Abstract
One of the most important challenges in treating cancer is the invasion and the angiogenesis of cancer cells. The synthesis of green nanoparticles (NPs) and their use in therapeutic fields is one of the most effective methods with minimal side effects in cancer treatment. In this study, cytotoxic and anti‐angiogenic effects of silver NPs (AgNPs) coated with palm pollen extract [Ag–PP(NPs)] were evaluated. For this purpose, the cells were treated with NPs and then were subjected to trypan blue testing (48 h). Then, the cancer invasion was evaluated by the scratch procedure and the expressions of the vascular endothelial growth factor (VEGF) and its receptor (VEGF‐R) genes were estimated using real‐time PCR assay. Also, the angiogenesis effect of the NPs was investigated with chick chorioallantoic membrane (CAM) assay. The Ag–PP(NPs) induced cytotoxicity on MCF7 cells. The findings also showed that Ag–PP(NPs) inhibit invasive cancer cells and reduce the expression of VEGF and VEGF‐R and significantly reduced the number and vessels lengths and the lengths and weights of the embryos in CAM assay. Ag–PP(NPs) with the induction of cytotoxic effects, metastatic inhibition and anti‐angiogenesis properties should be considered as an appropriate option for treatment of cancer
Inspec keywords: nanomedicine, genetics, cellular biophysics, toxicology, patient treatment, silver, cancer, biochemistry, biomedical materials, nanoparticles, nanofabrication, membranes
Other keywords: minimal side effects, cancer treatment, silver NPs, cancer invasion, vascular endothelial growth factor, receptor genes, VEGF‐R, real‐time polymerase chain reaction assay, angiogenesis effect, chick chorioallantoic membrane assay, MCF7 cells, invasive cancer cells, cytotoxic effects, putative mechanism, anticancer properties, antiangiogenic effects, antiangiogenesis properties, Ag–PP‐induced cytotoxicity, metastatic inhibition, palm pollen extraction, trypan blue testing, time 48.0 hour, Ag
1 Introduction
Since the cancer cells need to receive oxygen and food to continue their growth and reproduction, so one of the important challenges in cancer treatment is to inhibit the growth of these cells and reduce their nutrition. Angiogenesis is one of the ways that allows cancer cells to absorb more food, dispose of waste and metastases to other tissues. Since cancer is considered as one of the recurrent pathologies, inhibition of angiogenesis is considered as a major goal and an effective way of preventing cancer recurrence by Bhat and Singh [1] and Prabhu et al. [2]. Several factors and genes are involved in the angiogenesis process, and the balance between these factors causes the growth and development of new vessels in physiological conditions [3] and imbalance in the production of these factors can lead to pathological angiogenesis [4]. Hypoxia is one of the factors that activate inductive factors and ultimately leads to the use of nearby endothelial cells, detachment of endothelium periosteum and vessel instability [5]. The vascular endothelial growth factor (VEGF) factor is an angiogenesis promoting factor that activates and migrates endothelial cells to tumours which leads to the activation and migration of endothelial cells toward the tumours. VEGF and its receptor (VEGF‐R) in endothelial cells regulate and induce growth and proliferation and migration of endothelial cells. In tumour cells, the increased expression of these factors causes the growth, migration and metastasis of cancer cells to the surrounding tissues [6, 7]. Breast cancer is one of the most common cancers and one of the most important causes of death in women, which various factors such as growth factors, oncogenes and hormones are involved in its creation and development. Despite the advancements of surgical procedures, chemotherapy, radiation therapy and hormone therapy, one of the major problems in improving of these patients is the relapse after treatment, which factors such as metastasis of breast cancer cells through blood vessels and lymph nodes to liver and bone tissues play an important role in relapse of the disease [8, 9]. Therefore, using effective therapies and effective methods, it is possible to prevent cancer growth and the migration of cancer cells to other tissues. Therefore, the use of anti‐angiogenesis can be an important tool in the treatment of breast cancer. Nanotechnology has been able to play an important role in improving the quality of the effects of anticancer drugs by producing nano‐drugs and targeting tumour cells [10]. Therefore, the aim of this paper was to investigate the cytotoxicity capacity of silver nanoparticles (AgNPs) on human breast cancer cells and evaluating the anti‐angiogenesis effect of these NPs by assessing the expression of the angiogenic genes in the treated cells as well as the anti‐angiogenesis effects on chorioallantoic membrane.
2 Materials and methods
2.1 Materials
Ag nitrate (99.98%) was purchased with Merck (Darmstadt, Germany) brand. Pollen of Iranian palm pollen (PP) was obtained from a market in Iran. Cell culture medium and all the culture reagents were purchased with sigma (Sigma‐Aldrich Co., St. Louis, MO, USA) brand. Cancer cells were purchased from the Institute Pasteur Institute of Tehran, Iran.
2.2 Cultivation of human breast cancer cells
Human breast cancer (MCF‐7) cells were used in this paper. At first, the cells were cultured in a humidified atmosphere of 95% air and 5% carbon dioxide at 37°C with suitable culture medium, and after reaching the logarithmic phase, to evaluate, the effect of NP toxicity was used.
2.3 Cytotoxic effects of Ag–PP(NPs)
The cells were first cultured under appropriate conditions to reach the logarithmic phase, then were transferred to 96 plates with 5 × 103 density of cells in each well. After 24 h incubation, the cells were exposed to Ag–PP(NPs) [11] at different concentrations for 48 h. Then, cytotoxicity of Ag–PP(NPs) was investigated on cancer cells using trypan blue method. For this purpose, 10 µl of trypan blue was added to 10 µl cells suspension and then 10 µl of mixture counted by microscope. The number of live cells was counted in control and treatment groups [12].
2.4 Scratch assay
First, one NP was selected for this test, and after determining its inhibitory concentrations on the cancer cells, the NPs were used at appropriate concentrations for scratch test. To do a scratch test, first, the bottom of the 24‐well plate was covered by gelatin (20 μM/ml). Then, the cancer cells were cultured on collagen, and after 24 h scratches were created at the bottom of the plate. The cells were treated with an intermediate inhibitory concentration of (40 μM/ml) Ag–PP(NPs) for 10 h. Then, the scratch filling of the control and treatment groups was investigated by reverse microscope [13].
2.5 Evaluations of VEGF and VEGF‐R gene expressions
After 24 h treatment of the cancerous cells with inhibitor concentrations of Ag–PP(NPs), total RNA was extracted from treated and untreated cells with High Pure RNA Isolation Kit (Roche, Germany). After determining the RNA concentration, the cDNA was synthesised further using VEGF and VEGF‐R primers, the level of angiogenic gene expressions was evaluated by Cyber Green Kit and real‐time polymerase chain reaction (PCR) technique [14].
2.6 Angiogenesis effect of Ag–PP(NPs) on chorioallantoic membrane
About 60 egg shells were purchased from Tous Mashhad Company and randomly divided into six groups including control (without treatment), laboratory test (treated with solvent) and four treatment groups with different concentrations of NPs. On the second day of incubation, a patch was created on one side of the egg and then closed with paraffin and glue and placed on the eighth day in the incubator. On the eighth day, the windows were removed and a gelatin sponge consisting of egg albumin and agar was placed on the chorioallantoic membrane. Then, each of the samples was treated with specific concentrations of the NP. The control group did not receive treatment and the laboratory group was treated with solvent. Then, the window was closed and the eggs were transferred to the incubator. On the 12th day of incubation, images of the sponge location were prepared using a stereomicroscope. The length and number of vessels were evaluated by image J software. The length and the weight of the embryos were measured with calliper and scales [15].
2.7 Statistical analysis
The number and length of the vessels were evaluated by image J software. Data were presented as mean±standard deviation (SD). Data were analysed by SPSS software using one‐way analysis of variance and significant levels of p ≤ 0.05*, P ≤ 0.01** and P ≤ 0.001***.
3 Results
3.1 Cytotoxic effects of Ag–PP(NPs)
The results of the trypan blue assay showed that the Ag–PP(NPs) could inhibit the proliferation of breast cancer cells and induced cytotoxicity in cancer cells, depending on the dose. As shown in Fig. 1. Ag–PP(NPs) at various doses with p ≤ 0.001 reduce the survival of cancer cells and cause cytotoxic effects. The trypan blue results show that the Ag–PP(NPs) with IC50 about 10 μM/ml inhibit cancer cells.
Fig. 1.
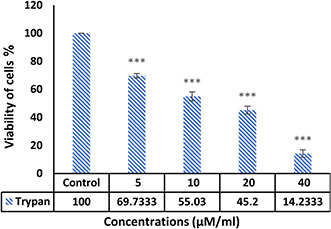
Detection of cell survival by trypan blue method after 48 h treatment with different concentrations of Ag–PP(NPs):***p<0.001 (n = 3, mean±SD)
3.2 Evaluation of migration of Michigan Cancer Foundation‐7, cells exposed with Ag–PP(NPs)
Morphological observation (Fig. 2.) of cells that exposed with 5 µg/ml concentration of NP and untreated cells; 10 h after scratching showed that untreated cells have been able to fill the gap and migrate during this period, while treated cells were unable to migrate and fill the gap. These results indicate that NPs are capable of suppressing the metastasis of breast cancer cells at inhibitory concentrations.
Fig. 2.
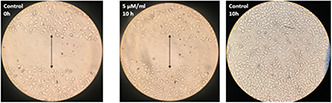
Inhibitory effect of the Ag–PP(NPs) on invasion of MCF‐7 breast cancer cells 10 h after treatment
3.3 Evaluations of expression levels of VEGF and VEGF‐R by real‐time PCR
The evaluations of the VEGF and VEGF‐R gene expressions in control and treatment groups showed that one of the mechanisms employed by the Ag–PP(NPs) in this paper is inhibition of VEGF and VEGF‐R expression (Fig. 3), and thus prevents metastasis and angiogenesis of tumour cells As the diagram shows, in cells that treated with different doses of Ag–PP(NPs) compared with untreated, the expression level of genes related to angiogenesis is significantly reduced, which confirms the anti‐angiogenesis effect of the Ag–PP(NPs).
Fig. 3.
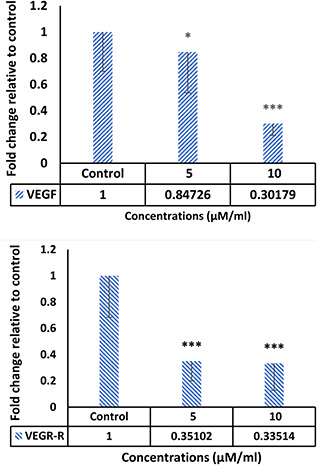
Reducing the expression of angiogenesis‐related genes in treated cells with different concentrations of Ag–PP(NPs)compared with control cells
3.4 Anti‐angiogenesis effect of Ag–PP(NPs) on chorioallantoic membrane
The morphological examination findings showed that the vessels length and a number of branches significantly reduced in the treatment group compared control (Fig. 4). Comparison of mean vessels length, number of branches, lengths and weights of the embryos in the control sample were not significantly different from laboratory control (p ≥ 0.05). Comparison of the mean number and length of vessels in treated groups with different concentrations of NPs with the control group showed that the length of the vessels (Fig. 5) in the treated group with 100 μM/ml of Ag–PP(NPs) was significantly different at the level of p ≤ 0.05 and in the index of number the vessels (Fig. 6) showed a significant difference at the level of p ≤ 0.001 compared with the control group in same concentration. The results of length and weight measurements of embryos (Figs. 7 and 8) showed that in all treated groups, the heights and weights of embryos showed a significant decrease compared with the control group P ≤ 0.01 and P ≤ 0.001.
Fig. 4.
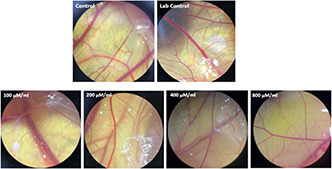
Morphological alteration of vessels in treated samples with different concentrations of Ag–PP(NPs) compared with control samples
Fig. 5.
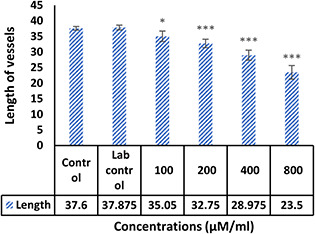
Mean length of blood vessels treated with Ag–PP(NPs) compared with untreated controls (*P<0.05 and ***P<0.001)
Fig. 6.
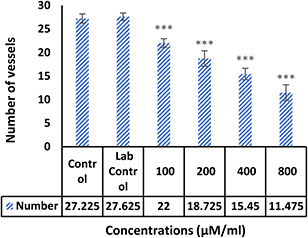
Average number of blood vessels treated with Ag–PP(NPs) compared with untreated controls (***P<0.001)
Fig. 7.
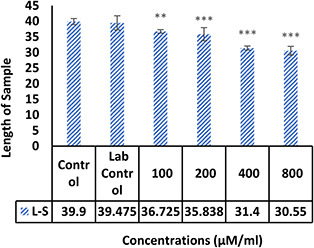
Mean height of embryos treated with NPs compared with control without treatment (**P<0.01 and ***P<0.001)
Fig. 8.
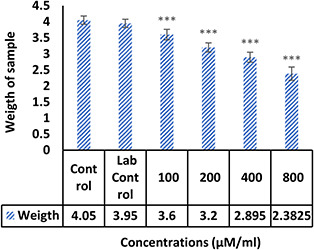
Mean weight of embryos treated with NPs compared with non‐treated controls (***P<0.001)
4 Discussion
The synthesis of NPs is done by various chemical, physical and biological methods. The synthesis of green NPs by using plant compounds as coatings in the NP resuscitation reaction reduces the side effects of chemical compounds. Medicinal plants are rich from of the biological molecules and have been considered due to their high tendency to absorb metallic NPs, strong stability and biodegradability in the synthesis of metal NPs. Therefore, biosynthetic methods using plant extracts have been considered as a simple, effective and appropriate technique in the synthesis of NPs [8]. In this paper, PP extract was used as a stabilising agent in the synthesis of AgNPs. The cytotoxic, anti‐angiogenic and anti‐rejection effects of these NPs were studied. Many factors including the size and shape of NPs are effective on the cellular mechanisms of NPs. Studies show that the sizes of the NPs are inversely correlated with the absorption rate by the cell. On the other hand, the morphology of NPs is another important factor in this regard. Studies show that spherical NPs have a better adsorption than rod NPs [16]. Studies have shown that palm extract has high antioxidant properties due to the presence of flavonoids [17]. Ag and zinc oxide NPs were synthesised from PP, and their antibacterial effects were investigated [11, 18]. In this paper, the effect of AgNPs on the breast cancer cells was evaluated and then the anti‐angiogenesis and antimetastatic effects of AgNPs from PP were studied. The results showed that the AgNP had a more toxic effect on breast cancer cells (Fig. 1) and could also prevent the migration of cancer cells (Fig. 2). Further study results showed that AgNPs were able to inhibit angiogenesis by reducing the expression of the angiogenesis inducing genes (VEGF and VEGF‐R) (Fig. 3) and also in the chorioallantoic membrane model (Figs. 4, 5, 6, 7, 8) Various studies have been done on the biological effects of synthesised NPs from different sources, which is comparable with that of the present paper. The cytotoxic effects of zinc oxide NPs synthesised by the green method from different plants including brown seaweed aqueous extract [19, 20] and Musa ornate; Zea mays aqueous extract [20] shows that these NPs are capable of inhibiting different cancer cells that are comparable with the present paper. Studies on AgNPs have also shown the role of this NP in inhibiting cancer cells. For example, the results of a 2010 study on human‐resistant breast cancer cells (T47D) showed that AgNPs could inhibit 75% of cells at a concentration of 15 μg/ml [21]. Other studies showed that AgNPs synthesised from various plants including Erythrina suberosa (Roxb.) Leaf extract [22], Syzygium cumini fruit extract [23] and Origanum Heracleoticum L. leaf extract [24] have cytotoxic effects against cancer cells. The chick chorioallantoic membrane (CAM) assay results showed the anti‐angiogenesis effects of this NP as concentration dependent. The results showed that these NPs at concentrations of 100 μg/ml significantly reduced the number and length of the vessels and decreased the size and weight of the embryo body. Similar results have been reported with the present study on the role of AgNPs in vasodilatation. An anti‐angiogenic effect of AgNPs was investigated in 2011, and the results showed that this NP is capable of inhibiting angiogenesis in the chorioallantoic membrane [25]. Similar results in the matrigel test confirmed the anti‐angiogenic effects of AgNPs in another study, and also confirmed the anti‐angiogenic effects of AgNPs in the chorioallantoic membrane [26]. In another study conducted in 2017 by Divya et al. [27], the angiogenic effect of zinc oxide NPs coated with gelatin was investigated using the CAM test. Results showed that the NP significantly decreased angiogenesis. The results of two studies conducted by Baharara et al. [15, 28] on the anti‐angiogenic effects of AgNPs synthesised from Achillea biebersteinii and Saliva officinalis showed that these NPs are capable of inhibiting angiogenesis and that these results are similar to the results of this paper. There are several ways to investigate cell migrations. One of the easiest methods is to use a scratch test. In this paper, the non‐migration of NP‐treated cells was demonstrated by this method, which indicates the role of NPs in the non‐migration of cancer cells and thus preventing the metastasis of these cells in the body.
5 Conclusion
In summary, the findings of this paper indicate that the NPs coated with PP extract have cytotoxic effects in breast cancer cells and can prevent invasions of these cancers and these NPs also inhibit angiogenesis in the CAM assay and, by reducing the expression of VEGF‐R, reduces survival and angiogenesis.
6 References
- 1. Bhat T.A. Singh R.P.: ‘Tumor angiogenesis – a potential target in cancer chemoprevention’, Food Chem. Toxicol., 2008, 46, (4), pp. 1334 –1345 [DOI] [PubMed] [Google Scholar]
- 2. Prabhu V.V. Chidambaranathan N. Gopal V.A..: ‘Historical review on current medication and therapies for inducing and inhibiting angiogenesis’, J. Chem. Pharm. Res., 2011, 3, pp. 526 –533 [Google Scholar]
- 3. Nishida N. Yano H. Nishida T. et al.: ‘Angiogenesis in cancer’, Vasc. Health Risk Manage., 2006, 2, (3), p. 213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ucuzian A.A. Gassman A.A. East A. et al.: ‘Molecular mediators of angiogenesis’, J. Burn Care Res., 2010, 31, (1), pp. 158 –175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maxwell P.H. Dachs G.U. Gleadle J.M. et al.: ‘Hypoxia‐inducible factor‐1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth’, Proc. Natl. Acad. Sci. USA, 1997, 94, (15), pp. 8104 –8109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reinmuth N. Liu W. Jung Y.D. et al.: ‘Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival’, FASEB J., 2001, 15, (7), pp. 1239 –1241 [DOI] [PubMed] [Google Scholar]
- 7. Baharara J. Amini E. Mousavi M.: ‘The anti‐proliferative and anti‐angiogenic effect of the methanol extract from brittle star’, Rep. Biochem. Mol. Biol., 2015, 3, (2), p. 68 [PMC free article] [PubMed] [Google Scholar]
- 8. Otrock Z.K. Hatoum H.A. Musallam K.M. et al.: ‘Is VEGF a predictive biomarker to anti‐angiogenic therapy’, Crit. Rev. Oncol. Hematol., 2011, 79, (2), pp. 103 –111 [DOI] [PubMed] [Google Scholar]
- 9. Li J. Qiu D.M. Chen S.H. et al.: ‘Suppression of human breast cancer cell metastasis by coptisine in vitro’, Asian Pac. J. Cancer Prev., 2014, 15, (14), pp. 5747 –5751 [DOI] [PubMed] [Google Scholar]
- 10. Banerjee D. Harfouche R. Sengupta S.: ‘Nanotechnology‐mediated targeting of tumor angiogenesis’, Vasc. Cell, 2011, 3, (1), p. 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Namvar F. Azizi S. Mohamad R. et al.: ‘Nanosized silver–palm pollen nanocomposite, green synthesis, characterization and antimicrobial activity’, Res. Chem. Intermediate, 2016, 42, (3), pp. 1571 –1581 [Google Scholar]
- 12. Sawadogo W.R. Cerella C. Al‐Mourabit A. et al.: ‘Cytotoxic, antiproliferative and pro‐apoptotic effects of 5‐hydroxyl‐6, 7, 3′, 4′, 5′‐pentamethoxyflavone isolated from Lantana ukambensis ’, Nutrients, 2015, 7, (12), pp. 10388 –10397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amini E. Nabiuni M. Baharara J. et al.: ‘Metastatic inhibitory and radical scavenging efficacies of saponins extracted from the brittle star (Ophiocoma erinaceus)’, Asian Pac. J. Cancer Prev., 2015, 16, pp. 4751 –4758 [DOI] [PubMed] [Google Scholar]
- 14. Soltani M. Parivar K. Baharara J. et al.: ‘Transcriptional analysis of VEGF‐d and TGFβ genes in MCF7 cells exposed to saponin isolated from Holothuria leucospilota (sea cucumber)’, Rep. Biochem. Mol. Biol., 2015, 4, (1), p. 25 [PMC free article] [PubMed] [Google Scholar]
- 15. Baharara J. Namvar F. Ramezani T. et al.: ‘Green synthesis of silver nanoparticles using Achillea biebersteinii flower extract and its anti‐angiogenic properties in the rat aortic ring model’, Molecules, 2014, 19, (4), pp. 4624 –4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang T. Wang L. Chen Q. et al.: ‘Cytotoxic potential of silver nanoparticles’, Yonsei Med. J., 2014, 55, (2), pp. 283 –291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bahmanpour S. Kavoosi F. Talaei T. et al.: ‘Effects of date palm (Phoenix dactylifera) gemmule extract on morphometric parameters of reproductive tissues, hormones and sperm quality in rat’, Aesthet. Surg. J., 2013, 10, (3), pp. 144 –150 [Google Scholar]
- 18. Azizi S. Namvar F. Mohamad R. et al.: ‘Facile biosynthesis and characterization of palm pollen stabilized ZnO nanoparticles’, Mater. Lett., 2015, 148, pp. 106 –109 [Google Scholar]
- 19. Namvar F. Rahman H.S. Mohamad R. et al.: ‘Cytotoxic effects of biosynthesized zinc oxide nanoparticles on murine cell lines’, Evidence‐Based Complementary Altern. Med., 2015, 2015, p. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saranya S. Vijayaranai K. Pavithra S. et al.: ‘In vitro cytotoxicity of zinc oxide, iron oxide and copper nanopowders prepared by green synthesis’, Toxicol. Rep., 2017, 4, pp. 427 –430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ostad S.N. Dehnad S. Nazari Z.E. et al.: ‘Cytotoxic activities of silver nanoparticles and silver ions in parent and tamoxifen‐resistant T47D human breast cancer cells and their combination effects with tamoxifen against resistant cells’, Avicenna J. Med. Biotechnol., 2010, 2, (4), p. 187 [PMC free article] [PubMed] [Google Scholar]
- 22. Ostad S.N. Dehnad S. Nazari Z.E. et al.: ‘Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (roxb.)’, Front. Mol. Biosci., 2017, 4, p. 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mittal A.K. Bhaumik J. Kumar S. et al.: ‘Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential’, J. Colloid Interface Sci., 2014, 415, pp. 39 –47 [DOI] [PubMed] [Google Scholar]
- 24. Rajenran R. Ganesan N.I.T.H.Y.A. BaluSK A.S. et al.: ‘Green synthesis, characterization, antimicrobial and cytotoxic effects of silver nanoparticles using Origanum heracleoticum L leaf extract’, Int. J. Pharm. Pharm. Sci., 2015, 7, (4), pp. 288 –293 [Google Scholar]
- 25. Will S.E.A. Favaron P.O. Pavez M.A. et al.: ‘Bactericidal silver nanoparticles present an antiangiogenic effect in the chorioallantoic membrane model (CAM)’, Sci. Microbial Pathogens: Commun. Current Res. Technol. Adv., 2011, 1, pp. 219 –227 [Google Scholar]
- 26. Gurunathan S. Lee K.J. Kalishwaralal K. et al.: ‘Antiangiogenic properties of silver nanoparticles’, Biomaterials, 2009, 30, (31), pp. 6341 –6350 [DOI] [PubMed] [Google Scholar]
- 27. Divya M. Vaseeharan B. Abinaya M. et al.: ‘Biopolymer gelatin‐coated zinc oxide nanoparticles showed high antibacterial, antibiofilm and anti‐angiogenic activity’, J Photochem. Photobiol. B., 2018, 178, pp. 211 –218 [DOI] [PubMed] [Google Scholar]
- 28. Baharara J. Namvar F. Mousavi M. et al.: ‘Anti‐angiogenesis effect of biogenic silver nanoparticles synthesized using Saliva officinalis on chick chorioallantoic membrane (CAM)’, Molecules, 2014, 19, (9), pp. 13498 –13508 [DOI] [PMC free article] [PubMed] [Google Scholar]


