Abstract
Breast cancer is a major cause of cancer mortality. Regarding the advantages of polymeric nanoparticles as drug delivery systems with targeting potential, in this study the antitumor mechanism of targeted docetaxel polymeric nanoparticles of Ecoflex® was exploited. Since the overexpression of HER‐2 receptor in breast cancer cases is associated with poor prognosis and more aggressive disease, the proposed nanoparticles were conjugated to HER‐2 specific aptamer molecules. In vitro cytotoxicity was evaluated by MTT assay. Flow‐cytometry analysis was performed to evaluate the cellular uptake of nanoparticles loaded with a fluorescent probe. Anti‐migration effects of samples were studied. Annexin IV‐FITC and propidium iodide were implemented to investigate apoptosis induction and cell cycle analysis. Enhanced cytotoxicity compared with free docetaxel was explained considering improved cellular uptake of the nanoparticles and induced apoptosis in a larger portion of cells. Lower relative migration demonstrated enhanced anti‐migration effect of nanoparticles, and cell cycle was arrested in G2/M phase using both formulations so the anti‐microtubule mechanism of the drug was not altered. Therefore, this system could offer a potential substitute for the currently marketed docetaxel formulations, which may reduce adverse effects of the drug, while further in vivo and clinical investigations are required.
Inspec keywords: cancer, molecular biophysics, drug delivery systems, fluorescence, biomedical materials, drugs, tumours, nanomedicine, proteins, toxicology, biochemistry, nanoparticles, diseases, cell motility, polymers
Other keywords: antitumor mechanism, targeted docetaxel polymeric nanoparticles, HER‐2 specific aptamer molecules, MTT assay, flow‐cytometry analysis, annexin IV‐FITC, apoptosis induction, cell cycle, lower relative migration, cancer mortality, drug delivery systems, aggressive disease, in vitro cytotoxicity, cellular uptake, breast cancer cell apoptosis, antimetastatic effect, HER‐2 aptamer‐targeted Ecoflex nanoparticles, antimigration effect, antimicrotubule mechanism, HER‐2 receptor, fluorescent probe, propidium iodide
1 Introduction
Breast cancer is the fifth cause of cancer death with 12.9% mortality rate reported in 2012, and almost 25% metastatic relapse. Genetic, reproductive, and hormonal risk factors could be involved in the aetiology of this disease. Breast cancer could be considered as a collection of separate diseases with different molecular, epidemiologic, and phenotypic characteristics. Therefore, identification of the subtype of each case would help in providing prognostic evaluation of the disease, and determining the therapeutic targets [1, 2]. Regarding the molecular aspects, different subtypes of breast cancer have different origins; thus, they differ in presence of receptors, for instance, oestrogen receptor (ER) progenitors are the origins of luminal subtypes; while, hormone receptors are not expressed in basal subtype, which could be due to originating at early steps of differentiation and lack of breast cancer gene 1 (BRCA1) function, that is required for expression of ER in the progenitors [2, 3]. The presence of human epidermal growth factor receptor 2 (HER‐2 orErbB2) receptor is another variable regarded in classification of breast cancer subtypes. HER‐2 is a trans‐membrane epithelial growth factor receptor amplified in 20–25% of human breast cancer cases and is associated with poor prognosis and more aggressive disease [4].
A notable importance of identifying the subtype of breast cancer is to determine the treatment strategy. In case of HER‐2 positive breast cancers, humanised HER‐2 antibodies such as trastuzumab (Herceptin) and pertuzumab could be added as targeting agents to the drug delivery system [5]. Antibodies provide suitable targeting ligands due to their high affinity and specificity; however, in spite of their advantages, various investigations are focused on finding substitute affinity agents without the draw backs of antibodies, including extremely high production costs, sophisticated eukaryotic machinery needed in production procedure, and poor stability causing denaturation and limited shelf‐life [6, 7].
Aptamers are DNA or RNA oligonucleotides with specific three‐dimensional structures formed according to their sequence, providing the opportunity of binding to a determined target with high affinity and specificity, proposed as a more stable substitute for antibodies with easier manufacturing and modification procedures [8].
Among the numerous FDA approved chemotherapeutic agents, docetaxel (Taxotere® Sanofi Aventis) is a noteworthy broad spectrum antitumor drug with high clinical efficacy against breast, lung, ovarian, prostate, gastric, head, and neck cancers. Its unique characteristics include high cytotoxicity, linear pharmacokinetics, and extended intracellular retaining time, even in comparison with other taxane‐derived agents, such as paclitaxel [9]. Its mechanism of action is performed via blocking the microtubules, which play a critical role in mitotic process, causing cell cycle arrest and inhibition of cell proliferation. Furthermore, some signalling pathways such as apoptosis are promoted due to phosphorylation and inactivation of Bcl‐2 gene by the antimicrotubule agents, including docetaxel (DTX) [10]. Angiogenesis, an important process in tumour progression and metastasis is also inhibited by this drug. DTX affects the epidermal growth factor receptor (EGFR) signalling pathway, which influences cell cycle regulation, angiogenesis, and apoptosis. Rat sarcoma virus homologue G‐protein (Ras) and mitogen‐activated protein kinase (MAP kinase) are effector proteins in this pathway, which convey the signals from cell surface to the nucleus. Ras proteins are involved in cell proliferation and apoptosis, and are associated with HER‐2 overexpression [9].
DTX‐related adverse effects, including neutropenic fever, anaemia, fluid retention, myalgia, peripheral neuropathy, skin and nail toxicity, and epiphora, are developed because healthy cells would also undergo the aforementioned mechanisms. These effects would even deprive the patient of adequate treatment [11, 12]. Therefore, a great deal of attention has been paid to development of new DTX delivery systems, implementing the advantages of targeted polymeric nanoparticles, which could offer the opportunity of passively targeting the tumour tissues through the enhanced permeation and retention (EPR) effect, and the possibility of addition of targeting ligands to direct the drug specifically to the site of action and eliminate its unspecific distribution throughout the body [13].
Polybutyleneadipate‐co‐butylene terephthalate (Ecoflex®, BASF) is a biodegradable and biocompatible co‐polyester. According to the previous studies, Ecoflex® is a non‐mutagenic and non‐toxic polymer with appropriate mechanical properties, and degradation time. This polymer was initially applied as packaging material, but its potential pharmaceutical application is notified recently [14].
The aim of current study was to investigate the mechanism of in vitro cytotoxicity of Ecoflex® nanoparticles (NPs) loaded with DTX and attached to HER‐2‐specific aptamer molecules (Apt‐DTX‐NPs), against a HER‐2 over expressing breast cancer cell line. The aptamer molecule was the DNA version of the minimal SE 15‐8 RNA aptamer, introduced by Kim and Jeong [15]. The affinity and specificity of this DNA aptamer was investigated by Tabasi et al. [16]. The half maximal inhibitory concentration (IC50) value of the Apt‐DTX‐NPs was compared with free DTX as an index to evaluate the effects of implementing the proposed delivery system. The mechanism of its cytotoxic effects was also investigated considering induction of apoptosis, cell cycle arrest, and cell migration.
2 Materials and method
2.1 Materials
The chemical used in the current study included: docetaxel (Cipla, India), Ecoflex® (Mw = 100,000, BASF, Germany), Pluronic F‐127 (Sigma, US), PEG (Mw = 6000), acetonitrile, dichloromethane, N,N‐dimethyl formamide (DMF) (Merck, Germany), 3‐[4, 5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyl tetrazolium bromide (MTT) (Sigma Company, USA), Roswell Park Memorial Institute (RPMI) medium, foetal bovine serum (FBS), trypsin/EDTA, penicillin/streptomycin (Biosera Europe, ZI du Bousquet, France), rhodamine B (Sigma, US), Herceptin (Roche, Switzerland), N‐(3‐Dimethylaminopropyl)‐N′‐ethylcarbodiimide hydrochloride (EDC) (Sigma‐Aldrich, Germany), N‐Hydroxysuccinimide (NHS) (Merck, Germany), GIBCO® Dulbecco's Phosphate‐Buffered Saline (DPBS), DNA aptamer with base sequence of 5′‐AGCCGCGAGGGGAGGGATAGGGTAGGGCGCGGCT‐3′ (custom‐synthetised by Genfanavaran Co, Iran).
2.2 Preparation of DTX‐loaded nanoparticles
DTX‐loaded NPs were prepared and attached to HER‐2‐specific aptamer according to the method reported by Ghassami et al. [17]. Briefly, 1:3 mixture of DTX and polymer was dissolved in a 2.7:1 mixture of dichloromethane and N,N‐dimethyl formamide. The obtained organic phase was broken into nano‐sized droplets in the electrical field of an electrospraying device (voltage of 20 kV, 12 cm distance between electrodes, feeding rate of 1 ml/h). The droplets were dried out on their way to opposite electrode, placed inside the aqueous phase containing 1% Pluronic F127. Eventually, an aqueous suspension of Ecoflex® NPs loaded with DTX (DTX‐NPs) was obtained. Aptamer molecules were attached to the DTX‐NPs via an amide bond formed via the following reactions; pretreatment of the –COOH functional groups on Ecoflex® were incubated with EDC (400 mM) and NHS (100 mM) solutions for 20 min at room temperature. Then, the activated –COOH groups of NPs were interacted with NH2 functional groups on the aptamer molecules after about 90 min incubation at room temperature to achieve HER‐2 aptamer‐targeted DTX‐NPs (Apt‐DTX‐NPs). In this reaction, sufficient amount of 100 pM aqueous solution of aptamer was used with the same volume of a 2% (w/w) of NPs dispersion [18, 19].
2.3 DTX release profile
In order to determine the appropriate incubation time of NPs with tumour cells, drug release from Apt‐DTX‐NPs was studied in PBS (pH 7.4 ± 0.2). The release medium contained 0.5% (w/v) Tween 80 for solubilisation of DTX. Dialysis bag (cut off 12000Da) was used, containing adequate aqueous suspension of Apt‐DTX‐NPs to reach the final concentration of 1.3 µg/mL DTX, which is equal to 15% of saturation solubility of DTX tri‐hydrate in the release medium, to keep the sink condition.
At predetermined time points, 100 µL samples were withdrawn from the medium and replaced with fresh medium to keep the volume constant. Each sample was filtered, using Amicon ultra centrifuge filters and analysed by HPLC system (waters, USA) equipped with a UV detector at 230 nm wavelength and using the C18 column (Waters Spherisorb®, 5 μm ODS2 4.6 × 250 mm). A mixture of acetonitrile:water (65:35) at 1 mL/min flow rate was used as mobile phase. Calibration curve was plotted in range of 0.25 to 40 µg/mL considering the inter‐day and intra‐day standard derivations [20].
2.4 In vitro cytotoxicity assay
BT‐474 and MDA‐B‐468 cells were used as HER‐2 positive and negative breast cancer cell lines, respectively [21], which were maintained in RPMI 1640 culture medium (Biosera, France) containing 10% fetal bovine serum (FBS) and 1% mixture of penicillin‐streptomycin (100 IU/ml–100 μg/ml) (Gibco, UK), and incubated at 98% relative humidity and 5% CO2. In 96‐well culture plates, cell suspension containing 6 × 104 cell/mL were seeded and maintained for 24‐h incubation period to let the cells attach to plates. Afterwards, different treatment and control samples including Apt‐DTX‐NPs, free DTX, blank Apt‐NPs (which did not contain DTX), and DMSO (in the maximum concentration needed to solubilise free DTX) were added to the plates and incubated for further 24 h, determined according to the DTX release profile. Then, 20 µL of MTT solution (5 mg/mL) was added to each well and incubated for 3 more hours to let the live cells produce formazan crystals, which were dissolved in DMSO in the next step and the absorbance of each well was analysed using an ELISA plates reader (Biotek instrument, USA) at 570 nm. All assays were performed in triplicate.
The cell survival in each treatment group was calculated using the following equation;
Cell Survival% = (absorbance of test group‐absorbance of blank)/(absorbance of control group‐absorbance of blank) × 100
The IC50 values were determined in each cell line and the differences between IC50 values of each treatment sample were investigated by statistical analysis.
2.5 Cellular uptake
Rhodamine B (RhB) was used as fluorescent probe, which was loaded instead of DTX inside the NPs (RhB‐NPs and Apt‐RhB‐NPs) to measure the cellular uptake of the aptamer‐targeted NPs by BT‐474 and MDA‐MB‐468 cell lines [22]. Following 2 h incubation of cells with RhB‐loaded NPs, the cells were washed to remove any free RhB and a FACS Calibur flow‐cytometric instrument (Becton Dickinson, USA) was implicated to quantify the amount of NPs up‐taken by the cells.
In order to make sure that any detected fluorescence was caused by the cells that had up‐taken the NPs, not the released fluorochrome, the release profile of the RhB from Apt‐NPs was evaluated using the same procedure as described for DTX release, using a fluorescence spectrometer instrument (Perkin Elmer, USA) to measure the amount of released RhB in the medium.
2.6 Cell migration assay
BT‐474 cells were collected in a serum‐free medium and incubated for 24 h under 98% humidity and 5% CO2 condition. After this starvation period, cell suspension containing 2 × 105 cells was transferred to the upper compartment of Transwell® Insert (24 mm diameter insert, 8.0 μm pore size, polycarbonate membrane, Costar, Corning Inc. USA) with 8 micron porosity. The insert was placed over the lower compartment filled with culture medium containing 10% FBS. Different control and treatment samples including blank Apt‐NPs, free DTX, and Apt‐DTX‐NPs were added to the upper compartment. Following 10 h of incubation, the cells that had migrated through the insert membrane were stained with tripane blue to differentiate live and dead cells. The live cells were counted by the microscopic examination in 10 fields and the ratio of mean number of migrated cells relative to the control group (non‐treated cells) was reported as the relative migration in each treatment group [23].
2.7 Apoptosis assay
In order to investigate the occurrence of apoptosis and/or necrosis, binding of annexin V and uptake of propidium iodide (PI) was studied using a Phosphatidyl Serine Detection Kit (IQ product, Netherlands). Then, 1 mL of a 360 × 104 cell /ml of BT‐474 cell suspension was seeded in each well of a 12‐well cell culture plate. After a 12‐h incubation period, treatment and control samples were added to the plate. Samples included free DTX, Apt‐DTX‐NPs and blank Apt‐NPs containing 40 ng/mL of DTX, almost equal to its IC50 value. Following 15 h incubation, the cells were trypsinised using trypsin‐EDTA (0.25%:0.01%) and transferred into round‐bottomed test tubes. Afterwards, the doubled staining procedure was performed using Annexin V‐ FITC/PI kit (IQ product, Netherland) according to the manufacturer description. Final analysis was performed by flow‐cytometry (BD FACS calibular, Germany) using unstained cells as negative control for cells treated with free drug and the cells treated with blank Apt‐NPs as negative control for the cells treated with Apt‐DTX‐NPs.
2.8 Cell cycle arrest
In each well of a 6‐well cell culture plate, 1 mL of a 106 cell/mL suspension of BT‐474 cells was seeded and incubated in the same condition as mentioned for MTT assay to let the cells attach to the plate and pass the lag‐phase. Then, 100 μL of the supernatant of each well was replaced with 100 μL of different samples, including free DTX, Apt‐DTX‐NPs, and blank Apt‐NPs at the concentration equal to the IC50 value of DTX on BT‐474 cell line. Following a 12‐hour incubation period, the cells were detached from the plate using trypsin and transferred to round‐bottomed tubes. Cells were washed with DPBS, fixed by slowly re‐suspending in 70% ice‐cold ethanol and placed at −20°C, overnight. When ethanol was removed, the cells were washed with DPBS, re‐suspended in 200 μL of Tali® cell cycle kit (Invitrogen, UK), and analysed by flow‐cytometer using FACS Calibur (Becton Dickinson, USA), after 30 min incubation at room temperature in a dark place.
2.9 Statistical analysis
Statistical analysis of data was performed by SPSS software (version19, SPSS Inc, USA). Data are expressed as mean ± standard deviation. Comparison between groups was performed via ANOVA or independent t ‐test; with P ‐value <0.05 considered as statistical significance.
3 Results
3.1 DTX release profile form Apt‐DTX‐NPs
The drug release profile from Apt‐DTX‐NPs is demonstrated in Fig. 1. Release efficiency was 47.69 ± 1.64%, and almost 60% of drug cargo was released in ∼30 h.
Fig. 1.
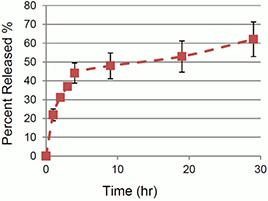
Release profile of DTX from Apt‐DTX‐Nps
3.2 In vitro cytotoxicity assay
The IC50 values of DTX‐NPs, aptamer‐targeted DTX‐NPs, and free drug on BT‐474 and MDA‐MB‐468 cells are presented in Fig. 2. On both cell lines, significant differences were observed between the IC50 values of free DTX and DTX‐loaded NPs. Furthermore, in BT‐474, the HER‐2 positive cell line, significant difference was also observed between the IC50 values of DTX‐NPs and Apt‐DTX‐NPs.
Fig. 2.
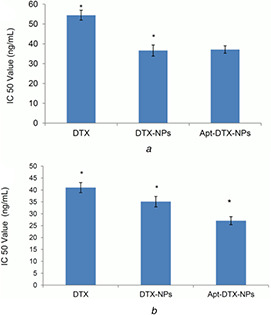
IC50 values of DTX‐NPs, Apt‐DTX‐NPs, and free drug on
(a) MDA‐MB‐468 cells, (b) BT‐474 cells (* indicates diagrams with significant difference p < 0.05)
3.3 Cellular uptake
Cellular uptake of blank NPs, free RhB, RhB‐NPs, and Apt‐RhB‐NPs, was measured in BT‐474 and MDA‐MB‐468 cell lines by flow‐cytometry analysis. The obtained diagrams are presented in Figs. 3 a and b, which illustrate a notable right shift in the diagram when RhB was entrapped in the aptamer‐targeted NPs in comparison with free RhB.
Fig. 3.
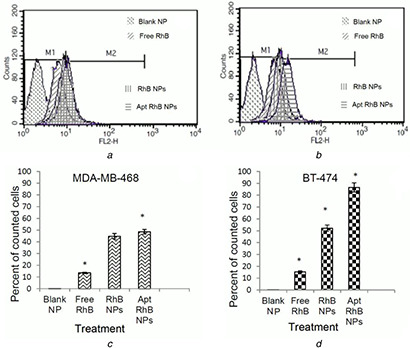
Flow‐cytometry diagrams indicating the fluorescence intensity and percent of cells counted as fluorescence event in flow‐cytometry analysis after treatment of cells with blank NPs, free RhB, RhB‐NPs, and AptRhB‐NPs
(a, c) MDA‐MB‐468, (b, d) BT‐474 cells
The M1 portion of the diagrams represents the auto‐fluorescence of the non‐treated cells and the additional intensity of fluorescence caused by uptake of probe is demonstrated as M2 portion of the diagrams. Figs. 3 c and d represent the percentage of counted cells detected with fluorescence in M2 portion of the flow‐cytometric diagrams, confirming significantly higher fluorescence intensity in cells treated with the proposed drug delivery system comparing to free RhB, and also significantly higher fluorescence intensity in BT‐474 cells in comparison with MDA‐MB‐474 cells when the targeting ligand was used on the drug delivery system.
3.4 Cell migration assay
Migration of BT‐474 cells from the upper serum‐free compartment of Transwell towards the lower compartment was evaluated following incubation with blank Apt‐NPs, free DTX, and Apt‐DTX‐NPs. The ratio of migrated cells in each group divided by the non‐treated group was reported as relative migration (Fig. 4). According to the results statistical analysis of variance, ANOVA, significant difference was observed between the treatment groups (p < 0.05).
Fig. 4.
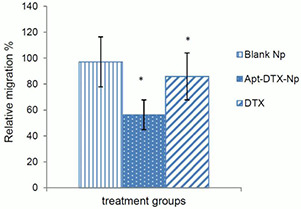
Relative migration of BT‐474 cells treated with blank NPs, Apt‐DTX‐NPs, and free DTX. (*p < 0.05)
3.5 Apoptosis assay
Fig. 5 illustrates the cellular apoptosis induced by Apt‐DTX‐NPs compared with blank‐NPs (as its control group) and apoptosis induced by free DTX compared with non‐treated cells (as its control group). It could be observed in this quadrant diagrams that cells are separated into four populations including top left: necrotic cells; top right: late apoptotic cells; bottom left: live cells; bottom right: early apoptotic cells [24].
Fig. 5.
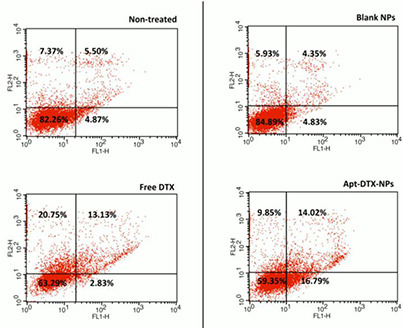
Cellular apoptosis induced by free DTX in comparison to non‐treated cells and apoptosis induced by Apt‐DTX‐NPs in comparison to blank‐NPs. The flow‐cytometric analysis divided cells into four separated groups and the percent of cells counted in each group are demonstrated on the quadrant charts. Top left: necrotic cells; top right: late apoptotic cells; bottom left: live cells; bottom right: early apoptotic cells
In this technique, cells are stained with Annexin V‐FITC (AxF), and PI and according to the type of fluorescence detected in the resulted cells, it could be distinguished whether the cells are live (AxF negative; PI negative), early apoptotic (AxF + , PI‐), late apoptotic (AxF + , PI + ) or necrotic (AxF‐, PI + ). As demonstrated in Fig. 5, treatment with free DTX resulted in 18.97% reduction of the live cells and, respectively, 13.38 and 7.63% increment of the portion of necrotic and late apoptotic cells in comparison with the non‐treated cells, which were used as negative control. In case of cells treated with Apt‐DTX‐NPs, the portion of live, apoptotic, and necrotic cells had the same alteration pattern as samples treated with free DTX. However, Apt‐DTX‐NPs caused a remarkably higher portion of early apoptotic cells (13.96% higher than free DTX) and lower portion of necrotic cells (10.9% lower than free DTX), while the amount of live cells and late apoptotic cells have almost remained the same.
3.6 Cell cycle assay
In order to study the possible effects of the proposed delivery system on cell cycle arrest caused by DTX, a flow‐cytometric analysis was performed. The results of treatment with Apt‐DTX‐NPs compared with blank Apt‐NPs and free DTX on distribution of DNA in the cell cycle are illustrated in Fig. 6.
Fig. 6.
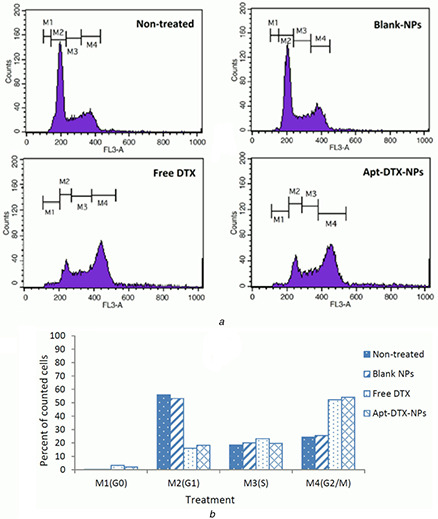
Cell cycle assay demonstrating the DNA distribution in cells treated with free DTX, Apt‐DTX‐NPs, and blank‐NPs in comparison to non‐treated cells
(a) Flow‐cytometry diagrams, (b) Effect of different treatments on cell cycle phases, measured by analysis of the related flow‐cytometry diagrams
According to the obtained flow‐cytometry diagrams, a major publication of non‐treated cells were in G1 phase (55.96%) and the rest of cells were in G2/M (24.40%) and S (18.51%) phases.
The percentage of cells in G0 phase was negligible (0.23%). Almost the same results were obtained when the cells were treated with blank Apt‐NPs. However, treatment with free DTX and Apt‐DTX‐NPs caused a noticeable increased cell population in G2/M phase, 52.26 and 54.14%, respectively. The results indicated cell cycle arrest in mitosis phase following treatment with these samples without any significant difference between free DTX and DTX which is entrapped in the proposed delivery system.
4 Discussion
Considering the remarkable advantages of aptamers, these molecules are investigated as targeting ligands providing suitable substitutes for monoclonal antibodies, with comparable affinity and specificity, besides simplicity of chemical synthesis procedure, versatility, and possibility of performing modifications required for each drug delivery system or target site [25]. Thus, in this study, a single‐stranded DNA aptamer was investigated to specifically guide the DTX‐loaded Ecoflex® nanoparticles, a biodegradable, and biocompatible polymer [26], to HER‐2 overexpressing tumour cells. Tabasi et al. [16] investigated the affinity and specificity of this aptamer molecule, which was the DNA version of the minimal SE 15‐8 RNA aptamer, introduced by Kim and Jeong [15]. Apart from providing higher stability, which is the important advantage of DNA molecule over the RNA, this aptamer has shorter sequence, and thus simple and cost‐effective manufacturing process in comparison with other HER‐2‐specific aptamers.
In order to compare the in vitro cytotoxicity of Apt‐DTX‐NPs with free DTX, MTT assay was performed on a HER‐2 positive and HER‐2 negative cell lines. Comparing the obtained IC50 values indicated that in both cell lines the cytotoxicity of Apt‐DTX‐NPs and DTX‐NPs was significantly higher than the free DTX (p < 0.05), while in the HER‐2 positive cell line, there was also a significantly lower IC50 value (p < 0.05), demonstrating higher cytotoxicity of the targeted delivery system (Fig. 2).
Significant increment of cellular uptake, observed in flow‐cytometry analysis (Fig. 3), was caused by implication of this drug delivery system and would explain its higher cytotoxic effects.
In order to investigate greater details of the cytotoxic effect of Apt‐DTX‐NPs in comparison with free DTX, apoptosis and cell cycle assays were performed. Apoptosis is defined as a programmed physiological cell death, which is a part of tissue homeostasis. One of the events that happen in the first stages of apoptosis is loss of plasma membrane asymmetry, which causes the phosphatidyl serine residues to be exposed at the outer leaflet of plasma membrane, regardless of the cell type. Due to high affinity of Annexin V to phosphatidyl serine residues, FITC‐labelled‐Annexin V could be implicated to detect loss of asymmetry in the membrane. Propidium iodide is also added simultaneously in order to discriminate the apoptotic cells from dead cells. PI is a DNA stain which is not permeable through cell membrane and is only able to be bound to DNA when the membrane has lost its integrity. Thus, induction of apoptosis could be evaluated via a double‐staining method, using AnxV‐FITC and PI, following analysis by flow‐cytometry or fluorescence microscopy [27, 28, 29].
In the cell cycle assay, PI was used to determine the DNA content of cells fixed in ethanol to evaluate the cell cycle phase of the cells following treatment. It could be interpreted from the flow‐cytometric analysis that a G2/M cell cycle arrest was induced when cells were treated with free DTX (Fig. 6), this finding is in accordance with the mechanism of action of this drug, which inhibits the function of microtubules and prevents new cell formation. The cell cycle arrest at G2/M phase was reported to be almost equal when HER‐2 positive cells were treated with Apt‐DTX‐NPs comparing to free DTX (Fig. 6). Therefore, the delivery system does not attenuate the effect of DTX on preventing the microtubule function.
The results of cell cycle (Fig. 6) and apoptosis assay (Fig. 5) were in accordance with MTT assay (Fig. 2). It is not possible to differentiate the necrotic and apoptotic cells with MTT assay, possibly because the initial steps of apoptosis is associated with alterations in the membrane of mitochondria [30], which could influence the formation of formazane crystals in the mitochondria. According to the results achieved in the present study, free DTX is able to propel the cells towards necrosis faster than the entrapped drug, and the portion of necrotic cells is higher when the cells are treated with free drug. This could be caused by the controlled release of DTX from the delivery system (Fig. 1). Since the percentage of apoptotic cells was also greater when they were treated with the targeted NP system, this would clarify higher cytotoxic effect of Apt‐DTX‐NPs in MTT assay, in which none of apoptotic and necrotic cells are taken into account as live cells.
Necrosis and apoptosis are major mechanisms of cell death; however, understanding of the molecular mechanisms involved in the clearance pathways for necrotic versus apoptotic cells would clarify the differences observed in the immunological outcomes. Necrotic cell death, which could occur as a result of trauma or toxins, is associated with rapid loss of integrity in cell membrane and stimulates host inflammatory response. These membrane‐damaged necrotic cells would directly stimulate an immunogenic response, triggered by various endogenous ‘danger’ signals, such as uric acid/MSU crystals, HMGB1, dsDNA, and spliceosome‐associated protein 130 (SAP130). In contrast, apoptosis is mediated by an intracellular cysteine‐dependent protease family, known as caspases, and apoptotic cells do not usually provoke inflammation, because cells would initially shrink to maintain the integrity of plasma membrane so the intracellular content, including proinflammatory signals are not released before being ingested by the phagocytes [31, 32, 33, 34, 35, 36].
In contrary to some previously reported delivery systems [37, 38, 39], treatment with the Apt‐DTX‐NPs investigated in the present study caused an approximately twofold increment in the portion of apoptotic cells (including cells in late and early stage), increased population of early apoptotic cells, and decreased population of necrotic cells in comparison with the free drug. Considering the above‐mentioned inflammatory response associated with necrosis which results in tissue damage [33], increment of early apoptosis and decreased necrosis by the Apt‐DTX‐NPs could be interpreted as an index of reduction of concomitant adverse effects associated with this drug delivery system; while treatment with the free drug resulted in remarkably higher necrotic cells that would consequently cause adverse effects due to the associated tissue damage.
Apart from playing the role of a drug carrier, NP delivery systems could participate in mediating biological effects of the active drug cargo; for instance, Luo et al. [39] investigated the molecular mechanism of apoptosis induced by DTX‐loaded oleic acid‐coated hydroxyapatite NPs. Cell cycle analysis demonstrated significant arrest of PC3 cells in G2/M phase, as a result of treatment with DTX or DTX‐NPs. While, void NPs did not have any influence on cell cycle, their DTX‐loaded NPs exhibited higher G2/M arrest in comparison with free drug. Besides, the results of apoptosis assay indicated that treatment with NPs loaded with DTX caused greater apoptotic cell population comparing to free drug; however, 12, 24, and 36 h treatment with these NPs resulted in higher percentage of late apoptotic cells than free DTX, while the early apoptotic portion of cells were almost equal in both treated cell groups.
Chen et al. [24] also reported G2/M phase cell cycle arrest by anti‐PSMA(prostate specific membrane antigen) aptamer‐decorated DTX‐loaded polymeric NPs prepared by solvent diffusion method using functional copolymer of poly(lactic‐co‐glycolic acid) and polyethylene glycol (PLGA‐b‐PEG) and sodium oleate in LNCap prostatic cancer cells. They reported higher in vitro antitumor effect, enhanced cellular uptake, and greater apoptotic induction by this drug delivery system comparing to the free DTX. High affinity of aptamer‐conjugated NPs to PSMA causing greater intracellular delivery of DTX could interpret these results.
Tran et al. [40] developed lipid polymer hybrid (LPH) NPs with a pH‐responsive PEG layer, using a modified emulsification method with an organic phase composed of Capryol 90, tocopherol polyethylene glycol succinate (TPGS), dimethyl dodecyl amido betaine (DDAB), and DTX an aqueous phase containing PEG‐b‐polyaspartic acid. According to their results, this delivery system loaded with DTX could effectively induce apoptosis in cancer cells, with a remarkable increment of early apoptotic cells. The results also exhibited high cellular uptake which was attributed to the electrostatic interactions between the negatively charged plasma membrane of cells and the positively charged LPH NPs.
It was suggested by Ouyang et al. [41] that DTX can inhibit the migration and invasion of breast cancer cells and this effect might be associated with suppression of filopodia formation. In order to investigate whether this effect was improved by Apt‐DTX‐NPs, and also to evaluate the effect of drug‐free delivery system on this mechanism, relative migration of BT‐474 breast cancer cells was compared in cells treated with Apt‐DTX‐NPs in comparison with free DTX and blank NPs. According to the obtained results (Fig. 3), an anti‐migration effect of DTX was observed significantly (p < 0.05). Besides, the proposed targeted delivery system was able to significantly decrease the relative migration percentage (p < 0.05) which means the anti‐migration effects of DTX was improved in the designed targeted NPs. Since the delivery system itself did not exhibit any effect on migration of cancer cell, these results could be justified by notifying the enhanced uptake of the targeted NPs by the cells that has probably led to inhibit the migration more efficiently comparing to the free drug.
5 Conclusion
Aptamers are sensitive, selective, stable, and non‐expensive molecules which could provide suitable affinity and specificity to replace monoclonal antibodies in targeting drug delivery. In this study, polymeric NPs loaded with DTX and attached to HER‐2‐specific aptamer ligand were evaluated in comparison with free DTX. Considering higher in vitro cytotoxicity and cellular uptake, in addition to the effect on cell migration, cell cycle arrest, and apoptosis, the proposed NPs seems to provide a potential delivery system for currently marketed formulations of DTX. Further investigations should be performed to confirm that the in vivo performance of this system is also superior regarding anti‐tumour efficacy and adverse effects. Also clinical investigations can elucidate the pharmacokinetic of the designed nanoparticles in comparison with the marketed drug.
6 References
- 1. Liu F.C. Lin H.T. Kuo C.F. et al.: ‘Epidemiology and survival outcome of breast cancer in a nationwide study’, Oncotarget, 2017, 8, (10), pp. 16939 –16950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bertucci F. Finetti P. Birnbaum D.: ‘Basal breast cancer: a complex and deadly molecular subtype’. CurrMol Med., 2012, 12, (1), pp. 96 –110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rouzier R. Perou C.M. Symmans W.F. et al.: ‘Breast cancer molecular subtypes respond differently to preoperative chemotherapy’, Clin. Cancer Res., 2005, 11, pp. 5678 –5685 [DOI] [PubMed] [Google Scholar]
- 4. Lewis Phillips G.D. Li G. Dugger D.L. et al.: ‘Targeting HER2‐positive breast cancer with trastuzumab‐DM1, an antibody‐cytotoxic drug conjugate’, Cancer Res., 2008, 68, (22), pp. 9280 –9290 [DOI] [PubMed] [Google Scholar]
- 5. Bauer K.R. Brown M. Cress R.D. et al.: ‘Descriptive analysis of estrogen receptor (ER)‐negative, progesterone receptor (PR)‐negative, and HER2‐negative invasive breast cancer, the so‐called triple‐negative phenotype’, Cancer, 2007, 109, (9), pp. 1721 –1728 [DOI] [PubMed] [Google Scholar]
- 6. Chames P. Van Regenmortel M. Weiss E. et al.: ‘Therapeutic antibodies: successes, limitations and hopes for the future’, Br. J. Pharmacol., 2009, 157, (2), pp. 220 –233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Sullivan C.K.: ‘Aptasensors – the future of biosensing’, Anal. BioanalChem., 2002, 372, (1), pp. 44 –48 [DOI] [PubMed] [Google Scholar]
- 8. Peyrin E.: ‘Nucleic acid aptamer molecular recognition principles and application in liquid chromatography and capillary electrophoresis’, J. Sep. Sci., 2009, 32, (10), pp. 1531 –1536 [DOI] [PubMed] [Google Scholar]
- 9. Herbst R.S. Khuri F.R.: ‘Mode of action of docetaxel – a basis for combination with novel anticancer agents’, Cancer Treat Rev., 2003, 29, (5), pp. 407 –415 [DOI] [PubMed] [Google Scholar]
- 10. Haldar S. Basu A. Croce C.M.: ‘Bcl‐2 is The Guardian of microtubule integrity’, Cancer Res., 1997, 57, pp. 229 –233 [PubMed] [Google Scholar]
- 11. Baker J. Ajani J. Scotte F. et al.: ‘Docetaxel‐related side effects and their management’, Eur. J. OncolNurs., 2008, 12, (3), pp. 253 –268 [DOI] [PubMed] [Google Scholar]
- 12. Esmaeli B. Valero V. Ahmadi M.A. et al.: ‘Canalicular stenosis secondary to docetaxel (Taxotere) a newly recognized side effect’, Am. Academy Ophthalmol., 2001, 108, (5), pp. 994 –995 [DOI] [PubMed] [Google Scholar]
- 13. Liua Q. Li R. Zhub Z. et al.: ‘Enhanced antitumor efficacy, biodistribution and penetration of docetaxel‐loaded biodegradable nanoparticles’, Int. J. Pharm., 2012, 430, pp. 350 –358 [DOI] [PubMed] [Google Scholar]
- 14. Varshosaz J. Ghassami E. Noorbakhsh A. et al.: ‘Poly (butylene adipate‐co‐butylene terephthalate) nanoparticles prepared by electrospraying technique for docetaxel delivery in ovarian cancer induced mice’, Drug DevInd Pharm, 2018, 44, (6), pp. 1012 –1022 [DOI] [PubMed] [Google Scholar]
- 15. Kim M.Y. Jeong S.: ‘In vitro selection of RNA aptamer and specific targeting of ErbB2 in breast cancer cells’, Nucleic. Acid. Ther., 2011, 21, pp. 73 –178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tabasi A. Noorbakhsh A. Sharifi E.: ‘Reduced graphene oxide‐chitosan‐aptamer interface as new platform for ultrasensitive detection of human epidermal growth factor receptor 2’, BiosensBioelectron, 2017, 95, pp. 117 –123 [DOI] [PubMed] [Google Scholar]
- 17. Ghassami E. Varshosaz J. Jahanian‐najafabadi A. et al.: ‘Pharmacokinetics and in vitro/in vivo antitumor efficacy of aptamer‐targeted ecoflex® nanoparticlesfor docetaxel delivery in ovarian cancer’, Int. J. Nanomed., 2018, 13, pp. 493 –504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farokhzad O.C. Jon S. Khademhosseini A. et al.: ‘Nanoparticle‐aptamerbioconjugates: a new approach for targeting prostate cancer cells’, Cancer, 2004, 64, (21), pp. 7668 –7672 [DOI] [PubMed] [Google Scholar]
- 19. Farokhzad O.C. Cheng J. Teply B.A. et al.: ‘Targeted nanoparticle‐aptamerbioconjugates for cancer chemotherapy in vivo’, Proc. Natl. Acad. Sci., 2006, 103, (16), pp. 6315 –6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varshosaz J. Taymouri S. Hassanzadeh F. et al.: ‘FolatedSynperonic‐CholesterylHemisuccinate polymeric micelles for the targeted delivery of docetaxel in melanoma’, BioMed Res. Int., 2015, 2015, pp. 1 –17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anido J. Matar P. Albanell J. et al.: ‘ZD1839, a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulinsignaling in her2‐overexpressing breast cancer cells’, Clin. Cancer Res., 2003, 9, (4), pp. 1274 –1283 [PubMed] [Google Scholar]
- 22. Xu Z. Chen L. Gu W. et al.: ‘The performance of docetaxel‐loaded solid lipid nanoparticles targeted to hepatocellular carcinoma’, Biomaterials, 2009, 30, (2), pp. 226 –232 [DOI] [PubMed] [Google Scholar]
- 23. Le X.F. Almeida M.I. Mao W. et al.: ‘Modulation of MicroRNA‐194 and cell migration by HER2‐targeting trastuzumab in breast cancer’, Plos one, 2012, 7, (7), p. e41170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Z. Tai Z. Gu F. et al.: ‘Aptamer‐mediated delivery of docetaxel to prostate cancer through polymeric nanoparticles for enhancement of antitumor efficacy’, Eur. J. Pharm Biopharm., 2016, 107, pp. 130 –141 [DOI] [PubMed] [Google Scholar]
- 25. Chen Z. Li L. Zhao H. et al.: ‘Electrochemical impedance spectroscopy detection of lysozyme based on electrodeposited gold nanoparticles’, Talanta, 2011, 83, (5), pp. 1501 –1506 [DOI] [PubMed] [Google Scholar]
- 26. Varshosaz J. Riahi S. Ghassami E. et al.: ‘Transferrin‐targeted poly(butylene adipate) terephthalate nanoparticles for targeted delivery of 5‐fluorouracil in HT29 colorectal cancer cell line’, J. BioactCompatPolym, 2017, 32, (5), pp. 503 –527 [Google Scholar]
- 27. van Engeland M. Nieland L.J. Ramaekers F.C. et al.: ‘Annexin V‐affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure’, Cytometry, 1998, 31, (1), pp. 1 –9 [DOI] [PubMed] [Google Scholar]
- 28. Pethig R. Talary M.S.: ‘Dielectrophoretic detection of membranemorphology changes in jurkat T‐cells undergoingetoposide‐induced apoptosis’, IET Nanobiotechnol.., 2007, 1, (1), pp. 2 –9 [DOI] [PubMed] [Google Scholar]
- 29. EdalatFard S. Tafvizi F. BikhofTorbati M.: ‘Silver nanoparticles biosynthesised usingCentellaasiatica leaf extract: apoptosisinduction in MCF‐7 breast cancer cell line’, IET Nanobiotechnol.., 2018, 12, (7), pp. 994 –1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kang R. Zeh H.J. Lotze M.T. et al.: ‘The beclin 1 network regulates autophagy and apoptosis’, Cell Death Differ., 2011, 18, (4), pp. 571 –580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Majno G. Joris I.: ‘Apoptosis, oncosis, and necrosis. An overview of cell death’, Am. J. Pathol., 1995, 146, (1), pp. 3 –15 [PMC free article] [PubMed] [Google Scholar]
- 32. Manjo G. La Gattuta M. Thompson T.E.: ‘Cellular death and necrosis: chemical, physical and morphologic changes in rat liver’, Virchows Arch PatholAnatPhysiolKlin Med, 1960, 333, pp. 421 –465 [DOI] [PubMed] [Google Scholar]
- 33. Rock K.L. Kono H.: ‘The inflammatory response to cell death’, Annu Rev. Pathol., 2008, 3, pp. 99 –126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huynh M.L. Fadok V.A. Henson P.M.: ‘Phosphatidylserine‐dependent ingestion of apoptotic cells promotes TGF‐beta1 secretion and the resolution of inflammation’, J. Clin. Invest, 2002, 109, (1), pp. 41 –50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dorn G.W.: ‘Molecular mechanisms that differentiate apoptosis from programmed necrosis’, Toxicologic Pathol., 2013, 41, pp. 227 –234 [DOI] [PubMed] [Google Scholar]
- 36. Poon I.K.H. Hulett M.D. Parish C.R.: ‘Molecular mechanisms of late apoptotic/necrotic cell clearance’, Cell Death Differ., 2010, 17, pp. 381 –397 [DOI] [PubMed] [Google Scholar]
- 37. Li L. Tang F. Liu H. et al.: ‘In vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy’, ACS Nano., 2010, 4, (11), pp. 6874 –6882 [DOI] [PubMed] [Google Scholar]
- 38. Liu D. Wang L. Zhihong Liu Z. et al.: ‘Preparation, characterization, and in vitro evaluation of docetaxel‐loaded poly(lactic acid)‐poly(ethylene glycol) nanoparticles for parenteral drug delivery’, J. Biomed. Nanotech., 2010, 6, pp. 675 –682 [DOI] [PubMed] [Google Scholar]
- 39. Luo Y. Ling Y. Guo W. et al.: ‘Docetaxel loaded oleic acid‐coated hydroxyapatite nanoparticles enhance the docetaxel‐induced apoptosis through activation of caspase‐2 in androgen independent prostate cancer cells’, J. Control Release, 2010, 147, (2), pp. 278 –288 [DOI] [PubMed] [Google Scholar]
- 40. Tran T.H. Ramasamy T. Choi J.Y. et al.: ‘Tumor‐targeting, pH‐sensitive nanoparticles for docetaxel delivery to drug‐resistant cancer cells’, Int. J. Nanomed., 2015, 10, pp. 5249 –5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ouyang L. Li L. Zhu K. et al.: ‘Docetaxel inhibits the migration and invasion of breast cancer cells by suppressing filopodia formation’, Tumor, 2013, 33, (9), pp. 776 –780 [Google Scholar]


