Abstract
Sheath blight disease in rice has caused major crop losses worldwide. Managing the causal agent of disease Rhizoctonia solani Kühn is difficult because of its broad host range and formation of sclerotia which can survive in harsh environmental conditions; therefore developing innovative disease management methods without application of hazardous chemicals has been considered as the main concern to maintain sustainable agriculture. This presented research has revealed the negative impact of silver nanoparticles (SNPs) on R. solani and disease progress both in vitro and in vivo. The adverse effects of the SNPs on R. solani are significantly dependent on the quantity of SNPs, sprayed at different concentrations in vitro. The highest inhibition level against sclerotia formation and mycelia growth are 92 and 85%, respectively, at a SNPs concentration of 50 ppm. In vivo glasshouse experiments also showed that SNPs at the same concentration favourably affects both the fresh and dry weight of rice plants with a remarkable suppressive effect on the lesion development in leaves.
Inspec keywords: antibacterial activity, silver, nanoparticles, nanomedicine, plant diseases, crops
Other keywords: in vitro antifungal properties, in vivo antifungal properties, silver nanoparticles, rice sheath blight disease, major crop losses, causal agent, Rhizoctonia solani Kühn, sclerotia, harsh environmental conditions, disease management methods, hazardous chemicals, sustainable agriculture, negative impact, inhibition level, mycelia growth, in vivo glasshouse experiments, rice plants, suppressive effect, lesion development, leaves
1 Introduction
Application of nano‐materials has widely influenced drug delivery, cancer therapy [1], energy [2], biomedical [3], agriculture [4] and many other high‐tech industries over recent years [5]. Nanotechnology has led to the new ways to control diseases using atomic‐scale materials [6, 7]. The extremely small‐scale particles have emerged as modern agents owing to their large surface to volume ratio which provides a large contact surface with pathogen sources [8]. Nanotechnology can have a great impact on natural processes and agriculture by introducing small scale tools [9]; plant protection products [10]; fertilizers [11]; water purification and pollutant remediation [12]; nanosensors, diagnostic devices [13]; and plant genetic modification [14].
Among nanoparticles (NPs), silver NPs (SNPs) can attack microorganisms, including the cell membrane structure in large‐scale biological processes [15, 16]. The antibacterial activity of silver ions has been well established and attributed to the ability of ionised SNPs to penetrate into the bacterial cell wall and to modulate cellular signalling [17]. SNPs with fungistatic, bacteriostatic and plasmonic properties are among the eco‐friendly inhibitors against plant‐pathogens compared with synthetic fungicides [18]; however the antifungal ability of SNPs has received less attention compared with medical and pharmaceutical sciences with only few studies undertaken against phytopathogenic fungi such as Alternaria alternata, Botrytis cinerea [19] and Colletotrichum gloeosporioides [20].
Rice (Oryza sativa L.) is a major food for a large proportion of the world's population, and is an important primary crop in muddy farmlands [21]. Sheath blight disease caused by Rhizoctonia solani Kühn AG1 (Teleomorph: Thanatephorus cucumeris; anastomosis group 1 IA, AG1 IA), is a common destructive disease of rice in all rice‐growing regions in the world. Sclerotia germination is a key factor in the dispersion of rice sheath blight disease, hence any potential inhibitor of sclerotia germination, i.e. SNPs, would be essential in order to decrease the inoculum. This impels rice farmers to use a large amount of anti‐nature and harmful chemicals annually to control sheath blight disease, which not only adds further costs in the short term but increases devastative damages in the long term to the human health and environment.
In this study, in order to control rice sheath blight disease with emphasis on the cleaner production at a lower cost, different concentrations of SNPs were examined as a new antifungal substance to suppress the pathogenic activity of R. solani under in vitro (to evaluate the inhibitory effects of SNPs on sclerotia formation and mycelia growth) and in vivo (to investigate the effects of antifungal activity of SNPs on the rice plant in a glasshouse trial) conditions.
2 Materials and methods
2.1 Reagent, rice seeds and fungal pathogen source
SNPs suspension was obtained from Nanocide Co., Tehran, with a concentration of 4000 ppm and an average particle size of 5–10 nm, in dark brown colloid physical form. Rice seeds of O. sativa L. var Hashemi and pure culture of R. solani AG‐1 IA were obtained from Iran Rice Research Institute (IRRI), Rasht [22]. The O. sativa L. var Hashemi potentially has a high yield, and is susceptible to sheath blight disease. The fungus was maintained on potato dextrose agar (PDA, Merck Co.) at room temperature.
2.2 In vitro examination of inhibitory effects of SNPs on mycelia and sclerotia of R. solani
To evaluate the in vitro antifungal effects of SNPs against R. solani AG1, four different concentrations of SNPs suspension (5, 10, 25 and 50 ppm) were added to Petri dishes before pouring plates with PDA. Uniform agar plugs with a diameter of 6 mm containing fungal mycelia were inoculated simultaneously at the centre of each Petri dish containing SNPs, followed by incubation at 28 ± 1°C for three days. The mycelia growth inhibition rate was calculated using (1) [4]
| (1) |
The parameter RH is the inhibition rate, R for the mycelium inhibition growth is the expansion in diameter of the mycelial fungus in the control dish (cm) and for the sclerotia formation growth under the inhibition process, R is the weight of the sclerotia in the control dish (mg). The parameter r for mycelium inhibition growth is the expansion in diameter of the fungus mycelial when treated by SNPs (cm), and for the sclerotia formation growth under the inhibition process, r is the weight of sclerotia when treated by SNPs (mg).
The antifungal effect of SNPs against R. solani sclerotia formation was measured after adding the four concentrations of SNPs to the PDA media content. Inoculated R. solani plates were maintained at room temperature for two weeks to manifest sclerotia formation, and the sclerotia formation inhibition rate was calculated using (1). All tests were carried out in triplicate.
The effect of SNPs on the germination of sclerotia was assayed using the following procedure [23]. The sclerotia of R. solani were formed on PDA at 15°C through incubating the inoculated plates for a week. Uniform sclerotia were collected from PDA plates, and the surface was sterilised in 1.5% sodium hypochlorite solution for 3 min. Then, three surface sterilised sclerotia were treated by SNPs of various concentrations and placed in a petri dish, and then they were incubated for a week at 25°C in the dark. The germination rates of sclerotia were measured and compared with the control (without SNPs). The percentage of inhibition against sclerotia was calculated using (1).
2.3 In vivo examination of SNPs on sheath blight disease under glasshouse conditions
Rice seeds were sown 3–4 cm below the soil surface of the pots (1 L) and they were separated into six groups with four pots in each group as follows: (a) pathogen alone, (b) pathogen + SNPs (5 ppm), (c) pathogen + SNPs (10 ppm), (d) pathogen + SNPs (25 ppm), (e) pathogen + SNPs (50 ppm) and (f) control (without SNPs). Rice plants were grown in pots under glasshouse conditions at 30°C and 85–95% relative humidity. As the plants reached their late tiller stage (three‐week‐old plants), they were treated by the inoculation process with R. solani. To achieve this, mycelia suspension of R. solani (5 × 108 CFU/ml) was evenly sprayed using a hand sprayer on the rice plants [24]. To maintain a fair coverage of SNPs on the foliage throughout the evaluation period, two sprays applied including 24 h post inoculation and 7 days, subsequently. After inoculating with pathogen and SNPs spraying process, the seedlings were covered with plastic bags for three days to maintain the high humidity. After 15 days, the disease severity was recorded via measuring the fresh weight, dry weight and relative lesion height (RLH), according to the 1996 IRRI standard. The RLH of each tiller was calculated using (2) [25]
| (2) |
2.4 Statistical analysis
Recorded data were subjected to analysis of variance with SAS software (SAS Institute, version 9). Duncan's Multiple Range Test was utilised to compare means.
3 Results
3.1 In vitro examination of inhibitory effects of SNPs on mycelia and sclerotia of R. solani
The effects of tested SNPS concentrations on mycelium growth, sclerotia formation and germination are presented in Fig. 1. Plates treated with 50 ppm SNPs revealed the minimum number of sclerotia. The RHs of 12, 23, 68 and 92% were found for the 5, 10, 25 and 50 of SNPs concentrations, respectively. With regard to the mycelia growth, RHs of 8, 35, 67 and 85 were recorded for the SNPs of concentrations of 5, 10, 25 and 50 ppm, respectively. The RHs of 15, 26, 57 and 98% were found for the SNPs concentrations of 5, 10, 25 and 50 ppm, respectively, related to sclerotia germination. These results indicate that SNPs has strongly suppressed R. solani under in vitro condition (Fig. 2).
Fig. 1.
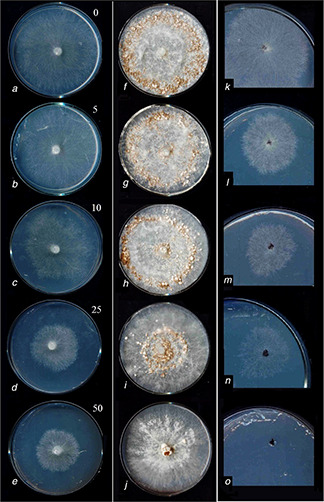
In vitro inhibitory effects of different concentrations of SNPs (indicated in the top right of left column) on R. solani AG1
Mycelia growth stage [left column (a) – (e) ], Sclerotia formation [middle column (f) –(j) ], Sclerotia germination [right column (k) –(o) ]
Fig. 2.
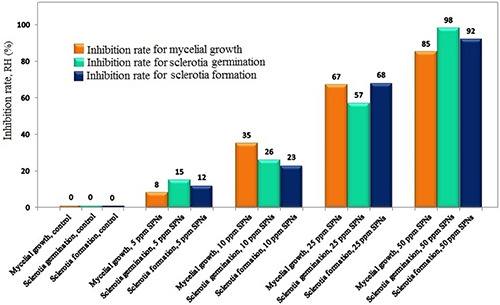
In vitro RH of SNPs effects on mycelia growth, sclerotia germination and sclerotia formation of R. solani AG1‐IA
3.2 In vivo examination of SNPs on sheath blight disease under glasshouse conditions
Fig. 3 shows the rice seedlings at the late tiller stage of 90 days under the greenhouse conditions (30°C, 85–95% humidity). The in vivo results of SNPs against R. solani, the causal agent of sheath blight disease, are presented in Fig. 4. Fig. 4 a indicates the leaf symptoms resulting from infection by R. solani alone, and Figs. 4 b–e indicate the pathogen plus the applied 5, 10, 25 and 50 ppm of SNPs and their different degrees of inhibition in the leave‐lesion development. The treatment of plants with the pathogen without SNPs resulted in typical sheath blight symptoms, but the treated plants with pathogen + SNPs show different levels of inhibitory effects. According to the results of analysis of variance for different traits, all traits were different at significance level at P ≤ 0.05. There are significant reductions in the symptoms of pathogen in pots treated with SNPs. The 5 ppm SNPs concentration has a small effect on the dry weight of the rice plants (Table 1); by increasing the concentration of SNPs to 50 ppm, the fresh weight and dry weight increased significantly. At 50 ppm SNPs concentration, the RLH of each tiller decreased which evidence that applying SNPs has a strong antifungal influence on and minimises lesion in the rice tillers. The comparative results of the inhibition activity of SNPs against sheath blight revealed significant reduction of lesions on the rice sheath (Fig. 5).
Fig. 3.

Rice seedlings after 90 days under greenhouse conditions, with a temperature of 30°C and constant humidity of 85–95%
Fig. 4.

Effect of SNPs treatment on the lesion development by R. solani on rice leaves
(a) Pathogen alone, (b) Pathogen + SNPs (5 ppm), (c) Pathogen + SNPs (10 ppm), (d) Pathogen + SNPs (25 ppm), (e) Pathogen + SNPs (50 ppm), (f) Untreated control
Table 1.
In vivo inhibitory effect of SNPs on rice sheath blight under glasshouse conditions
| Treatment | Fresh weight, g | Dry weight, g | RLH, % |
|---|---|---|---|
| control | 22.6*a | 5.8a | — |
| pathogen | 12.5b | 2.3b | 95% |
| pathogen + SNPs (5 ppm) | 13.7b | 2.5b | 90% |
| pathogen + SNPs (10 ppm) | 14.2bc | 2.7bc | 80% |
| pathogen + SNPs (25 ppm) | 18.3d | 3.8d | 55% |
| pathogen + SNPs (50 ppm) | 20.3ad | 4.4ad | 15% |
*The presented data are the means of four replications, and they are subjected to the analysis with the variance n = 5.
The labels a–d means followed by a different letter(s) in the same column differ significantly (p ≤ 0.05) according to Duncan's Multiple Range Test.
Fig. 5.

Effect of SNPs on the lesion development by R. solani on rice sheath
(a) Pathogen alone, (b) Pathogen + SNPs (5 ppm), (c) Pathogen + SNPs (10 ppm), (d) Pathogen + SNPs (25 ppm), (e) Pathogen + SNPs (50 ppm), (f) Untreated control
3.3 Discussion
SNPs can denature cells by attacking their membranes and structures. A previous research found that SNPs disrupts transport systems, including ion efflux [26]. The dysfunction of ion efflux causes a rapid accumulation of silver ions, interrupting cellular processes such as respiration and metabolism by reacting with the molecules. Ji Seon et al. (2009) showed that upon the treatment by SNPs spray, the hyphal walls were seriously damaged and resulted in the plasmolysis of hyphae. Considering the cellular effects of silver ions, SNPs mediated the collapse in Sclerotinia sclerotiorum hyphae which damaged the hyphal walls [27].
Silver ions are known to deactivate cellular enzymes and DNA by coordinating with electron‐donating groups such as thiols, carboxylates, indoles, amides, hydroxyls and so on. [28, 29] According to past studies on SNPs, the smaller the SNPs, the more Ag+ ions they release which affects the performance of microorganisms [30, 31]. As discovered in our investigation, SNPs in an aqueous solution with small sizes can penetrate into the cells of microorganisms and destroy their membrane integrity [32, 33, 34].
NPs have a vast surface to volume ratio which significantly enhances their property of cell membrane permeability compared with non‐NPs forms of the same material [35, 36, 37]. NPs are able to penetrate the membranes of microorganisms, leading to cell deformation [38]. NPs, with their large surface to volume ratio, exhibit active antimicrobial properties due to their higher ability to interact with cellular membranes through disruption of the cell wall structure, affecting the respiratory chain and cell division in DNA and proteins as a microorganism [39]. It is likely that the size of SNPs similarly plays a key role in their permeability and antifungal activity. In short, SNPs have an active antifungal activity with great biocide properties, thus, they have the potential to be considered as an economical and eco‐friendly pesticide. The application of chemical fungicides adds additional indirect long‐term and hidden costs as it causes dangerous side effects in both human health and the environment [40]. The editors of Nature estimated that any technology takes some 20 years to emerge from the laboratory and be commercialised [41]. Application of nanotechnology in agriculture might take a few decades to move from laboratory to land however reasonable expectations would be crucial for this nascent field to blossom [42].
The efficacy of SNPs is increased by conjugating the antifungal drug miconazole with SNPs which exhibits significant fungicidal activity [43]. SNPs with chitin inhibit the spore germination of the examined pathogens [44, 45]. Moreover, bioactive capped SNPs were found to be able to control the endophytic fungus of C. gloeosporioides, in vitro [46]. The antifungal activity of SNPs is comparable with those of ionic SNPs; however, ionic silver remains cytotoxic at the concentrations that inhibit the growth of the examined yeasts [47]. According to previous research, both positive and negative effects on plant growth and development were suggested [48]. In some plant species (e.g. Brassica juncea), it has been indicated SNPs affect total plant growth factors including shoot and root length and biochemical attributes such as chlorophyll, carbohydrate and protein contents and rate of antioxidant enzymes [49].
4 Conclusion
In vitro and in vivo study of the antifungal activity of SNPs at concentrations of 5, 10, 25 and 50 ppm was conducted against fungal pathogen R. solani to reduce and prevent the sheath blight in rice seedlings. The in vitro results showed the RHs for mycelial growth, sclerotia formation and sclerotia germination were, respectively, (8, 35, 67, 85), (12, 23, 68, 92) and (15, 26, 57, 98) for their corresponding SNPs concentrations (5, 10, 25 and 50 ppm). The results clearly show that the RHs strongly depend on SNPs concentration, and substantially increase upon an increase in SNPs concentration. As indicated in the in vitro test, we can conclude an increasing trend in the inhibition rate for mycelial growth, sclerotia germination and sclerotia formation with the increasing amount of SPNs. By spraying SNPs on the rice plants, the sheath blight disease's symptoms on the leaves decreased, and at 50 ppm SNPs concentration, the symptoms completely vanished.
The in vivo results show that the SNPs solution created an antimicrobial layer around the rice plants which protected the plants from pathogens. It was also demonstrated that SNPs highly affect sclerotia formation and germination. SNPs can penetrate into the fungal cell membrane and cell wall, killing microorganism cells. This investigation suggests SNPs can replace chemical pesticides in controlling and inhibiting sheath blight, a common disease in rice.
5 Acknowledgements
This paper was dedicated to the late A. Afzalipour and the late Fakhereh Saba, the founders of Universities in Kerman province. The authors were thankful to the Shahid Bahonar University of Kerman for providing the research facilities.
6 References
- 1. Mrinmay C. Raisa K. Alexey V. et al.: ‘Carbon nanomaterials for drug delivery and cancer therapy’, J. Nanosci. Nanotechnol., 2015, 15, (8), pp. 5501 –5511 [DOI] [PubMed] [Google Scholar]
- 2. Amini A. Yang C. Cheng C. et al.: ‘Nanoscale variation in energy dissipation in austenitic shape memory alloys in ultimate loading cycles’, J. Intell. Mater. Syst. Struct., 2014, doi: 10.1177/1045389X14560365 [Google Scholar]
- 3. Amini A. Hameed N. Church J.S. et al.: ‘Effect of grahpene on thermomechanical behaviour of NiTi shape memory alloy during nano‐scale phase transition’, Scr. Mater., 2013, 68, pp. 420 –423 [Google Scholar]
- 4. Khatami M. Pourseyedi S. Khatami M. et al.: ‘Synthesis of silver nanoparticles using seed exudates of Sinapis arvensis as a novel bioresource, and evaluation of their antifungal activity’, Bioresources Bioprocessing, 2015, 2, (19), pp. 1 –7 [Google Scholar]
- 5. Schrofel A. Gabriela K. Ivo Š. et al.: ‘Applications of biosynthesized metallic nanoparticles – a review’, Acta Biomater., 2014, 10, pp. 4023 –4042 [DOI] [PubMed] [Google Scholar]
- 6. Afreen R. V R. E R.: ‘Synthesis of monodispersed silver nanoparticles by Rhizopus stolonifer and its antibacterial activity against MDR strains of Pseudomonas aeruginosa from burnt patients’, Int. J. Environ. Sci., 2011, 1, pp. 1583 –1592 [Google Scholar]
- 7. Soltani Nejad M. Khatami M. Bonjar G.H.S.: ‘ Streptomyces somaliensis mediated green synthesis of silver nanoparticles’, Nanomed. J., 2015, 2, (3), pp. 233 –238 [Google Scholar]
- 8. Kathiraven T. Sundaramanickam A. Shanmugam N. et al.: ‘Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens’, Appl. Nanosci., 2015, 5, (4), pp. 499 –504 [Google Scholar]
- 9. Parisi C. Vigani M. Rodríguez‐Cerezo E.: ‘Agricultural nanotechnologies: what are the current possibilities?’, Nano Today, 2015, 10, (2), pp. 124 –127 [Google Scholar]
- 10. Anjali C.H. Sharma Y. Mukherjee A. et al.: ‘Neem oil (Azadirachta indica) nanoemulsion – a potent larvicidal agent against Culex quinquefasciatus ‘, Pest Manage. Sci., 2012, 68, (2), pp. 158 –163 [DOI] [PubMed] [Google Scholar]
- 11. Milani N. McLaughlin M.J. Stacey S.P. et al.: ‘Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles’, J. Agric. Food Chem., 2012, 60, (16), pp. 3991 –3998 [DOI] [PubMed] [Google Scholar]
- 12. McMurray T.A. Dunlop P.S.M. Byrne J.A.: ‘The photocatalytic degradation of atrazine on nanoparticulate Tio2 films’, J. Photochem. Photobiol. A, Chem., 2006, 182, (1), pp. 43 –51 [Google Scholar]
- 13. Vamvakaki V. Chaniotakis N.A.: ‘Pesticide detection with a liposome‐based nano‐biosensor’, Biosens. Bioelectron., 2007, 22, (12), pp. 2848 –2853 [DOI] [PubMed] [Google Scholar]
- 14. Torney F. Trewyn B.G. Lin V.S.Y. et al.: ‘Mesoporous silica nanoparticles deliver DNA and chemicals into plants’, Nat. Nano, 2007, 2, (5), pp. 295 –300 [DOI] [PubMed] [Google Scholar]
- 15. Pal S. Tak Y. Song J.: ‘Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle: a study of the gram‐negative bacterium Escherichia Coli ‘, Appl. Environ. Microbiol., 2007, 73, pp. 1712 –1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dibrov P. Dzioba J. Gosink K.: ‘Chemiosmotic mechanism of antimicrobial activity of Ag (+) in Vibrio cholerae ‘, Antimicrob. Agents Chemother., 2002, 46, pp. 2668 –2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braydich‐Stolle L. Hussain S. Schlager J.J. et al.: ‘ In vitro cytotoxicity of nanoparticles in mammalian germline stem cells’, Toxicol. Sci., 2005, 88, pp. 412 –419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kabir L. Kim S.W. Jung J.H. et al.: ‘Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field’, Mycobiology, 2011, 39, (3), pp. 194 –199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ouda S.M.: ‘Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea ‘, Res. J. Microbiol., 2014, 9, pp. 34 –42 [Google Scholar]
- 20. Aguilar‐Méndez M.A. Martín‐Martínez E.S. Ortega‐Arroyo L. et al.: ‘Synthesis and characterization of silver nanoparticles: effect on phytopathogen Colletotrichum gloesporioides ‘, J. Nanoparticle Res., 2011, 13, (6), pp. 2525 –2532 [Google Scholar]
- 21. Kazemi H. Kamkar B. Lakzaei S. et al.: ‘Energy flow analysis for rice production in different geographical regions of Iran’, Energy, 2015, 84, pp. 390 –396 [Google Scholar]
- 22. Padasht‐Dehkaei F. Willocquet L. Ebadi A.A. et al.: ‘Study on partial resistance to sheath blight disease (Rhizoctonia Solani Ag1‐ Ia) in Iranian and selected exotic cultivars of rice’, Iran. J. Plant Prot. Sci, 2013, 44, pp. 307 –317 [Google Scholar]
- 23. Kazempour M.N.: ‘Biological control of Rhizoctonia solani, the causal agent of rice sheath blight by antagonistic bacteria in greenhouse and field conditions’, Plant Pathol., 2004, 3, pp. 88 –96 [Google Scholar]
- 24. Park D.‐S. Sayler R.J. Hong Y.‐G. et al.: ‘A method for inoculation and evaluation of rice sheath blight disease’, Plant Dis., 2008, 92, (1), pp. 25 –29 [DOI] [PubMed] [Google Scholar]
- 25. Yadav S. Anuradha G. Kumar R.R. et al.: ‘Identification of Qtls and possible candidate genes conferring sheath blight resistance in rice (Oryza sativa L.)’, SpringerPlus, 2015, 4, p. 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morones J.R. Elechiguerra J.L. Camacho A. et al.: ‘The bactericidal effect of silver nanoparticles’, Nanobiotechnology, 2005, 16, pp. 2346 –2353 [DOI] [PubMed] [Google Scholar]
- 27. Ji‐Seon M. Kyoung‐Su K. Sang‐Woo K. et al.: ‘Effects of colloidal silver nanoparticles on sclerotium‐forming phytopathogenic fungi’, Plant Pathol., 2009, 25, (4), pp. 376 –380 [Google Scholar]
- 28. Thenmozhi M. Kannabiran K. Kumar R. et al.: ‘Antifungal activity of Streptomyces sp. VITSTK7 and its synthesized Ag2 O/Ag nanoparticles against medically important Aspergillus pathogens’, de Mycologie Médicale, 2013, 23, pp. 97 –103 [DOI] [PubMed] [Google Scholar]
- 29. Feng Q. Wu J. Chen G. et al.: ‘A mechanistic study of the antibacterial effect of silver ions on Escherichia Coli and Staphylococcus Aureus ‘, Biomed. Mater., 2000, 52, pp. 662 –668 [DOI] [PubMed] [Google Scholar]
- 30. Espinosa‐Cristóbal L.F. Martínez‐Castañón G.A. Martínez‐Martínez R.E. et al.: ‘Antibacterial effect of silver nanoparticles against Streptococcus mutans ‘, Mater. Lett., 2009, 63, pp. 2603 –2606 [Google Scholar]
- 31. Shenashen M.A. El‐Safty S.A. Elshehy E.A.: ‘Synthesis, morphological control, and properties of silver nanoparticles in potential applications’, Part. Part. Syst. Charact., 2014, 31, pp. 293 –316 [Google Scholar]
- 32. Ogar A. Tylko G. Turnau K.: ‘Antifungal properties of silver nanoparticles against indoor mould growth’, Sci. Total Environ., 2015, 15, pp. 305 –314 [DOI] [PubMed] [Google Scholar]
- 33. Choi O. Deng K. Kim N.: ‘The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth’, Water Res., 2008, 42, pp. 3066 –3074 [DOI] [PubMed] [Google Scholar]
- 34. Gogoi S. Gopinath P. Paul A.: ‘Green fluorescent protein expressing Escherichia coli as a model system for investigating the antimicrobial activities of silver nanoparticles’, Langmuir, 2015, 22, pp. 9322 –9328 [DOI] [PubMed] [Google Scholar]
- 35. Kvitek L. Panacek A. Soukupova J. et al.: ‘Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (Nps)’, J. Phys. Chem. C, 2008, 112, pp. 5825 –5834 [Google Scholar]
- 36. Narayanan K.B. Park H.H.: ‘Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens’, Eur. J. Plant Pathol., 2014, 140, (2), pp. 185 –192 [Google Scholar]
- 37. Gurunathan S. Raman J. Malek S.N.A. et al.: ‘Green synthesis of silver nanoparticles using Ganoderma neo‐japonicum imazeki: a potential cytotoxic agent against breast cancer cells’, Int. J. Nanomed., 2013, 8, pp. 4399 –4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marambio‐Jones C. Hoek E.M.: ‘A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment’, Nanoparticle Res., 2010, 12, pp. 1531 –1551 [Google Scholar]
- 39. Ahmad T. Wani I. Manzoor N. et al.: ‘Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles’, Colloids Surf. B, Biointerfaces, 2013, 107, pp. 227 –234 [DOI] [PubMed] [Google Scholar]
- 40. Qin J. He H. Luo S. et al.: ‘Effects of rice‐water chestnut intercropping on rice sheath blight and rice blast diseases’, Crop Prot., 2013, 43, pp. 89 –93 [Google Scholar]
- 41.‘The long game’, Nature, 2011, 473, (7348), pp. 419 –419 [DOI] [PubMed] [Google Scholar]
- 42. Mukhopadhyay S.S.: ‘Nanotechnology in agriculture: prospects and constraints’, J. Nanotechnol. Sci. Appl., 2014, 7, pp. 63 –71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar C.G. Poornachandra Y.: ‘Biodirected synthesis of miconazole‐conjugated bacterial silver nanoparticles and their application as antifungal agents and drug delivery vehicles’, Colloids Surf. B, Biointerfaces, 2015, 125, pp. 110 –119 [DOI] [PubMed] [Google Scholar]
- 44. Ifuku S. Tsukiyama Y. Yukawa T. et al.: ‘Facile preparation of silver nanoparticles immobilized on chitin nanofiber surfaces to endow antifungal activities’, Carbohydr. Polym., 2015, 117, pp. 813 –817 [DOI] [PubMed] [Google Scholar]
- 45. Kotzybik K. Gräf V. Kugler L. et al.: ‘Influence of different nanomaterials on growth and mycotoxin production of Penicillium verrucosum ’, PLoS ONE, 2016, 11, pp. e0150855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muthuramalingam T.R. Shanmugam C. Gunasekaran D. et al.: ‘Bioactive bile salt‐capped silver nanoparticles activity against destructive plant pathogenic fungi through in vitro system’, RSC Adv., 2015, 5, pp. 71174 –71182 [Google Scholar]
- 47. Roe D. Karandikar B. Bonn‐Savage N. et al.: ‘Antimicrobial surface functionalization of plastic catheters by silver nanoparticles’, J. Antimicrob. Chemother., 2008, 61, pp. 869 –876 [DOI] [PubMed] [Google Scholar]
- 48. Siddiqui M.H. Al‐Whaibi M.H. Firoz M. et al.: ‘Role of nanoparticles in plants’, in Siddiqui H.M. Al‐Whaibi H.M. Mohammad F. (eds.): ‘Nanotechnology and plant sciences: nanoparticles and their impact on plants’ (Springer International Publishing, Switzerland, 2015) [Google Scholar]
- 49. Sharma P. Bhatt D. Zaidi M.G.H. et al.: ‘Silver nanoparticle‐mediated enhancement in growth and antioxidant status of Brassica juncea ’, Appl. Biochem. Biotechnol., 2012, 167, (8), pp. 2225 –2233 [DOI] [PubMed] [Google Scholar]


