which the solution was placed in a Teflon‐lined stainless‐steel autoclave and was maintained at a temperature of 160°C for 12 h. The solution was thawed to the room temperature gradually. The prepared Fe3 O4 NPs (magnetite NPs) were collected using continuous centrifugation and washed with deionised water to remove all the remains of leaf extract. The concentrated NPs were dried in an oven at a temperature 80°C for 24 h and stored in an air‐tight container for further analysis.
2.4 Preparation of hybrid Chi‐Fe3 O4 nanocomposite
About 200 mg of the synthesised Fe3 O4 NPs were added to 20 mL of chitosan solution (20 mg of chitosan in 20 mL of 1% acetic acid) under continuous stirring at room temperature for 2 h. The obtained hybrid chitosan‐magnetite nanocomposites (Chi‐Fe3 O4) were washed with double distilled water and concentrated by centrifugation at 5000 rpm for 15 min. The NPs were calcinated at 40°C for 1 h to remove excess chitosan and dried in an oven at 60°C for 24 h.
2.5 Characterisation techniques
The techniques used for the characterisation of hybrid Chi‐Fe3 O4 nanocomposite are as follows: The formation of hybrid Chi‐Fe3 O4 nanocomposite was primarily confirmed using ultraviolet–visible (UV‐Vis) spectroscopy (JASCO‐V‐670), the spectrum recorded a strong peak at the range of 200–600 nm at a resolution of 1 nm. The transmission electron microscopy (TEM, Technai G2, at 200 kV) was used for analysing the morphology and size of the hybrid Chi‐Fe3 O4 nanocomposite. The crystalline nature of hybrid Chi‐Fe3 O4 nanocomposite was confirmed using a Rigaku Miniflex diffractometer with Cu‐Kα radiation and the X‐ray diffraction (XRD) diffraction pattern was analysed at the range of 20°–80°. The surface morphology of the hybrid Chi‐Fe3 O4 nanocomposite was studied using field emission scanning microscopy (HITACHI SU6000 FESEM). Energy‐dispersive X‐ray microanalysis (EDS, R Model Quan Tax 200, Germany) was used to analyse the chemical composition of the hybrid Chi‐Fe3 O4 nanocomposite. The functional group examination of hybrid Chi‐Fe3 O4 nanocomposite was analysed by Fourier transform infrared (FTIR) spectroscopic analysis (Shimadzu IR‐Prestige‐21), read at the range of 400–4000 cm−1. The zeta potential was studied with Zeta sizer‐NanoZs (Malvern Instruments) at 25°C to understand the surface charge of hybrid Chi‐Fe3 O4 nanocomposite. Dynamic light scattering (DLS) spectroscopy was used for calculating the average size of hybrid Chi‐Fe3 O4 nanocomposite, by dispersing hybrid Chi‐Fe3 O4 nanocomposite samples in de‐ionised water.
2.6 Minimum inhibitory concentration (MIC) determination
The MIC of hybrid Chi‐Fe3 O4 nanocomposite against S. marcescens and S. aureus was studied using the protocols reported by CLSI‐Clinical and Laboratory Standards Institute [42]. The bacterial pathogens were cultured in Mueller–Hinton broth (MHB) at 37°C overnight and diluted to produce 5 × 105 CFU/mL. Sterile culture media of 200 µL was used as control. Chi‐Fe3 O4 nanocomposite of different concentrations (0.5, 1, 10, 25, 50, 75, 100 μg/mL) was introduced to 200 µL of overnight grown cultures taken in a polypropylene 96‐well microplate and was incubated at 37°C for 24 h. The absorbance was noted before and after incubation at 550 nm. In the intervening time, the minimum bactericidal concentration (MBC) was also determined using the lowest concentrations.
2.6.1 Antibacterial activity determination
The hybrid Chi‐Fe3 O4 nanocomposite was studied for its antibacterial activity against S. marcescens and S. aureus using standard agar well diffusion method. Briefly, the pathogenic bacterial strains S. marcescens and S. aureus (1.5 × 108 CFU/mL) were swabbed on agar plates containing Muller–Hinton medium. Hybrid Chi‐Fe3 O4 nanocomposite was dispersed in sterile distilled water at the concentration of 1 mg/1 mL. Wells of 5 mm in diameter were made on agar plates using a well puncher and were loaded with different concentrations of 10, 25, 50 and 100 μL of hybrid Chi‐Fe3 O4 nanocomposites. The antimicrobial activity was determined by measuring the zone of inhibition around the wells of the agar plates incubated for 24 h at 37°C.
In addition, the surface morphology of the bacterial cells was observed using light microscopy and FESEM. Overnight grown S. marcescens and S. aureus cultures of 200 μL were incubated with 100 μL of hybrid Chi‐Fe3 O4 nanocomposite (1 mg/1 mL sterile water) for 3 h, where the untreated cultures served as control. The bacterial cells were collected by centrifugation after the incubation, washed twice with 0.85% NaCl, and fixed on to a glass slide with 2% glutaraldehyde at room temperature (∼25°C). The fixed cells were resuspended in double‐distilled water and dehydrated on silicon substrate chips.
2.6.2 Congo red agar assay
The formation of bacterial biofilms was confirmed using Congo red agar method [43]. The overnight bacterial cultures of S. marcescens and S. aureus were inoculated on brain heart infusion agar plate containing 5% sucrose supplement and 0.8 µg of Congo red and incubated for 24 h at 37°C. Black‐coloured colonies indicate the positive formation of biofilm with a dry crystalline consistency.
2.6.3 Antibiofilm assay
The antibiofilm activity of hybrid Chi‐Fe3 O4 nanocomposite was done by using the method of Nithya et al. [44]. The bacterial pathogenic S. marcescens and S. aureus biofilms were grown in LB medium supplemented with 5% sucrose in a 24‐well polystyrene plate at 30°C for 24 h. After the incubation, the contents in the wells were discarded and the plates were washed with phosphate‐buffered saline twice. Further, they were washed with distilled water for the removal of the remaining non‐adhered cells. The adhered immobile cells with biofilms were stained with 0.4% (w/v) crystal violet stain for 5 min and were washed with distilled water. Different concentrations of hybrid Chi‐Fe3 O4 nanocomposite (ranging from 5 to 50 µg/mL) were added to the wells, where untreated well with cells and biofilm served as the control. The plates were incubated for 24 h at 37°C. In total, 1 mL of absolute ethanol was pipetted to each well to dissolve the crystal violet staining the cells and biofilms. The absorbance was noted at 570 nm and the percentage of biofilm inhibition was calculated using the following formula:
| (1) |
2.6.4 Statistical analysis
All the experiments were done in triplicates, and the results were presented as mean ± standard deviation (n = 4). The experimental data were analysed using Statistical Package for the Social Sciences (SPSS) (SPSS version 14. IBM Corporation). P < 0.05 was considered as statistically significant.
3 Results and discussions
3.1 Preparation of Chi‐Fe3 O4 nanocomposite
The development of Fe3 O4 NPs (magnetite NPs) was preliminarily confirmed by the colour change from pale yellow to black colour when kept in a Teflon‐lined stainless‐steel autoclave (see Fig. 1 a). In discussion with the results reported by Kouhbanani et al., [45] the synthesised Fe3 O4 NPs formed a black colour solution. The occurrence of various phytocompounds from T. portulacifolium leaf extract that gives the ability to reduce FeCl2 and FeCl3 to Fe3 o4 NPs
Fig. 1.
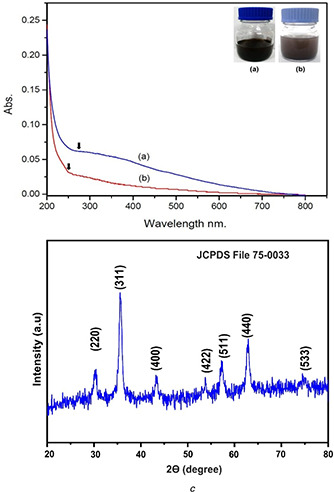
UV‐Vis spectrum of Chi‐Fe3 O4 nanocomposites
(a) Fe3 O4 NPs, (b) Chi‐Fe3 O4 nanocomposites, (c) XRD analysis of Chi‐Fe3 O4 nanocomposites
Buazar et al. [46] studied the mechanism of Fe3 O4 ‐NPs formation using a potato. He showed that extract plays an important role in reducing agents and as capping in the Fe3 O4 ‐NPs formation. The reaction started in addition of NaOH and produced the oxidation of starch in alkaline solution these oxidations produced electrons that reduced Fe+ ions to Fe0 NPs. In the interim, the starch primary hydroxyl groups were oxidised to the carboxyl group. He also showed that the problem of aggregation of NPs in water was overcome as Fe3 O4 ‐NPs dissolved in potato extract easily.
After the addition of chitosan solution with the synthesised Fe3 O4 NPs, a change in colour from black suspension to brown was noted directly in the reaction mixture (see Fig. 1 b). The change of brown colour indicates the formation of Chi‐Fe3 O4 nanocomposite. These results are supported by the work of Nehra et al., [47] who observed similar colour change during the preparation of chitosan‐coated magnetic NPs. The hydroxyl and amine groups of the chitosan is a suitable capping agent for the synthesis of metal NPs and the presence of amine group in chitosan has a strong affinity towards metal ions hence it helps the binding of chitosan to the metal [48].
3.2 Characterisation techniques
3.2.1 UV‐Vis spectroscopy
The UV‐Vis spectrum of both Fe3 O4 NPs and the hybrid Chi‐Fe3 O4 nanocomposite were measured at 200–800 nm range. The absorbance peak characteristic to Fe3 O4 NPs was obtained at 260 nm (see Fig. 1 a). The previous report states the spectral absorbance around 260 nm is a characteristic feature of Fe3 O4 NPs [49]. After the adding of chitosan solution into the synthesised Fe3 O4 NPs, the change in absorbance shift was noticed at 272 nm and the broadening absorbance spectral alterations initially support the surface modifications of FeO NPs [50].
3.2.2 XRD analysis
XRD pattern of the hybrid Chi‐Fe3 O4 nanocomposite is shown in Fig. 1 c. Major diffraction peaks 311, 220, 400, 422, 511 and 440 were noted at the planes 35°, 30°,43°, 53°, 57° and 62°, respectively. This confirms that the magnetite‐Fe3 O4 NPs possess a spinel structure with polycrystalline nature. The 2θ value of the diffraction peak 311 was considered as the confirmation for Fe3 O4 NPs. As reported in various previous literature works, the standard diffraction peak 311 at 35.423° corresponds to for magnetite formation [51]. The obtained XRD analysis data of hybrid Chi‐Fe3 O4 nanocomposite was reliable with standard JCPDS reference code–75‐0033 [15, 28]. The crystallite size of the hybrid Chi‐Fe3 O4 nanocomposite was calculated by the Scherrer ((2)) [52]
| (2) |
where D = average crystallite size, K ‐shape constant, λ– wavelength of X‐ray, β– full width half maximum (FWHM) of refection (in radians) located at 2θ and θ– angle of reflection (in degrees). The crystalline size of the composite was determined to be 7.13 nm, derived from the FWHM of the corresponding peak at 311.
3.2.3 TEM analysis
TEM images of hybrid Chi‐Fe3 O4 nanocomposite were shown in Figs. 2 a and b. The obtained TEM results of hybrid Chi‐Fe3 O4 nanocomposite reveals that most of the particles are spherical in shape with less agglomeration. These also show the polycrystalline nature of the composite with a particle size distribution of 3.78–46.40 nm (see Fig. 2 d). Similar findings were reported by Pati et al. [53] who have synthesised spherical‐shaped Chitosan‐functionalised Fe3 O4 at Au nanomaterials with different size range from 10 to 40 nm.
Fig. 2.
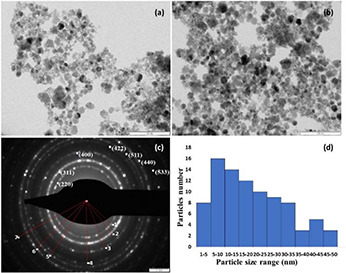
TEM analysis of Chi‐Fe3 O4 nanocomposites
(a) , (b) TEM images of hybrid Chi‐Fe3O4, (c) SAED pattern, (d) Particle size distribution
3.2.4 FE‐SEM and EDS analysis
Surface morphology and elemental composition the of hybrid Chi‐Fe3 O4 nanocomposite was determined by FESEM and energy dispersive spectroscopy, respectively, shown in Figs. 3 a and b. The images attained using FE‐SEM shows the predominantly spherical‐shaped particles with high aggregation, this may be due to the strong inter‐particle's Van der Waals force and magnetic attraction among the Fe3 O4 NPs [54]. The chitosan was strongly bound on the surface of the hybrid Chi‐Fe3 O4 nanocomposite. Similar observations were noticed in the previous report by Unsoy et al. [55].
Fig. 3.

FESEM analysis of Chi‐Fe3 O4 nanocomposites
(a) FESEM image at 3.14 kx magnification, (b) FESEM image at 408 X magnification
In addition, the EDS spectrum of the nanocomposite exhibited the presence of Fe and O, C and Cl (see Fig. 4 b). This clearly states the formation of Fe3 O4 and the absence of any other peaks indicating the purity of hybrid Chi‐Fe3 O4 nanocomposite. The presence of Fe, O and C in the EDS spectrum of chitosan‐coated nanomaterials supports the precursor material in the synthesis of hybrid Chi‐Fe3 O4 nanocomposite. The weak carbon (C) signals produced may be due to the presence of chitosan that encapsulates the Fe3 O4 [56, 57]. The detection of C in the EDS spectrum also supports the presence of chitosan polymer in the prepared nanocomposite. Similar findings were reported by Manikantan et al., [52] who have documented the presence of C in the prepared copper‐chitosan NPs. The functional groups Cl and O signals in the EDS spectrum corresponds to the X‐ray emission from proteins bound to the NPs surface, which were then removed by centrifugation followed by repeated washing of the composite [58].
Fig. 4.
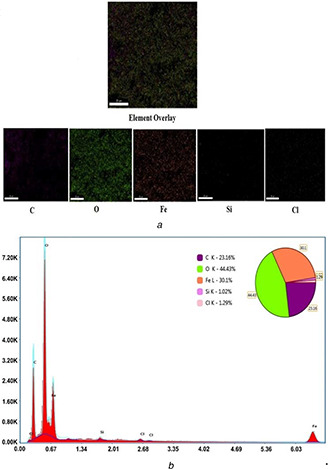
EDS analysis and elemental mapping
(a) Elemental mapping, (b) EDS analysis of Chi‐Fe3 O4 nanocomposites
3.2.5 FTIR analysis
The FTIR spectrum of hybrid Chi‐Fe3 O4 (see Fig. 5) showed sharp peaks (cm−1) 3502.73, 3329.14, 2303.03, 1743.65, 1643.35, 1519.91, 1369.46 and 1215.15 corresponds to O–H stretching vibrations, hydroxy group (H‐bonded OH stretch), N–H stretching (for chitosan), alkyl carbonate, carbonyl groups, aromatic nitro compounds, carboxylate (carboxylic acid salt) stretching and C–C aromatic ring/C–OH stretching vibrations, respectively, attributing to the functional groups from the reduction of NPs. The peaks 636.51 attributes to the iron oxide skeleton, the peaks 597.93, 540.07, 501.49 are characteristic to Fe3 O4 NPs corresponding to the aliphatic Iodo compounds, C–I stretch. In addition 466.77 indicated aryl disulphides (S–S stretch) attribute the intrinsic stretching vibrations of the Fe at a tetrahedral site. In discussion with the works of Nasrollahzadeh et al. (2016) [59] and Sathishkumar et al. [60] similar characteristic peaks were found at the range of 500–600 with the iron oxide skeleton. Zulfikar et al. [61] and Tiwari et al. [62] also showed a similar range of peaks that are characteristic to Fe3 O4 and Chitosan. The increased intensities of N–H vibrations for hybrid Chi‐Fe3 O4 nanocomposite determine the surface coating of chitosan on negatively charged Fe3 O4 NPs, which indicates a strong interaction between the amino group on chitosan molecules and Fe3 O4 NPs.
Fig. 5.
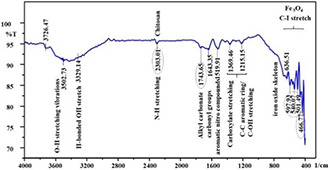
FTIR analysis of Chi‐Fe3 O4 nanocomposites
3.2.6 Zeta potential analysis and DLS studies
The zeta potential measurements for synthesised hybrid Chi‐Fe3 O4 nanocomposite were presented in Figs. 6 a and b). The Zeta potential value of −78.9 mV shows that the surface of the hybrid Chi‐Fe3 O4 nanocomposite is negatively charged, and this constitute interaction of the particles with each other and consequently contributing to a stable particle size of the sample. Our results were similar to the previous report by Banerji et al. [63]. The DLS studies showed the Z average size diameter of 1288.0 nm for hybrid Chi‐Fe3 O4 nanocomposite (see Fig. 6 c). The results of DLS analysis shows the increased size of the nanocomposite in comparison to TEM results, this is because DLS measures the hydrodynamic size of the synthesised NPs [64, 65].
Fig. 6.
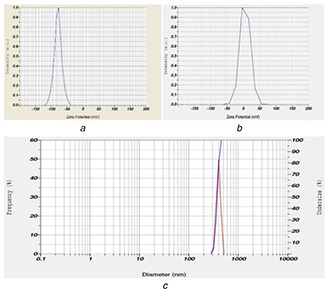
Zeta potential and DLS analysis
(a) Zeta potential analysis of Fe3 O4, (b) Zeta potential analysis of Chi‐Fe3 O4 nanocomposites, (c) DLS studies of Chi‐Fe3 O4 nanocomposites
3.3 MIC and MBC determination
MIC and MBC of hybrid Chi‐Fe3 O4 nanocomposite against S. marcescens and S. aureus were determined to be 47 and 39 µg/mL, respectively (see Table 1). The MIC and MBC results showed the hybrid Chi‐Fe3 O4 nanocomposite were effective against both Escherichia. coli and S. aureus, making it a good nanomaterial for the treatment against pathogenic bacteria.
Table 1.
MIC and MBC Chi‐Fe3 O4 nanocomposites against tested organisms
| Bacterial samples | MIC, µg/mL | MBC, µg/mL |
|---|---|---|
| S. marcescens | 47 | 45 |
| S. aureus | 39 | 36 |
3.4 Antibacterial activity
On evaluating the antibacterial activity of the nanocomposite against the bacterial strains using standard agar well diffusion method, strong antibacterial activity was observed against S. marcescens at 21 mm at the concentration of 100 μL, followed by S. aureus at 13 mm at the concentration of 100 μL. Whereas 10 and 25, 50 μL concentration showed moderate activity of 1, 2 and 11 mm, respectively, against S. marcescens and 0, 4 and 6 mm, respectively, against S. aureus (see Fig. 7).
Fig. 7.
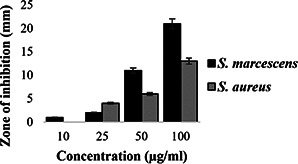
Antibacterial activity of Chi‐Fe3 O4 nanocomposites against S. marcescens, S. aureus
Furthermore, the morphological changes of hybrid Chi‐Fe3 O4 nanocomposite treated and untreated S. marcescens and S. aureus cultures were observed using light microscopy and FESEM analysis (see Fig. 8). At the highest concentration of 100 μL of hybrid Chi‐Fe3 O4 nanocomposite treated against both test, organisms exhibited significant morphological changes of cell membranes, where no morphological changes were observed in the untreated bacterial cultures. The morphological changes on the bacterial membrane surface on treatment with hybrid Chi‐Fe3 O4 nanocomposite (Scheme 1), indicates its ability to break and penetrate the cell membrane. It is also considered that the composite can also damage the DNA and denatured the cellular proteins [66, 67].
Fig. 8.
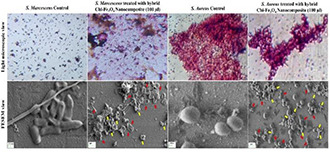
FESEM and Light microscopic view of Chi‐Fe3 O4 nanocomposites treated and control pathogens of S. aureus and S. marcescens
3.5 Congo red method
The Congo red agar method confirmed the formation of biofilms after 48 h indicated by the growth of black colour colonies. 92.20 and 87.32% of the colonies respective to S. marcescens (see Fig. 9 a) and S. aureus (see Fig. 9 b) grown was observed to be black in colour with dry crystalline consistency.
Fig. 9.
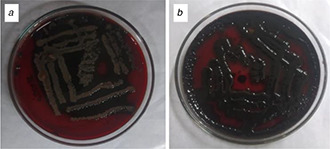
Congo red method of biofilm confirmation
(a) S. marcescens, (b) S. aureus
3.6 Antibiofilm activity
The antibiofilm activity of hybrid Chi‐Fe3 O4 nanocomposite against S. marcescens and S. aureus pathogens was done using the crystal violet assay. After the 24 h of treatment, nanocomposite at the concentration of 5–50 µg/mL resulted in greater inhibition of 72.8–85.5% (see Figs. 10 a and b).
Fig. 10.
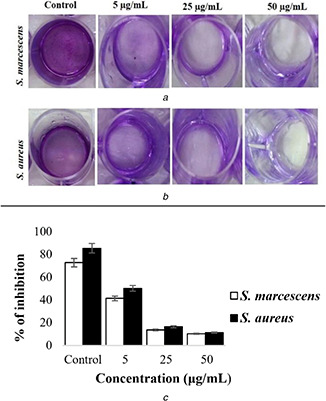
Antibiofilm activity of Chi‐Fe3 O4 nanocomposites against
(a) S. marcescens, (b) S. aureus, (c) % of inhibition of biofilm formation by Chi‐Fe3 O4 nanocomposites
On treating S. marcescens and S. aureus using 5 µg/mL hybrid Chi‐Fe3 O4 nanocomposite significant inhibition of 41.4 and 50.2% was observed, respectively, where 13.7 and 16.4% of inhibition, respectively, was observed when the nanocomposite concentration increased to 20 µg/mL. At a concentration of 50 μg/mL nanocomposite treatment on the pathogens for 24 h, only 10 and 11% bacterial colonies were observed. Hence it is reasonable that the biofilm inhibition activity of hybrid Chi‐Fe3 O4 nanocomposite directly proportional to the concentration used (see Fig. 10). The previous reports also showed similar activity on the increase of the respective materials [68].
4 Conclusion
In conclusion, experimental results showed that the hybrid Chi‐Fe3 O4 nanocomposite prepared using solvothermal‐assisted green synthesis method possesses significant physical and chemical properties, with potential antibacterial and antibiofilm nature. Thereby, opening doors for the use of this hybrid Chi‐Fe3 O4 nanocomposite for several biological and medical applications like antimicrobial formulations, packaging of food materials, water treatment and so on. In future, hybrid Chi‐Fe3 O4 nanocomposite can be considered as a substantial material to treat rapidly increasing antibiotic‐resistant pathogens.
5 Acknowledgments
The authors would like to thank Department of Science and Technology (DST‐FIST), India for the grants provided (Grant No. DST‐FIST/120/2012) to establish laboratory facilities at Department of Biotechnology Kongunadu Arts and Science College, Coimbatore, Tamilnadu, India. The authors gratefully acknowledge CNR RAO Research centre, Avinasilingam Institute for Home science and Higher Education for Women for the XRD, EDS, FESEM and FTIR analysis. We also extend our thanks to SAIF, IIT Bombay, India for the TEM analysis. P.S., S.B., V.J., K.J., G.Y., D.S.R.S.K. contributed equally to this work.
6 References
- 1. Revia R.A., Zhang M.: ‘Magnetite nanoparticles for cancer diagnosis, treatment and treatment monitoring: recent advances’, Mater. Today, 2016, 19, pp. 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ling D., Lee N., Hyeon T.: ‘Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications’, Acc. Chem. Res., 2015, 48, pp. 1276–1285 [DOI] [PubMed] [Google Scholar]
- 3. Seabra A.B., Haddad P., Duran N.: ‘Biogenic synthesis of nanostructured iron compounds: applications and perspectives’, IET Nanobiotechnol., 2013, 7, (3), pp. 90–99 [DOI] [PubMed] [Google Scholar]
- 4. Pérez N., Moya C., Tartaj P. et al.: ‘Aggregation state and magnetic properties of magnetite nanoparticles controlled by an optimized silica coating’, J. Appl. Phys., 2017, 121, p. 044304 [Google Scholar]
- 5. Gavilán H., Posth H., Bogart O. et al.: ‘How shape and internal structure affect the magnetic properties of anisometric magnetite nanoparticles’, Acta Mater., 2017, 15, pp. 416–424 [Google Scholar]
- 6. Easo S.L., Mohanan P.V.: ‘Dextran stabilized iron oxide nanoparticles: synthesis, characterization and in vitro studies’, Carbohydr. Polym., 2013, 92, (1), pp. 726–732 [DOI] [PubMed] [Google Scholar]
- 7. Lu Y., Yin Y., Mayers B.T. et al.: ‘Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol–gel approach’, Nano Lett., 2002, 2, (3), pp. 183–186 [Google Scholar]
- 8. Hufschmid R., Arami H., Ferguson R.M. et al.: ‘Synthesis of phase‐pure and monodisperse iron oxide nanoparticles by thermal decomposition’, Nanoscale, 2015, 7, (25), pp. 11142–11154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ge S., Shi X., Sun K. et al.: ‘Facile hydrothermal synthesis of iron oxide nanoparticles with tunable magnetic properties’, J. Phys. Chem. C, 2009, 113, (31), pp. 13593–13599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jamshidiyan M., Shirani A.S., Alahyarizadeh G.H.: ‘Solvothermal synthesis and characterization of magnetic Fe3 O4 nanoparticle by different sodium salt sources’, Mater. Sci., 2017, 35, pp. 50–57 [Google Scholar]
- 11. Solano E., Perez‐Mirabet L., Martinez‐Julian F. et al.: ‘Facile and efficient one‐pot solvothermal and microwave‐assisted synthesis of stable colloidal solutions of MFe2O4 spinel magnetic nanoparticles’, J. Nanopart. Res., 2012, 14, (8), p. 1034 [Google Scholar]
- 12. Kataria N., Garg V.K.: ‘Green synthesis of Fe3 O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: regeneration and mechanism’, Chemosphere, 2018, 208, pp. 818–828 [DOI] [PubMed] [Google Scholar]
- 13. Cai Y., Shen Y., Xie A. et al.: ‘Green synthesis of soya bean sprouts‐mediated superparamagnetic Fe3 O4 nanoparticles’, J. Magn. Magn. Mater., 2010, 322, (19), pp. 2938–2943 [Google Scholar]
- 14. Yew Y.P., Shameli K., Miyake M. et al.: ‘Green synthesis of magnetite (Fe3 O4) nanoparticles using seaweed (kappaphycus alvarezii) extract’, Nanoscale Res. Lett., 2016, 11, (1), p. 276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arokiyaraj S., Saravanan M., Prakash N.U. et al.: ‘Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. Leaf extract: an in vitro study’, Mater. Res. Bull., 2013, 48, (9), pp. 3323–3327 [Google Scholar]
- 16. Venkateswarlu S., Kumar B.N., Prathima B. et al.: ‘A novel green synthesis of Fe3 O4 ‐Ag core shell recyclable nanoparticles using Vitis vinifera stem extract and its enhanced antibacterial performance’, Physica B: Condens. Matter., 2015, 457, pp. 30–35 [Google Scholar]
- 17. Park Y., Hong Y.N., Weyers A. et al.: ‘Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles’, IET Nanobiotechnol., 2011, 5, (3), pp. 69–78 [DOI] [PubMed] [Google Scholar]
- 18. Zhang Z., Li L., Liu C. et al.: ‘Solvothermal synthesis of mesoporous Fe3 O4 nanoparticles in mixed solvent of ethylene glycol and water: structure and magnetic properties’, J. Supercond. Nov. Magn., 2016, 32, p. 757 [Google Scholar]
- 19. Thalapaneni N.R., Chidambaram K.A., Ellappan T. et al.: ‘Inhibition of carbohydrate digestive enzymes by Talinum portulacifolium (forssk) leaf extract’, J. Complement. Integr. Med., 2008, 5, pp. 1553–3840 [Google Scholar]
- 20. Babu R.K., Vinay K., Sameena S.K. et al.: ‘Antihyperglycemic and antioxidant effects of Talinum portulacifolium leaf extracts in streptozotocin diabetic rats: A dose‐dependent study’, Pharmacogn. Mag., 2009, 5, pp. 1–10 [Google Scholar]
- 21. Mohapatra S., Mallick S.K., Maiti T.K. et al.: ‘Synthesis of highly stable folic acid conjugated magnetite nanoparticles for targeting cancer cells’, Nanotechnology, 2007, 18, p. 385102 [Google Scholar]
- 22. Kmetz A.A., Becker M.D., Lyon B.A. et al.: ‘Improved mobility of magnetite nanoparticles at high salinity with polymers and surfactants’, Energy Fuel, 2016, 30, pp. 1915–1926 [Google Scholar]
- 23. Muxika A., Etxabide A., Uranga J. et al.: ‘Chitosan as a bioactive polymer: processing, properties and applications’, Int. J. Biol. Macromol., 2017, 105, pp. 1358–1368 [DOI] [PubMed] [Google Scholar]
- 24. Ng W.L., Yeong W.Y., Nain M.W.: ‘Polyelectrolyte gelatin‐chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering’, Int. J. Bioprinting, 2016, 2, pp. 53–62 [Google Scholar]
- 25. Xing K., Zhu X., Peng X. et al.: ‘Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review’, Agron. Sustain. Dev., 2015, 35, pp. 569–588 [Google Scholar]
- 26. Senthilkumar P., Surendran L., Sudhagar B. et al.: ‘Hydrothermal assisted Eichhornia crassipes mediated synthesis of magnetite nanoparticles (E‐Fe3 O4) and its antibiofilm activity’, Mater. Res. Express, 2019, 6, p. 095405 [Google Scholar]
- 27. Illés E., Tombácz E., Szekeres M. et al.: ‘Novel carboxylated PEG‐coating on magnetite nanoparticles designed for biomedical applications’, J. Magn. Magn. Mater., 2015, 380, pp. 132–139 [Google Scholar]
- 28. Arakha M., Pal S., Samantarrai D. et al.: ‘Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle‐bacteria interface’, Sci. Rep., 2015, 5, p. 14813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parandhaman T., Pentela T., Ramalingam B. et al.: ‘Metal nanoparticle loaded magnetic‐chitosan microsphere: water dispersible and easily separable hybrid metal nano‐biomaterial for catalytic applications’, ACS Sustainable Chem. Eng., 2016, 5, pp. 489–501 [Google Scholar]
- 30. Ding Y., Shen S.Z., Sun H. et al.: ‘Design and construction of polymerized‐chitosan coated Fe3 O4 magnetic nanoparticles and its application for hydrophobic drug delivery’, Mater. Sci. Eng. C, 2015, 48, pp. 487–498 [DOI] [PubMed] [Google Scholar]
- 31. Vaghari H., Jafarizadeh‐Malmiri H., Mohammadlou M. et al.: ‘Application of magnetic nanoparticles in smart enzyme immobilization’, Biotechnol. Lett., 2016, 38, pp. 223–233 [DOI] [PubMed] [Google Scholar]
- 32. Khalkhali M., Sadighian S., Rostamizadeh K. et al.: ‘Synthesis and characterization of dextran coated magnetite nanoparticles for diagnostics and therapy’, BioImpacts: BI, 2015, 5, p. 141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Senthilkumar P., Yaswant G., Kavitha S. et al.: ‘Preparation and characterization of hybrid chitosan‐silver nanoparticles (chi‐Ag NPs); A potential antibacterial agent’, Int. J. Biol. Macromol., 2019, 141, pp. 290–298 [DOI] [PubMed] [Google Scholar]
- 34. Sharma V.K., Johnson N., Cizmas L. et al.: ‘Review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes’, Chemosphere, 2016, 1, pp. 702–714 [DOI] [PubMed] [Google Scholar]
- 35. Blair J.M., Webber M.A., Baylay A.J. et al.: ‘Molecular mechanisms of antibiotic resistance’, Nat. Rev. Microbiol., 2015, 13, p. 42 [DOI] [PubMed] [Google Scholar]
- 36. Blanco P., Hernando‐Amado S., Reales‐Calderon J. et al.: ‘Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants’, Microorganisms., 2016, 4, p. 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Høiby N., Bjarnsholt T., Givskov M. et al.: ‘Antibiotic resistance of bacterial biofilms’, Int. J. Antimicrobiol. Agents, 2010, 35, pp. 322–332 [DOI] [PubMed] [Google Scholar]
- 38. Fabres‐Klein M.H., Santos M.J., Klein R.C. et al.: ‘An association between milk and slime increases biofilm production by bovine Staphylococcus aureus ’, BMC Vet. Res., 2015, 11, p. 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dufour D., Leung V., Lévesque C.M.: ‘Bacterial biofilm: structure, function, and antimicrobial resistance’, Endod. Topics, 2010, 22, pp. 2–16 [Google Scholar]
- 40. Markovíc D., Deeks C., Nunney T. et al.: ‘Antibacterial activity of Cu‐based nanoparticles synthesized on the cotton fabrics modified with polycarboxylic acids’, Carbohydrates, 2018, 200, pp. 173–182 [DOI] [PubMed] [Google Scholar]
- 41. Fereshteh F., Jebela S., Almasib H.: ‘Morphological, physical, antimicrobial and release properties of ZnO nanoparticles‐loaded bacterial cellulose films’, Carbohydrates, 2016, 149, pp. 8–19 [DOI] [PubMed] [Google Scholar]
- 42. Wayane P.: ‘Performance standard for antimicrobial susceptibility testing, clinical and laboratory standards institute (CLSI)’. 26th Informational supplement, Wayne, PA, USA, 2016, vol. 33, p. M100‐S23 [Google Scholar]
- 43. Freeman D.J., Falkiner F.R., Keane C.Y.: ‘New method for detecting slime production by coagulase negative staphylococci’, J. Clin. Pathol., 1989, 42, pp. 872–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nithya C., Devi M.G., Karutha Pandian S.: ‘A novel compound from the marine bacterium Bacillus pumilus S6‐15 inhibits biofilm formation in gram‐positive and gram‐negative species’, Biofouling, 2011, 27, pp. 519–528 [DOI] [PubMed] [Google Scholar]
- 45. Kouhbanani M.A.J., Beheshtkhoo N., Amani A.M. et al.: ‘Green synthesis of iron oxide nanoparticles using Artemisia vulgaris leaf extract and their application as a heterogeneous Fenton‐like catalyst for the degradation of methyl orange’, Mater. Res. Express, 2018, 5, p. 115013 [Google Scholar]
- 46. Buazar F., Baghlani‐Nejazd M.H., Badri M. et al.: ‘Facile one‐pot phytosynthesis of magnetic nanoparticles using potato extract and their catalytic activity’, Starch‐Starke, 2016, 68, pp. 796–804 [Google Scholar]
- 47. Nehra P., Chauhan R.P., Garg N. et al.: ‘Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles’, Br. J. Biomed. Sci., 2018, 75, pp. 13–18 [DOI] [PubMed] [Google Scholar]
- 48. Jayakumar R., Menon D., Manzoor K. et al.: ‘Biomedical applications of chitin and chitosan‐based nanomaterials – A short review’, Carb. Pol., 2010, 82, pp. 227–232 [Google Scholar]
- 49. Awwad A.M., Salem N.M.: ‘A green and facile approach for synthesis of magnetite nanoparticles’, J. Nanosci. Nanotechnol., 2016, 2, pp. 208–213 [Google Scholar]
- 50. Elsherbini A.A., Saber M., Aggag M. et al.: ‘Laser and radiofrequency‐induced hyperthermia treatment via gold‐coated magnetic nanocomposites’, Int. J. Nanomed., 2011, 6, p. 2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Inbaraj B.S., Tsai T.Y., Chen B.H.: ‘Synthesis, characterization and antibacterial activity of superparamagnetic nanoparticles modified with glycol chitosan’, Sci. Technol. Adv. Mater., 2012, 13, p. 015002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manikantan J., Ramalingam H.B., Shekar B.C. et al.: ‘Physical and optical properties of HfO2 NPs–synthesis and characterization in finding its feasibility in opto‐electronic devices’, Adv. Powder. Technol., 2017, 28, pp. 1636–1646 [Google Scholar]
- 53. Pati S.S., Singh L.H., Guimarães E.M. et al.: ‘Magnetic chitosan‐functionalized Fe3 O4 @ Au nanoparticles: synthesis and characterization’, J. Alloys Compd., 2016, 684, pp. 68–74 [Google Scholar]
- 54. Takai Z.I., Mustafa M.K., Asman S.: ‘Preparation of high‐performance conductive polyaniline magnetite (PANI/Fe3 O4) nanocomposites by sol‐gel method’, Asian J. Chem., 2018, 30, pp. 2625–2630 [Google Scholar]
- 55. Unsoy G., Yalcin S., Khodadust R. et al.: ‘Synthesis optimization and characterization of chitosan‐coated iron oxide nanoparticles produced for biomedical applications’, J. Nanopart. Res., 2012, 14, p. 964 [Google Scholar]
- 56. Tokarek K., Hueso J.L., Kuśtrowski P. et al.: ‘Green synthesis of chitosan‐stabilized copper nanoparticles’, Eur. J. Inorg. Chem., 2013, 28, pp. 4940–4947 [Google Scholar]
- 57. Jayaseelan C., Ramkumar R., Rahuman A.A. et al.: ‘Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity’, Ind. Crop. Prod., 2013, 45, pp. 423–429 [Google Scholar]
- 58. Senthilkumar P., Kumar D.R., Sudhagar B. et al.: ‘Seagrass‐mediated silver nanoparticles synthesis by Enhalus acoroides and its α‐glucosidase inhibitory activity from the gulf of mannar’, J. Nanostructure Chem., 2016, 6, pp. 275–280 [Google Scholar]
- 59. Nasrollahzadeh M., Atarod M., Sajadi S.M.: ‘Green synthesis of the Cu/Fe3 O4 nanoparticles using Morinda morindoides leaf aqueous extract: a highly efficient magnetically separable catalyst for the reduction of organic dyes in aqueous medium at room temperature’, Appl. Surf. Sci., 2016, 364, pp. 636–644 [Google Scholar]
- 60. Sathishkumar G., Logeshwaran V., Sarathbabu S. et al.: ‘Green synthesis of magnetic Fe3 O4 nanoparticles using Couroupita guianensis aubl. Fruit extract for their antibacterial and cytotoxicity activities’, Artif. Cell. Nanomed. Biotechnol., 2018, 46, pp. 589–598 [DOI] [PubMed] [Google Scholar]
- 61. Zulfikar M.A., Afrita S., Wahyuningrum D. et al.: ‘Preparation of Fe3 O4 ‐chitosan hybrid nano‐particles used for humic acid adsorption, environ’, Nanotechnol. Monit. Manage., 2016, 6, pp. 64–75 [Google Scholar]
- 62. Tiwari I., Singh M., Pandey C.M. et al.: ‘Electrochemical genosensor based on graphene oxide modified iron oxide–chitosan hybrid nanocomposite for pathogen detection’, Sensor. Actuators B, 2015, 206, pp. 276–283 [Google Scholar]
- 63. Banerji B., Pramanik S.K., Mandal S. et al.: ‘Synthesis, characterization and cytotoxicity study of magnetic (Fe3 O4) nanoparticles and their drug conjugate’, RSC Adv., 2012, 2, pp. 2493–2497 [Google Scholar]
- 64. Berne B.J., Pecora R.: ‘Dynamic light scattering: with applications to chemistry, biology, and physics’ (Courier Dover Publications, New York, Mineola, 2000) [Google Scholar]
- 65. Gittings M.R., Saville D.A.: ‘The determination of hydrodynamic size and zeta potential from electrophoretic mobility and light scattering measurements’, Colloids Surf. A, 1998, 141, pp. 111–117 [Google Scholar]
- 66. Slavin Y.N., Asnis J., Häfeli U.O. et al.: ‘Metal nanoparticles: understanding the mechanisms behind antibacterial activity’, J. Nanobiotechnol., 2017, 15, p. 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Auffan M., Rose J., Wiesner M.R. et al.: ‘Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro’, Environ. Pollut., 2009, 157, pp. 1127–1133 [DOI] [PubMed] [Google Scholar]
- 68. Agarwala M., Choudhury B., Yadav R.N.: ‘Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogens’, Indian J. Microbiol., 2014, 54, pp. 365–368 [DOI] [PMC free article] [PubMed] [Google Scholar]


