Abstract
High‐quality colloidal silver nanoparticles (AgNP) were synthesised via a green approach by using hydroalcoholic extracts of Malva sylvestris. Silver nitrate was used as a substrate ion while the plant extract successfully played the role of reducing and stabilising agents. The synthesised nanoparticles were carefully characterised by using transmission electron microscopy, atomic‐force microscopy, energy dispersive X‐ray spectroscopy, Fourier transform infrared spectroscopy and UV–vis spectroscopy. The maximum absorption wavelengths of the colloidal solutions synthesised using 70 and 96% ethanol and 100% methanol, as extraction solvents, were 430, 485 and 504 nm, respectively. Interestingly, the size distribution of nanoparticles depended on the used solvent. The best particle size distribution belonged to the nanoparticles synthesised by 70% ethanol extract, which was 20–40 nm. The antibacterial activity of the synthesised nanoparticles was studied on Escherichia coli, Staphylococcus aureus and Streptococcus pyogenes using disk diffusion, minimum inhibitory concentrations and minimum bactericidal concentrations assays. The best antibacterial activity obtained for the AgNPs produced by using 96% ethanolic extract.
Inspec keywords: silver, nanoparticles, nanofabrication, antibacterial activity, colloids, particle size, transmission electron microscopy, atomic force microscopy, X‐ray chemical analysis, Fourier transform spectra, infrared spectra, ultraviolet spectra, visible spectra, microorganisms, nanomedicine, biomedical materials
Other keywords: Green synthesis, flower extract, Malva sylvestris, antibacterial activity, high‐quality colloidal silver nanoparticles, hydroalcoholic extracts, plant extract, reducing agents, stabilising agents, transmission electron microscopy, atomic‐force microscopy, energy dispersive X‐ray spectroscopy, Fourier transform infrared spectroscopy, UV– vis spectroscopy, colloidal solutions, particle size distribution, Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes, disk diffusion, minimum inhibitory concentrations, minimum bactericidal concentrations assays, ethanolic extract, size 430 nm, size 485 nm, size 504 nm, size 20 nm to 40 nm, Ag
1 Introduction
Nanotechnology is science, engineering and technology conducted at the nanoscale, which is about 1–100 nm. Nanoscience and nanotechnology are the study and application of extremely small particles and can be used across all other scientific fields such as chemistry, biology, physics, materials science, and engineering [1]. Nanomaterials manifest extremely fascinating and useful properties, which can be exploited for a variety of structural and non‐structural applications [2].
Silver nanoparticles (AgNPs) can be synthesised by several physical, chemical and biological methods [3]. One such promising process is green synthesis [4]. AgNPs received the highest attention for commercialisation as 55.4% of the total nanomaterial‐based products available on the market (313, out of 565 products) are produced based on the AgNPs [5]. Unique antimicrobial properties of AgNPs have attracted great attention for developing biomedical devices such as wound dressings [6], surgical instruments and bone substitute materials [7].
In this study, a biological method was used for the synthesis of nanoparticles [8]. Plants provide a better platform for the synthesis of nanoparticles as they are free from toxic chemicals and provide natural capping agents [9]. Moreover, the use of plant extracts also considerably reduces the cost of microorganism's isolation and cell culture required for nanoparticles synthesis [10]. Sometimes, the synthesis of nanoparticles using various plants and their extracts can be advantageous over other biological synthesis processes, which involve very complex procedures of maintaining microbial cultures [11]. The synthesis of nanoparticles using plant extracts is the most adopted method for green and eco‐friendly production of nanoparticles with the special advantage that the plants are widely distributed, easily available, much safer to handle and act as a source of several metabolites [12]. The shape and size of the prepared nanoparticles using plants can be modulated by changing the type of plant, organic solvent, concentration of extract and AgNO3, pH and temperature [13]. As the plant tissues contain various metabolites with the ability to act as reducing and capping agents in the synthesis reaction of metal nanoparticles, they seem to be one of the best alternatives instead of the previous methods for large‐scale synthesis of nanoparticles [14]. The main mechanism considered for the process is plant‐assisted reduction due to the phytochemicals. The main phytochemicals involved are terpenoids, flavones, ketones, aldehydes, amides, and carboxylic acids [9]. Flavones, organic acids, and quinones are water‐soluble phytochemicals that are responsible for the immediate reduction of ions [14]. The reduction of metal ions using plants extract has been found to be much faster as compared to microorganisms, and formation of high colloidal metal nanoparticles has been reported, recently [15]. Malva sylvestris is a species of the mallow genus, Malva, in the family of Malvaceae, originated from Western Europe, North Africa and Asia through the English speaking world [16]. The extract of Malta sylvestris contains high amounts of phenols and flavonoids [17]. Quercetin, Malvidin 3‐glucoside and Scopoletin are three important components in this plant (see Fig. 1 a) [18]. The ability of Malta sylvestris extracts for synthesis of high‐quality AgNPs with appropriate antibacterial activity was evaluated in this study.
Fig. 1.
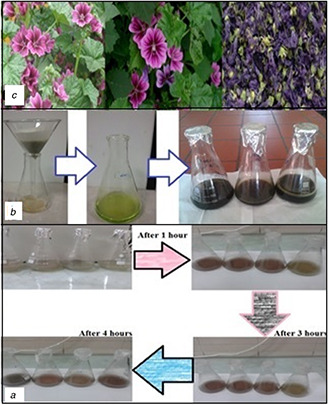
AgNP synthesis process
(a) Rapid change of the reaction color over time, (b) Preparation of mallow extracts, (c) Mallow flowers
2 Materials and methods
2.1 Preparation of plant extract
The Malva sylvestris’ extracts were prepared by suspending 30 g of dried flower powder in 200 ml of the solvent (96% ethanol, 70% ethanol or absolute methanol) following by sonication for 1 h, placing in the dark for 2 days, and finally filtering through Whatman No.1 filter paper and storing in −20°C before use [19]. The prepared extracts were used in separate experiments for green synthesis of AgNPs (see Fig. 1 b).
2.2 Synthesis of AgNPs
The stock solution of AgNO3 (50 mM) was prepared and used in the reactions. Different concentrations of AgNO3 were added to different flasks containing extracts and water for bio‐reduction. The addition of the aqueous AgNO3 solution to the extracts resulted in the change of colour within hours and the formation of AgNPs with its signatory colour. The bio‐reduction of Ag+ ions was monitored using UV–vis spectrophotometer by regular sampling within hours after addition of AgNO3. In order to optimise the synthesis reaction of AgNPs and to obtain stable colloidal nanoparticles with appropriate sizes and shapes, various parameters including extracts concentration, silver nitrate concentration, temperature and light were investigated in the separate reactions. The effect of extract solvent (methanol or ethanol), different amounts of extracts and various concentrations of silver nitrate were also investigated (see Table 1). The initial reactions were carried out at room temperature (25°C) under visible light. After careful investigation, it was found that the light is the most important factor and, in fact, the only factor driving these reactions so that the changes in temperature and pH could not affect the progress of the reaction, considerably. Thus, the effects of the nature of light on the synthesis of AgNPs were also studied.
Table 1.
Different compositions and reaction conditions used in the synthesis of AgNPs
| Extract type | Extract, ml | H2 O, ml | AgNO3, mM | Total volume |
|---|---|---|---|---|
| 96% ethanol | 0.25 | 19.50 | 0.25 | 20 |
| 96% ethanol | 0.50 | 19.00 | 0.50 | 20 |
| 96% ethanol | 0.75 | 18.50 | 0.75 | 20 |
| 96% ethanol | 1.00 | 18.00 | 1.00 | 20 |
| 70% ethanol | 0.25 | 19.50 | 0.25 | 20 |
| 70% ethanol | 0.50 | 19.00 | 0.50 | 20 |
| 70% ethanol | 0.75 | 18.50 | 0.75 | 20 |
| 70% ethanol | 1.00 | 18.00 | 1.00 | 20 |
| methanol | 0.25 | 19.50 | 0.25 | 20 |
| methanol | 0.50 | 19.00 | 0.50 | 20 |
| methanol | 0.75 | 18.50 | 0.75 | 20 |
| methanol | 1.00 | 18.00 | 1.00 | 20 |
2.3 Characterisation of AgNPs
Characterisation of nanoparticles was performed by using UV–vis spectroscopy, transmission electron microscopy (TEM), atomic‐force microscopy (AFM) and energy dispersive X‐ray spectroscopy (EDX). The rate of synthesis of nanoparticles at different reaction conditions was evaluated using Biocrom Biowave UV–vis spectrophotometer in the range between 200 and 800 nm at room temperature. Philips CM30‐150 TEM was used for direct imaging of the nanomaterials to obtain quantitative information about the particle and/or grain size, size distribution and morphology. Three‐dimensional characterisation of nanoparticles with sub‐nanometre resolution, and qualitative and quantitative information on many physical properties including size, morphology, surface texture and roughness were obtained by Dual Scope C‐26 AFM from DME Company. JASCO 6300 FTIR (Fourier transform infrared spectroscopy) was used to investigate the presence of functional groups on the surface of AgNPs and compared with the functional groups of the plant extracts. EDX (Croan 2012 JASCO EDX) was also used to confirm the presence of the related metal signals and to further investigate the chemical composition of nanoparticles.
2.4 Antibacterial activity assay
The antimicrobial activity of AgNPs was investigated against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Streptococcus pyogenes (S. pyogenes) using the disk diffusion assay. To this aim, the sterilised Whatman filter paper (No.1) discs of 6 mm diameter were impregnated with 20 μl of different colloidal solutions of SNSs. Each bacterial strain was swabbed uniformly onto individual plates. The impregnated disks were placed on the plates and after incubation at 37°C for 24 h, the diameter of inhibition zone was measured. Extracts were used individually as control.
3 Results and discussion
3.1 Visible observation for synthesis of SNSs
The reaction rate, reduction and stabilisation properties are important factors in nanoparticles synthesis. The synthesised nanoparticles, according to the size and surface plasmon resonance properties, showed different colours.
The concentration of substrate and plant extract, temperature, light and type of the extracts are important factors that should be considered in this study. By managing these items, the size and shape of nanoparticles can be controlled. Different experiments were conducted by using the extracts in order to investigate the effect of type of extract on the reaction rate and synthesis of SNSs. Time‐dependent monitoring of the colour of reactions and UV–vis spectroscopic analysis revealed that the reduction rate in the presence of 96% ethanol extract was higher than 70% ethanol and methanol extracts because changing the colour from milky to brown in the reactions containing 96% ethanol extract occurred significantly faster than the other extracts (see Fig. 1 c). The variation of maximum absorption wavelength versus time was evaluated in order to determine the optimum time for completeness of reactions. In fact, when the maximum wavelength was fixed and did not change, the synthesis reaction was completed, and nanoparticles were stable. A set of reactions containing various volumes of each extract was conducted and monitored using the UV–vis spectroscopy (see Table 1). 4 h after addition of silver nitrate, UV absorption peak was maximum and, as mentioned, the reaction rate was considerably faster in the presence of 96% ethanol extract.
By increasing the concentration of extracts, the rate of reaction was increased but aggregation was also observed. It seems that after the addition of silver nitrate, the organic compounds of the extract did not have enough time to stabilise the nanoparticles, and coarse particles were achieved. The intensity of the absorption peak of nanoparticles synthesised by 70% ethanol extract was high, and its maximum wavelength was lower than the other two extracts. Due to the surface plasmon resonance property of nanoparticles, we concluded that nanoparticles synthesised with 70% ethanol extract had greater stability and smaller size distribution, too. The spectral results showed that the size distribution of the particles synthesised by 96% ethanol extract is less than the particle size distribution obtained by methanol extract. After several experiments in different concentrations (see Table 1), the optimum concentration for the synthesis of nanoparticles was obtained. Maximum wavelength of three extracts was in the range of 400–600 nm. Four hours after adding silver nitrate to the solutions in different optimum concentrations, maximum absorption spectrum of the synthesised nanoparticles in the presence of 70% ethanol extract reached 430 nm (see Fig. 2), maximum wavelength at 96% ethanol extract reached about 480–490 nm (see Fig. 3) and a peak wavelength of 504 nm was obtained for the methanol extract (see Fig. 4). In the presence of methanol extract, absorption intensity was lower and had a wider peak. The results of UV–vis spectroscopy suggest that, due to the nature of surface plasmon resonance of the synthesised particles and their absorption peak, the size distribution of nanoparticles in the presence of 70% ethanol extract is narrow, and more stable nanoparticles than nanoparticles in 96% ethanol extract are obtained. Nanoparticles obtained by methanol extract are bigger and less stable than nanoparticles synthesised using 70% ethanol and methanol extract.
Fig. 2.
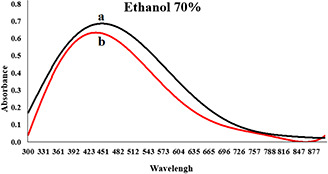
Absorption spectrum of colloidal solution of synthesised AgNPs in the presence of 5.00 ml 70% ethanol extract, 5.00 ml of silver nitrate (50 mM) and water (the total volume is 20 ml)
(a) Second hours and (b) Forth hours
Fig. 3.
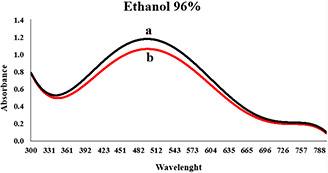
Absorption spectrum of colloidal solution of synthesised AgNPs in the presence of 5.00 ml 96% ethanol extract, 5.00 ml of silver nitrate (50 mM) and water (the total volume is 20 ml)
(a) Second hours and (b) Forth hours
Fig. 4.
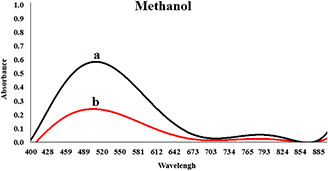
Synthesised AgNPs in the presence of 5.00 ml methanol extract, 5.00 ml of silver nitrate(50 mM) and water (the total volume is 20 ml)
(a) Second hours and (b) Forth hours
3.2 Fourier transmission infrared spectroscopy
The results of FTIR of the pure extracts indicate the presence of different functional groups such as carbon monoxide, hydrogen nitride, fats and biomolecules. In other words, spectroscopy was used to study the surface chemistry of nanoparticles. Band in 3400–3600 cm−1 correspond to the hydrogen stretching of alcohols and phenols while the band in 2850–3000 cm−1 attributed to the vibrational frequency of CH2. The band in 1450–1470 cm−1 is due to the vibrational frequency of CH3 and the band located in 1045–1055 cm−1 correspond to the vibrational frequency of CO. Indeed, during the growth stage, biomolecules, especially proteins and metabolites such as terpenoids, not only effectively participate in the reduction of silver ions but also surround them and prevented them from agglomeration. FTIR spectra of AgNPs showed high similarity with the spectra of the pure extracts, and there was no excess peak in the range of 500–540, corresponding to the metal ions of silver. This showed the fact that all the silver ions participated in the synthesis of the nanoparticle. This test was performed for 70% ethanol extracts (see Fig. 5 a), 96% ethanol extracts (see Fig. 5 b) and methanol extracts (see Fig. 5 c), and the aforementioned results were approved for all of them.
Fig. 5.
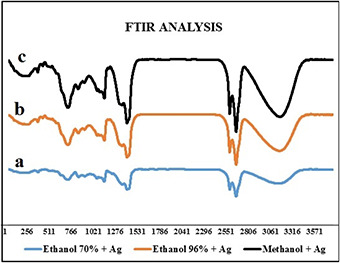
FTIR spectra of the AgNPs synthesised in the presence of extracts
(a) Ethanol 70%, (b) Ethanol 96%, (c) Methanol
3.3 Transmission electron microscope
The size of nanoparticles synthesised by using 70% ethanol extract was about 20–40 nm (Fig. 6). Nanoparticles were spherical and hexagonal with acceptable scattering and diffraction, and there was no aggregation and the size distribution of particles in the colloid solution was acceptable. In the presence of 96% ethanol extract, the size of nanoparticles was about 70–80 nm and larger nanoparticles with wide size distribution were obtained in the form of aggregates (see Fig. 7), compared to 70% ethanol extract. In the colloidal nanoparticles synthesised using methanol extract displayed large distribution size in the range of 90–100 nm (see Fig. 8) due to the significant aggregation. Due to the properties and surface plasmon absorption of the nanoparticles, particle size and absorption could be related to each other directly. In fact, regarding the UV–vis spectra of three extracts and their TEM tests, the obtained results can be related to each other.
Fig. 6.
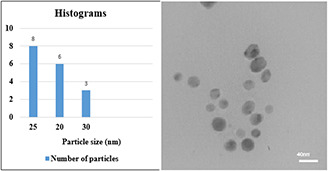
TEM image of nanoparticles synthesised using 70% ethanol extract
Fig. 7.
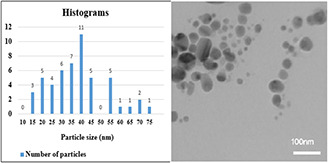
TEM image of nanoparticles synthesised using 96% ethanol extract
Fig. 8.
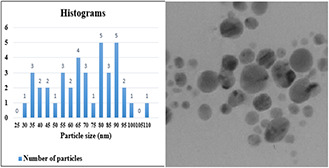
TEM image of nanoparticles synthesised using methanol extract
3.4 Atomic force microscope
AFM imaging was used to further confirm the results obtained from TEM. The obtained particle size and particle arrangement with AFM for a colloidal solution of 70% ethanol extract have coordination and consistency. The nanoparticles displayed spherical shape with appropriate dispersity and acceptable size distribution (see Fig. 9). The AFM images of nanoparticles synthesised using 96% ethanol extract (see Fig. 10) showed larger size and more aggregation than the former nanoparticles. Based on the AFM results, the nanoparticles synthesised using methanol extract displayed the largest size and highest aggregation (see Fig. 11). The overall results indicated the significant effect of the type of plant extract on the physical properties of nanoparticles especially size and colloidal stability.
Fig. 9.
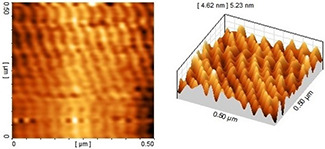
AFM image of nanoparticles synthesised using 70% ethanol extract
Fig. 10.
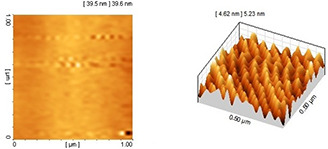
AFM image of nanoparticles synthesised using 96% ethanol extract
Fig. 11.
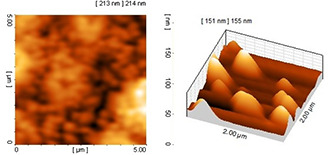
AFM image of nanoparticles synthesised using methanol extract
3.5 Energy dispersive analysis by X‐rays (EDX)
EDX analysis clearly showed the presence of a metallic silver signal in the samples containing synthesised AgNPs. The results indicated the successful conversion of silver ions to nanometal particles (see Fig. 12).
Fig. 12.
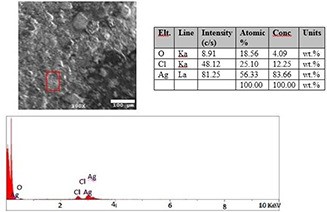
EDX spectra of AgNPs synthesised using extract of Malva sylvestris
3.6 Antibacterial assay of AgNPs
The results of antibacterial properties of nanoparticles are presented in Table 2. At first look, it shows that the extracts have only a minor antibacterial effect. However, all of the synthesised AgNPs exhibited appropriate antibacterial properties. Among them, AgNPs synthesised using methanol extract displayed highest anti‐bactericidal effect on E. coli and S. aureus. The highest anti‐bactericidal effect on S. pyogenes obtained from the nanoparticles synthesised in the presence of 96% ethanol extract. Therefore, we can conclude that the AgNPs synthesised biologically in this study have an acceptable antibacterial effect depending on the type of extract and bacterial strain.
Table 2.
Antibacterial effects of the AgNPs on three strains of bacteria
| Inhibition zone diameter, mm | |||
|---|---|---|---|
| Extract type | Bacterial stain | ||
| E. coli | S. aurous | S. pyogenes | |
| 96% ethanol extract containing nanoparticles | 0.5 ± 9.7 | 9.2 ± 0.5 | 9.4 ± 0.5 |
| 70% ethanol extract containing nanoparticles | 10.0 ± 0.5 | 9.0 ± 0.5 | 8.0 ± 0.5 |
| methanol extract containing nanoparticles | 10.2 ± 0.5 | 10.4 ± 0.5 | 8.1 ± 0.5 |
3.7 Minimum inhibitory concentrations (MIC) assay
Ethanol 70%, ethanol 96% and methanol samples were selected based on the results of disk diffusion assays to determine the MIC against all three bacterial strains tested.
3.8 Minimum bactericidal concentrations (MBC)
MBC obtained when the silver nitrate concentration was 0.125 mM. In this case, 96% ethanol was the best extract for inhibitory against S. pyogen while methanol was the best extract for inhibitory against S. aureus and E. coli (see Table 3).
Table 3.
Minimum bactericidal concentrations
| MBC | |||
|---|---|---|---|
| Extract type | Bacterial stain | ||
| E. coli | S. aurous | S. pyogenes | |
| 96% ethanol | 0.25 mM AgNO3 | 0.25 mM AgNO3 | 0.125 mM AgNO3 |
| 70% ethanol | 0.25 mM AgNO3 | 0.25 mM AgNO3 | 0.25 mM AgNO3 |
| methanol | 0.125 mM AgNO3 | 0.125 mM AgNO3 | 0.5 mM AgNO3 |
4 Conclusion
Plant‐based synthesis of metal nanoparticles is an emerging approach in nanoparticle synthesis because this method is easier and more efficient than the other methods. Malva sylvestris extracts contain excellent reducing and capping agents for the biogenic synthesis of high quality AgNPs. Application of 70% ethanol extract led to the synthesis of high stable nanoparticles with the best size distribution of ∼20–40 nm.
The investigation of the antibacterial effect of the produced nanoparticles on S. pyogenes, S. aureus and E. coli showed the highest antibacterial activity of AgNPs synthesised using 96% ethanol extract against S. pyogenes and methanol extract synthesised nanoparticles against S. aureus and E. coli.
5 Acknowledgment
The financial support of research council of Isfahan University was gratefully acknowledged.
6 References
- 1. De Franceschi S. Kouwenhoven L.: ‘Nanotechnology: electronics and the single atom’, Nature, 2002, 417, pp. 701 –702 [DOI] [PubMed] [Google Scholar]
- 2. Schulte P.A. Geraci C.L. Murashov V. et al.: ‘Occupational safety and health criteria for responsible development of nanotechnology’, J. Nanopart. Res., 2014, 16, pp. 1 –17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sobolev K. Gutiérrez M.F.: ‘How nanotechnology can change the concrete world’, Am. Ceram. Soc. Bull., 2005, 84, pp. 14 –15 [Google Scholar]
- 4. Kalkawi A. Abbasi Kajani A. Bordbar A.K.: ‘Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity’, IET Nanobiotechnol., 2017, 11, pp. 370 –376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker C. Pradhan A. Pakstis L. et al.: ‘Synthesis and antibacterial properties of silver nanoparticles’, J. Nanosci. Nanotechnol., 2005, 5, pp. 244 –249 [DOI] [PubMed] [Google Scholar]
- 6. Lee K.S. El‐Sayed M.A.: ‘Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition’, J. Phy. Chem. B., 2006, 110, pp. 220 –225 [DOI] [PubMed] [Google Scholar]
- 7. Beer C. Foldbjerg R. Hayashi Y. et al.: ‘Toxicity of silver nanoparticle or silver ion?’, Toxicol. Lett., 2012, 208, pp. 286 –292 [DOI] [PubMed] [Google Scholar]
- 8. Thakkar K.N. Mhatre S.S. Parikh R.Y.: ‘Biological synthesis of metallic nanoparticles’, Nanomed. Nanotech. Biol. Med., 2010, 6, pp. 257 –262 [DOI] [PubMed] [Google Scholar]
- 9. Veeraputhiran V.: ‘Bio‐catalytic synthesis of silver nanoparticles’, Int. J. ChemTech. Res., 2013, 5, pp. 55 –62 [Google Scholar]
- 10. Ghosh S. Patil S. Ahire M. et al.: ‘Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents’, Int. J. nanomed., 2012, 7, pp. 483 –496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh C. Baboota R.K. Naik P.K. et al.: ‘Biocompatible synthesis of silver and gold nanoparticles using leaf extract of Dalbergia sissoo ’, Adv. Mater. Lett., 2012, 3, pp. 279 –285 [Google Scholar]
- 12. Abbasi Kajani A. Zarkesh‐Esfahani S.H. Bordbar A.K. et al.: ‘Anticancer effects of silver nanoparticles encapsulated by Taxus baccata extracts’, J. Mol. Liq., 2016, 223, pp. 549 –556 [Google Scholar]
- 13. Awwad A.M. Salem N.M. Abdeen A.O.: ‘Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity’, Int. J. Ind. Chem., 2013, 4, pp. 1 –6 [Google Scholar]
- 14. Dwivedi A.D. Gopal K.: ‘Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract’, Colloid Surf. A, 2010, 369, pp. 27 –33 [Google Scholar]
- 15. Rout Y. Behera S. Ojha A.K. et al.: ‘Green synthesis of silver nanoparticles using Ocimum sanctum (Tulashi) and study of their antibacterial and antifungal activities’, J. Microbiol. Antimicrob., 2012, 4, pp. 103 –109 [Google Scholar]
- 16. Razavi S.M. Zarrini G. Molavi G. et al.: ‘Bioactivity of Malva sylvestris L., a medicinal plant from Iran’, Iranian. J. Med. Sci., 2011, 14, pp. 574 –579 [PMC free article] [PubMed] [Google Scholar]
- 17. Jeambey Z. Johns T. Talhouk S. et al.: ‘Perceived health and medicinal properties of six species of wild edible plants in north‐east Lebanon’, Public Health Nutr., 2009, 12, pp. 1902 –1911 [DOI] [PubMed] [Google Scholar]
- 18. Tabaraki R. Yosefi Z. Asadi G.H.A.: ‘Chemical composition and antioxidant properties of Malva sylvestris ’, J. Res. Agri. Sci., 2012, 1, pp. 59 –68 [Google Scholar]
- 19. Wang L. Weller C.L.: ‘Recent advances in extraction of nutraceuticals from plants’, Trends Food Sci. Technol., 2006, 17, pp. 300 –312 [Google Scholar]


