Abstract
Cisplatin treatment results in acute kidney injury (AKI) by the phosphorylation of mixed lineage kinase domain‐like protein (MLKL). The knockout of MLKL, which is a principle mediator of necroptosis, is believed to alleviate the AKI symptoms. The present study was aimed to improve the therapeutic efficacy in AKI. For this purpose, miR‐500a‐3P was identified as appropriate miRNA therapeutics and loaded in liposome delivery carrier. The authors have showed that the miR‐LIP directly controls the expression of RIPK3 and MLKL – a modulator of necroptosis and thereby reduces the severity of kidney injury. The miR‐LIP significantly controlled the phosphorylation of MLKL compared to that of CDDP‐treated HK2 cells. Similar results are observed with RIPK3. The miR‐LIP has also been demonstrated to control the inflammatory response in tubular cells. Western blot analysis further revealed that the phosphorylation of P‐65 was mainly responsible for the inflammatory response and miR‐LIP significantly decreased the CDDP‐induced NF‐kB phosphorylation. Overall, the present study explored the molecular mechanism behind the necroptosis in AKI and potential of miRNA in targeting MLKL pathways. Study further highlights the potential advantage of liposome as a delivery carrier for miRNA therapeutics.
Inspec keywords: medical disorders, biochemistry, cancer, cellular biophysics, kidney, enzymes, drugs, toxicology, patient treatment, injuries, genetics, molecular biophysics
Other keywords: current 500.0 A, functional role, microRNA‐500a‐3P‐loaded liposomes, cisplatin‐induced AKI, cisplatin treatment results, acute kidney injury, phosphorylation, mixed lineage kinase domain, necroptosis, AKI symptoms, therapeutic efficacy, appropriate miRNA therapeutics, liposome delivery carrier, miR‐LIP, RIPK3, inflammatory response, CDDP‐induced NF‐kB, MLKL pathways
1 Introduction
Cisplatin is a well‐known anticancer drug used in the treatment of multiple cancers including ovarian, head and neck, bladder, esophagus and cervical cancers [1]. However, one of the critical limitations of cisplatin is the nephrotoxicity of the kidney. A single administration of cisplatin (100 mg/m2) results in significant acute kidney injury (AKI) [2]. It has been reported that AKI suffers from a 50% mortality rate which is likely to develop chronic kidney diseases with time [3]. The cisplatin‐induced nephrotoxicity is marked by metabolic activation, oxidative injury and inflammatory tissue damage. Specifically, AKI is the result of inflammation and cell death in the proximal tube of the kidney [4]. Although the exact mechanism of AKI is not understood well, however necroptosis is believed to cause the inflammation [5, 6]. The necroptosis causes impaired plasma membrane which results in the escape of cellular contents and causes inflammation. It has been reported that receptor interacting protein kinase (RIPK)1 and RIPK3 and mixed lineage kinase domain‐like protein (MLKL) are a main contributing factors for necroptosis [7, 8]. MLKL is activated after interacting with RIPK3 in the necrosome and the phosphorylation of MLKL promotes the oligomerisation, transfer to the inner plasma membrane and membrane permeabilisation results in necroptosis‐based cell death [9]. Studies therefore demonstrate that the RIPK/MLKL might be playing a central role in the initiation of AKI. Inhibition of either RIPK1 or deficiency of RIPK3 might control the cisplatin‐induced AKI [10]. Recently, it has been confirmed that the knockout of MLKL prevents the necroptosis of proximal tubular cells in a cisplatin‐induced AKI model [11].
MicroRNA (miRNA) are short, non‐coding RNAs involved in the regulation of expression of target genes at post‐transcriptional level via binding to the 3′ untranslated region (3′UTR) of downstream target genes that results in either degradation or inhibition of miRNA [12]. MiRNAs could directly target the 3′‐UTR of the target gene and results in the therapeutic outcome of importance. Besides, miRNA was involved in many vital biological processes including apoptosis, cell proliferation, differentiation and biological properties and physiological functions [13]. The use of miRNA in treating kidney disease is one of the interesting and upcoming research areas. By functioning as regulators of gene expression miRNAs play a crucial role in a variety of molecular processes in multiple organs including the kidney [14]. One of the earliest report shows that miR‐34a (p53 target) is increased in the proximal tubular cells after the administration of cisplatin and therefore, silencing of miR‐34a increased cisplatin toxicity leading to the conclusion that miR‐34a has a protective role during kidney injury [15]. miR‐500a‐3p, as one of the original miRNAs discovered is implicated in anticancer drug resistance and targets RIPK1 in osteosarcoma [16, 17]. However, its potential to target the downstream MLKL pathway is still unexplored and present is an effort to understand the molecular mechanism of miR‐500a‐3p in AKI.
One of the limitations of miRNA is the high instability in the systemic circulation and enzymatic degradation. At the in vitro level, miRNA was used as a lipofectamine complex; however, a stable delivery system has to be designed for the successful clinical translation of miRNA. Nanoparticulate drug delivery system provides an interesting avenue for the delivery of therapeutics to the desired region of the body. Among many nanocarriers, the liposome is one of the most studied carrier system for the delivery of therapeutics [18, 19, 20, 21, 22, 23]. The importance of liposome lies in the fact that it contains hydrophilic and hydrophobic chambers which could be used to load respective drugs. Furthermore, liposome could be surface modified to include additional functional characteristics. In the present study, we have employed a cationic liposome to encapsulate the miRNA on the surface via electrostatic interactions [24, 25].
The main aim of the present study was to improve the therapeutic efficacy in AKI. miR‐500a‐3p was used as a novel approach for the treatment of AKI. The role of miR‐500a‐3p in controlling necroptosis and its effect on the downstream signalling pathways has been studied in detail. Molecular checkpoints have been studied by means of Western blotting and RT‐PCR analysis. The use of miRNA in treating kidney disease is one of the interesting and upcoming research areas.
2 Materials and methods
2.1 Preparation of miRNA‐500‐3P‐loaded liposomes
Cationic liposome was prepared by the thin‐film hydration method. Briefly, DOTAP, EPC and cholesterol were dissolved in chloroform in a molar ratio of 3:1:2. The solution mixture was vortexed and well mixed. The organic solvent was evaporated using a rotary evaporator at 60°C for 1 h. The lipid fraction was kept in a vacuum for 3 h to remove every trace of organic solvent. The lipid fraction was incubated with HEPES buffer (20 mM) for 1 h and ultrasonicated for 20 s. The liposome was extruded for 21 times using a mini‐extruder using a 100 nm filter. The so‐formed liposome was incubated with miR‐500a‐3p at 4°C overnight. The unloaded miRNA was removed by centrifugation.
The particle size distribution was determined by ZetaSizer using the principle of dynamic light scattering technique (Zetasizer, Nano‐ZS; Malvern Instruments, Malvern, UK). The particle morphology was determined by transmission electron microscopy (TEM; H7600, Hitachi, Tokyo, Japan) at an accelerating voltage of 100 kV.
2.2 Cell culture and miRNA‐liposome transfection
The HK2 kidney cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with fetal bovine serum (FBS) and 1% antibiotic mixture. The cells were grown in a conditioned humidified incubated maintained at 37°C. For all the experiments, 3 × 105 cells were seeded in each well of six‐well plate and divided into groups; untreated control; cisplatin; and cisplatin + miR‐LIP. Cisplatin was added in a concentration of 15 µM to the HK2 cells. For cisplatin + miR‐LIP group, cisplatin was added after 24 h of miR‐LIP transfection. The cells were then collected for further analysis. For hypoxia‐induced injury model, cells were treated with sodium azide (NaN3) for 4 h and then washed and replenished with regular culture medium.
2.3 Flow cytometer analysis
The apoptosis assay was carried out by flow cytometer analysis after staining with Annexin V/PI mixture. The cells were treated with groups as mentioned above and after 24 h, cells were extracted and stained with 2.5 µl of Annexin V and 2.5 µl of PI for 15 min. The cells were diluted with binding buffer and studied using flow cytometer (BD FACS Calibur, USA).
2.4 Hoechst 33342 staining assay
The apoptosis was further determined by Hoechst 33342 staining analysis. The cells were treated with groups as mentioned above and after 24 h cells were washed and fixed with 4% paraformaldehyde. The cells were stained with Hoechst 33342 (10 µg/ml) and observed under fluorescence microscope (Nikon A1, Japan).
2.5 Protein extraction and Western blot analysis
The treated cells were washed and extracted using a stripping buffer. The cells were lysed, and proteins were collected and quantified using Bradford assay. The proteins were separated on an 8–10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gel and transferred to a Trans‐Blot polyvinylidene difluoride membranes (Bio‐Rad Laboratories). The membranes were blocked using a 5% skim milk and incubated with respective primary antibodies of P‐P65, P‐MLKL, RIPK3, KIM1 and GAPDH. The antibodies are diluted at 1:1000 and incubated overnight at 4°C. Next day, membranes were incubated with secondary antibody (mouse or rabbit) for 1 h. Blots were developed by using a superenhanced chemiluminescence substrate according to the manufacturer's protocol.
2.6 RNA extraction and RT‐PCR
The treated cells were washed and RNA was collected by the addition of Trizol (Invitrogen). The concentration of RNA was detected by NanoDrop 2000 (Thermo Fischer Scientific). One microgram of RNA was reverse transcribed using a cDNA synthesis kit (Quanta Biosciences) using T100 Thermal Cycler (BioRad). Real‐time qPCR was performed using a SYBR Green Super Mix kit on a CFX connect Real‐Time System (BioRad). The gene‐specific primers of kidney injury molecule (KIM‐1), TNF‐α and IL‐8 were normalised against GAPDH as an internal control. Primers for the Kim‐1 gene (forward primer: 5′‐TGGCACTGTGACATCCTCAGA‐3′, reverse primer: 5′‐GCAACGGACATGCCAACATA‐3′). IL‐8, forward primer: 5′‐ATGACTTCCAAGCTGGCCGTGCT‐3′, reverse primer: 5′‐TCTCAGCCCTCTTCAAAAACTTCTC‐3′. TNF‐a, forward primer: 5′‐TGTGTCTCCTGTAGTAACTG‐3′, reverse primer: 5′‐ACGAATTCCTTCCAGCGCAA‐3′.
2.7 Statistical analysis
To assess the significance between different treatment groups, a Student's t ‐test (two‐tailed) was used to determine the significance with P values at least <0.05 categorised as statistically significant.
3 Results and discussion
3.1 Physicochemical characterisation of miR‐LIP
AKI is the result of inflammation and cell death in the proximal tube of the kidney. It has been reported that RIPK1 and RIPK3 and MLKL are the main contributing factors for necroptosis.
Studies demonstrated that the RIPK/MLKL might be playing a central role in the initiation of AKI. Inhibition of either RIPK1 or deficiency of RIPK3 might control the cisplatin‐induced AKI. However, its potential to target the downstream MLKL pathway is still unexplored and present is an effort to understand the molecular mechanism of miR‐500a‐3p in AKI. For this purpose, we have designed and synthesised a unique delivery system for the delivery of miRNA (Fig. 1 a). The cationic liposomes were prepared and miRNA was loaded using the electrostatic force of interaction. The negative charge of miRNA will bind to the positive surface charge of liposomes. The average particle size of miR‐LIP was 95.4 ± 1.42 nm with a surface charge of 21.5 ± 1.98 mV (Fig. 1 b). The miRNA was loaded on the cationic liposome at N/P ratio of 4. The small particle size of <100 nm will be beneficial for the cellular uptake of nanoparticles in the biological cells. Moreover, positive charge on the particle will allow the preferential binding to the cells. The particle morphology was monitored by TEM (Fig. 1 c). The particles were perfectly spherical shaped and distributed uniformly in the TEM grid.
Fig. 1.
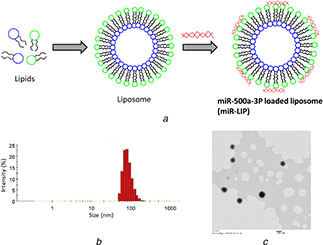
Physicochemical characterization of miRNA‐loaded liposomes
(a) Schematic presentation of preparation of miRNA‐loaded liposome, (b) Particle size distribution, (c) Particle morphology analysis by TEM
3.2 MiR‐LIP inhibits CDDP‐induced cell deaths in HK2 cells
The HK2 cells were treated with CDDP (15 µM) in a time‐dependent manner. Results show that CDDP significantly suppresses the expression of miR‐500a‐3P in HK2 cells in a time‐dependent manner. No differences were observed within the first 6 h whereas notable difference in miR‐500a‐3P was observed by 24 h (Fig. 2 a). The difference was more obvious after 48 h of incubation indicating the severe adverse effect of cisplatin in AKI. The impact of CDDP on the apoptosis of HK2 cell was evaluated by flow cytometer after staining the cells with Annexin V and PI (Fig. 2 b). Results clearly show that ∼45% of cells undergone the early or late apoptosis phase with 50% of cells viable. As expected, pretreatment with miR‐LIP significantly prevented the apoptosis and only 20% of cells were in apoptosis phase indicating the potential of miR‐500a‐3P in general and miR‐LIP in particular. The apoptosis was further confirmed by Hoechst 33342 assay (Fig. 2 c). As seen, control cells intact whereas cisplatin‐treated cells showed membrane rupturing with lot of apoptotic body formation and condensation. Consistent with flow cytometer analysis, miR‐LIP effectively prevented the apoptosis of HK2 cells.
Fig. 2.
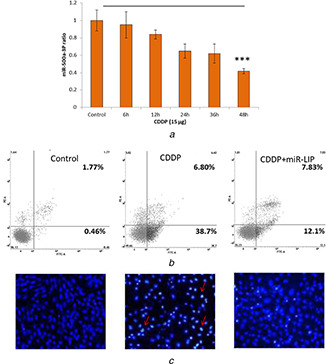
In vitro anticancer activity in HK2 cells
(a) Effect of cisplatin (CDDP) on the expression level of miR‐500a‐3P by PCR analysis, (b) Apoptosis analysis of HK2 cells after staining with Annexin V/PI using flow cytometer, (c) Qualitative apoptosis analysis of kidney cells after staining with Hoechst 33342 and observed under fluorescence microscope
3.3 MiR‐LIP inhibits CDDP‐induced inflammatory response in HK2 cells
Inflammatory markers TNF‐a and IL‐8 were evaluated after cisplatin exposure to the HK2 cells (Figs. 3 a and b). As seen, levels of TNF‐α and IL‐8 were significantly increased with regard to the non‐treated control. The remarkable increase in the inflammatory response indicates the severe adverse potential of cisplatin. In both the cases, miR‐LIP decreased the inflammatory response significantly. Western blot analysis further revealed that the phosphorylation of P‐65 was mainly responsible for the inflammatory response and miR‐LIP significantly decreased the CDDP‐induced NF‐kB phosphorylation (Figs. 3 c and d). Overexpression of miR‐500a‐3P effectively inhibited the NF‐kB‐based inflammation in the kidney cells. It can be explained by the fact that necroptotic cells undergo membrane rupturing and release the endogenous pro/inflammatory components resulting in severe inflammatory response. The overexpression of miR‐500a‐3P successfully reverses the membrane damage and inflammatory cells.
Fig. 3.

Gene expression and protein expression analysis upon treatment with respective formulations
(a, b) PCR analysis of effect of CDDP and CDDP + miR‐LIP on the protein levels of TNF‐a and IL8 expression, (c) Western blot analysis of expression level of P‐P65 after treatment with CDDP and CDDP + miR‐LIP in HK2 cells, (d) Quantification of western blot data
3.4 MiR‐LIP inhibits necroptosis by MLKL targeting
Western blot data clearly shows that phosphorylation of MLKL remarkably increased after CDDP treatment compared with control. The miR‐LIP significantly controlled the phosphorylation of MLKL compared to that of CDDP‐treated HK2 cells (Fig. 4 a). Similar results are observed with RIPK3. CDDP treatment increased the RIPK3 level while miR‐LIP significantly reduced the expression of respective protein almost to level on non‐treated cells (Fig. 4 b). The membrane translocation of MLKL is the main contributing factor of necroptosis and miR‐LIP effectively reduced the phosphorylation and thereby translocation of MLKL. Therefore, miR‐LIP might be directly responsible for the suppression of necroptosis in tubular epithelial cells by inhibiting the MLKL phosphorylation. Necroptosis is considered as a form of regulated cell death mechanism which is mediated by RIPK3 and its substrate MLKL. MLKL is phosphorylated at T357/S358 and forms the important component of RIPK3‐based downstream signalling pathway in TNF‐based necroptosis. The interaction between RIPK3 and phosphorylated MLKL results in cell death [26]. The role of CDDP in inducing AKI is believed to be via necroptosis. It has been reported that the suppression of RIPK1 or RIPK3 reduces the severity of ischaemia‐induced kidney injury or CDDP‐based nephropathy. Similarly, the inhibition of MLKL alleviates the tubular cell necroptosis in CDDP‐based nephropathy. Recently, Wogonin (Chinese medicine) has been shown to inhibit RIPK1 and suppressed the necroptosis and kidney injury [27]. Micro RNAs are reported to play a vital role in decreasing the severity of AKI by specifically targeting the components associated with necroptosis. The potential role of miR‐500a‐3P in controlling the RIPK3‐dependent necroptosis is a new finding [28]. Earlier, miR‐500a‐3P has been reported to play important role in the treatment of liver cancer and breast cancer.
Fig. 4.
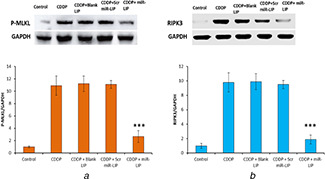
Targeting effect of miR‐LIP in HK2 cells
(a) Targeting effect of miR‐LIP on the expression of P‐MLKL, (b) RIPK3 proteins
Western blot analysis was performed to evaluate the inhibitory effect of miR‐LIP on the necroptosis
3.5 MiR‐LIP in hypoxia‐induced tubular injury
In order to determine whether miR‐LIP is effective in controlling hypoxia‐induced kidney cell injury, HK2 cells were exposed with sodium azide (NaN3) to sink anoxic environment. Results clearly showed that NaN3‐induced KIM‐1 expression was significantly downregulated by miR‐LIP (Figs. 5 a and b). Furthermore, miR‐LIP significantly controlled the NaN3‐induced inflammatory response. miR‐LIP significantly reduced the level of TNF‐a and IL‐8 (Figs. 5 c and d). The reduction of NaN3‐induced TNF‐α and IL‐8 is a clear indication of the influence of miR‐LIP on the hypoxia‐induced inflammatory response and necroptosis.
Fig. 5.
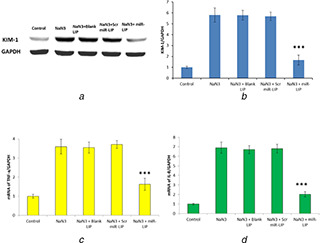
Effect of miR‐LIP on the tubular cell injury
(a) Western blot analysis of KIM‐1 protein level in HK2 cells after treatment with sodium azide, (b) Quantification of KIM‐1 protein expression, (c) PCR analysis of NaN3‐induced TNF‐a mRNA levels, (d) PCR analysis of NaN3‐induced IL‐8 mRNA levels
Overall, we have shown that miR‐500a‐3P‐loaded liposome (miR‐LIP) effectively controlled the CDDP or NaN3‐induced kidney conditions. The level of miR‐500a‐3P was significantly reduced after treatment with CDDP while replenish the cells with miR‐500a‐3P improved the conditions of AKI in terms of protein or gene expression. Especially, we have showed that miR‐500a‐3P controlled the expression of MLKL and RIPK3 and controlled the CDDP‐induced programmed cell death or necroptosis in kidney cells. We have further shown the exposure of miR‐500a‐3P effectively reduced the NF‐kB‐based inflammatory response. The miR‐LIP effectively controlled the cell necroptosis and prevented from the release of endogenous inflammatory or proinflammatory components of cells. The release of these components might result in excessive damage to the kidney cells and adjoining cells [29]. Therefore, miR‐LIP inhibited both the inflammation as well as kidney injury. One of the important aspect of the present is the use of liposome to deliver or transfect the kidney cells. Although miR‐500a‐3P has potent role in AKI, however, lack of appropriate delivery system will hamper its clinical translation. Study clearly showed that the liposome encapsulation of miR‐500a‐3P is effective in controlling the AKI.
4 Conclusions
The main aim of the present study was to improve the therapeutic efficacy in AKI. For this purpose, miR‐500a‐3P was identified as appropriate miRNA therapeutics and loaded in a liposome delivery carrier. We have shown that the miR‐LIP directly controls the expression of RIPK3 and MLKL – a modulator of necroptosis and thereby reduces the severity of kidney injury. The miR‐LIP significantly controlled the phosphorylation of MLKL compared to that of CDDP‐treated HK2 cells. Similar results are observed with RIPK3. The miR‐LIP has also been demonstrated to control the inflammatory response in tubular cells. Western blot analysis further revealed that the phosphorylation of P‐65 was mainly responsible for the inflammatory response and miR‐LIP significantly decreased the CDDP‐induced NF‐kB phosphorylation. Overall, the present study explored the molecular mechanism behind the necroptosis in AKI. The study further highlights the potential advantage of liposome as a delivery carrier for miRNA therapeutics.
5 Acknowledgments
The project was funded by the National Natural Science Foundation (20873999), Suzhou Science and Technology Bureau Technology Demonstration Project (SS201712, SS201812) and Shanghai Jiaotong University Humanities and Social Science Academic Fund (WKC20160412).
6 References
- 1. Berdis A.J.: ‘Inhibiting DNA polymerases as a therapeutic intervention against cancer’, Front. Mol. Biosci., 2017, 4, pp. 1 –12 10.3389/fmolb.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu X. Nie S. Liu Z. et al.: ‘Epidemiology and clinical correlates of AKI in Chinese hospitalized adults’, Clin. J. Am. Soc. Nephrol., 2015, 10, pp. 1510 –1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crona D.J. Faso A. Nishijima T.F. et al.: ‘A systematic review of strategies to prevent cisplatin‐induced nephrotoxicity’, Oncologist, 2017, 22, pp. 609 –619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozkok A. Edelstein C.L.: ‘Pathophysiology of cisplatin‐induced acute kidney injury’, BioMed Res. Int., 2014, 2014, Article ID 967826 pp. 1 –17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Legrand M. Dupuis C. Simon C. et al.: ‘Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study’, Crit. Care, 2013, 17, p. R278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyd J.H. Forbes J. Nakada T.A. et al.: ‘Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality’, Crit. Care Med., 2011, 39, pp. 259 –265 [DOI] [PubMed] [Google Scholar]
- 7. Galluzzi L. Kepp O. Chan F.K. et al.: ‘Necroptosis: mechanisms and relevance to disease’, Annu. Rev. Pathol., 2017, 12, pp. 103 –130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mulay S.R. Desai J. Kumar S.V. et al.: ‘Cytotoxicity of crystals involves RIPK3‐ MLKL‐mediated necroptosis’, Nat. Commun., 2016, 7, p. 10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai Z. Jitkaew S. Zhao J. et al.: ‘Plasma membrane translocation of trimerized MLKL protein is required for TNF‐induced necroptosis’, Nat. Cell Biol., 2014, 16, pp. 55 –65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang S. Zhang C. Hu L. et al.: ‘Necroptosis in acute kidney injury: a shedding light’, Cell Death Dis., 2016, 7, p. e2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linkermann A.: ‘Nonapoptotic cell death in acute kidney injury and transplantation’, Kidney Int., 2016, 89, pp. 46 –57 [DOI] [PubMed] [Google Scholar]
- 12. Selbach M. Schwanhäusser B. Thierfelder N. et al.: ‘Widespread changes in protein synthesis induced by microRNAs’, Nature, 2008, 455, pp. 58 –63 [DOI] [PubMed] [Google Scholar]
- 13. Hata A. Lieberman J.: ‘Dysregulation of microRNA biogenesis and gene silencing in cancer’, Sci. Signal., 2015, 8, p. re3 [DOI] [PubMed] [Google Scholar]
- 14. Calin G.A. Croce C.M.: ‘MicroRNA signatures in human cancers’, Nat. Rev. Cancer, 2006, 6, pp. 857 –866 [DOI] [PubMed] [Google Scholar]
- 15. Ren D. Wang M. Guo W. et al.: ‘Double‐negative feedback loop between ZEB2 and miR‐145 regulates epithelial‐mesenchymal transition and stem cell properties in prostate cancer cells’, Cell Tissue Res., 2014, 358, pp. 763 –778 [DOI] [PubMed] [Google Scholar]
- 16. Pavkovic M. Vaidya V.S.: ‘MicroRNAs and drug‐induced kidney injury’, Pharmacol. Ther., 2016, 163, pp. 48 –57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Novak J. Sana J. Stracina T. et al.: ‘Doxorubicin and liposomal doxorubicin differentially affect expression of miR‐208a and let‐7 g in rat ventricles and atria’, Cardiovasc. Toxicol., 2017, 17, pp. 355 –359 [DOI] [PubMed] [Google Scholar]
- 18. Chen Y. Gao D.Y. Huang L.: ‘In vivo delivery of miRNAs for cancer therapy: challenges and strategies’, Adv. Drug Delivery Rev., 2015, 81, pp. 128 –141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma D. Lin Q.M. Zhang L.M. et al.: ‘A star‐shaped porphyrin‐arginine functionalized poly(L‐lysine) copolymer for photo‐enhanced drug and gene co‐delivery’, Biomaterials, 2014, 35, (14), pp. 4357 –4367 [DOI] [PubMed] [Google Scholar]
- 20. Zhou X. Zheng Q. Wang C. et al.: ‘Star‐shaped amphiphilic hyperbranched polyglycerol conjugated with dendritic poly(l‐lysine) for the codelivery of docetaxel and MMP‐9 siRNA in cancer therapy’, ACS Appl. Mater. Interfaces, 2016, 8, (20), pp. 12609 –12619 [DOI] [PubMed] [Google Scholar]
- 21. Liu T. Chen S. Zhang S. et al.: ‘Transferrin‐functionalized chitosan‐graft‐poly(l‐lysine) dendrons as a high‐efficiency gene delivery carrier for nasopharyngeal carcinoma therapy’, J. Mater. Chem. B, 2018, 6, pp. 4314 –4325 [DOI] [PubMed] [Google Scholar]
- 22. Liu T. Li J. Wu X. et al.: ‘Transferrin‐targeting redox hyperbranched poly(amido amine)‐functionalized graphene oxide for sensitized chemotherapy combined with gene therapy to nasopharyngeal carcinoma’, Drug Deliv., 2019, 26, (1), pp. 744 –755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramasamy T. Ruttala H.B. Kaliraj K. et al.: ‘Polypeptide derivative of metformin with the combined advantage of a gene carrier and anticancer activity’, ACS. Biomater. Sci. Eng., 2019, 5, pp. 5159 –5168 [DOI] [PubMed] [Google Scholar]
- 24. Linkermann A. Bräsen J.H. Darding M. et al.: ‘Two independent pathways of regulated necrosis mediate ischemia‐reperfusion injury’, Proc. Natl. Acad. Sci. USA, 2013, 110, pp. 12024 –12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramasamy T. Ruttala H.B. Gupta B. et al.: ‘Smart chemistry‐based nanosized drug delivery systems for systemic applications: a comprehensive review’, J. Controlled Release, 2017, 258, pp. 226 –253 [DOI] [PubMed] [Google Scholar]
- 26. Meng X.M. Li H.D. Wu W.F. et al.: ‘Wogonin protects against cisplatininduced acute kidney injury by targeting RIPK1‐mediated necroptosis’, Lab. Invest., 2018, 98, pp. 79 –94 [DOI] [PubMed] [Google Scholar]
- 27. Brandenburger T. Salgado Somoza A. Devaux Y. et al.: ‘Noncoding RNAs in acute kidney injury’, Kidney Int., 2018, 94, pp. 870 –881 [DOI] [PubMed] [Google Scholar]
- 28. Von Mässenhausen A. Tonnus W. Linkermann A.: ‘Cell death pathways drive necroinflammation during acute kidney injury’, Nephron, 2018, 140, pp. 144 –147 [DOI] [PubMed] [Google Scholar]
- 29. Jiang C. Long J. Liu B. et al.: ‘Mir‐500a‐3p promotes cancer stemcells properties via STAT3 pathway in human hepatocellular carcinoma’, J. Exp. Clin. Cancer Res., 2017, 36, p. 99 [DOI] [PMC free article] [PubMed] [Google Scholar]


