Abstract
In the modern era of science and technology, nanotechnology is becoming popular science field because materials at nanoscale contain improved physical, chemical and biological properties. This study aimed to explore the capacity of bimetallic nanoparticle alloys of silver (Ag), copper (Cu), gold (Au) in different ratios to evaluate the effects on medicinally important plant Eruca sativa. Biochemical parameters of Eruca sativa were studied by applying bimetallic alloy nanoparticles. Seeds of Eruca sativa were germinated on Murashige and Skoog medium with various combinations of nanoparticles suspension employed in concentration of (30 µg/ml). Bimetallic alloys were considered as a stress inducing factor in plants while studying the phytotoxicity. Many secondary metabolites were released because defensive mechanism of plants was active in response to stress. Such secondary metabolites produced in medicinal plants have a great capability in treating the human diseases. In the authors’ study, nanoparticles of small size and of high toxicity effect produced more secondary metabolites like total protein content, total flavonoids and total phenolic content.
Inspec keywords: botany, biochemistry, nanoparticles, silver alloys, copper alloys, gold alloys, biomedical materials, nanomedicine
Other keywords: seed germination frequency, Eruca sativa biochemical characterisation, nanotechnology, bimetallic nanoparticle alloy, silver alloy, copper alloy, gold alloy, murashige, skoog medium, nanoparticles suspension, secondary metabolite, medicinal plant, human disease, total protein content, total flavonoid, total phenolic content, Au, Ag, Cu
1 Introduction
Nanotechnology is an emergent industry likely to leave significant influences on society, economy and environment. At present, almost 475 nanotechnology products are already in the U.S. market including tennis rackets, paints, and precision instruments [1]. Nanomaterials with dimension between 1 and 100 nm are gradually more in use for commercial purposes such as microelectronics, fillers, semiconductors, opacifiers, catalysts, cosmetics, and drug carriers [2]. Method of making bimetallic nanoparticles alloys for improving new materials that have novel fitness than their godparent metals. The major examples of bimetallic alloy nanoparticles are Ag–Cu, Ag–Au, Pd–Pt, Ag–Ti, and Au–Pd. Diverse basic and physical properties are present in materials at nanoscale which are different than these materials in bulk form [3]. The prominent factor of nanoparticles includes its minimal size and high solubility. It has been hypothetically and tentatively demonstrated that the limit of nanoparticles to solubilise is much greater than the material present in bulk form. Due to highly enhanced electronic, optical and catalytic properties, bimetallic nanoparticles are preferred over monometallic nanocrystals. Furthermore, the adjustment in the arrangement of metals gives another opportunity in customising the bimetallic nanoparticles properties other than the typical shape and size control. Bimetallic nanoparticles exist in many different structural arrangements, such as random, core–shell, cluster‐in‐cluster, and alloy structures. In bulk metals, two sort of metal components frequently give a composite structure. In the event that the nuclear sizes of two components are same, then they will arrange themselves as random alloy, whereas components with diverse nuclear size shape an intermetallic alloy. In actual, we have investigated that bimetallic alloy nanoparticles between light transition metals and valuable metals have intermetallic alloy like structures [4].
For development in drug industry new compounds of medicinal values have been immensely extracted from plants. Several naturally occurring molecules in higher plants have been used as food additives, pesticides, pharmaceuticals, flavour and fragrance ingredients and agrochemicals. Therefore, to find out novel plant derived compounds must be the main concern in present and upcoming struggles for maintainable protection and coherent utility of biodiversity. Most desired compounds having medicinal value can be produced on an industrial scale with the help of biotechnological interventions, specifically plant tissue culture, which is highly influential for basic agriculture in the production of biologically active plant secondary metabolites [5]. With the assistance of biotic and abiotic stresses secondary metabolites production can be enhanced in medicinal plants. These secondary metabolites have medicinal value and their consistent production at a larger level is needed. Elicitors like biomolecules, temperature, humidity, heavy metals and so on are already being exploited to produce the secondary metabolites [6]. Recently with the advancement in the field of nanotechnology, scientists are now focusing to use the nanomaterials as an elicitor to check the stress responses in different economically and medicinally important plants. For novel therapeutics, many pharmaceutical substances have been produced using the data of research in the area of plant tissue culture technology. A wide range of medicinal compounds such as steroids, alkaloids, phenolics, terpenoids, saponins, amino acids and flavonoids have been produced only because of advances in the area of cell cultures [7].
A very few studies showed both positive and negative effects of nanoparticles on higher plants Lin and Xing [8] studied five types of multi‐walled nanoparticles and their effect on six different species at seed germination and root growth level. Root growth inhibition showed variations among the nanoparticles used and is related to the concentration of the nanoparticles. According to Lee et al. [9] growth rate of Phaseolus radiatus and Triticum aestivum was reduced on employment of Cu nanoparticles and seedling length was negatively related to concentration of the nanoparticles. Effect of biologically synthesised silver nanoparticles (AgNPs) is also tested on seed germination and seedling growth of Boswellia ovalifoliolata, a narrow endemic and globally threatened tree species. The molecular mechanisms involved in nanoparticles effects on plants are unknown. It is, therefore, important to increase the knowledge about these mechanisms before implementation of these particles on a large‐scale agriculture or the delivery of pesticides and herbicides [10].
This study was conducted to evaluate the effects of bimetallic nanoparticles on medicinally important Eruca sativa and production of secondary metabolites under stress conditions. Phytotoxicity study of monometallic nanoparticles on Eruca sativa is estimated up till now [11]. These features of nanoparticles can be more or less due to the surface area, shape, size, or nature of the material from which they are designed [12]. Effect of AgNPs on morphology and proteomics of Eruca sativa has been reported [13]. A clear idea is necessary for understanding the role of bimetallic nanoparticles in altering the seed germination and affecting the biochemical profile of important medicinal plant Eruca sativa. Previously, effect of monometallic and bimetallic alloys on medicinally important plant species, Silybum marianum had been evaluated [14]. Suspension of nanoparticles was employed on seeds via Murashige and Skoog medium and seeds were germinated under controlled environment.
2 Materials and methods
2.1 Plant source and surface sterilisation
National Agriculture Research Centre (NARC), Islamabad was the source institute to obtain the seeds of Eruca sativa. Erucic acid is the vital compound of commercial importance found in Eruca sativa. Viability of seeds was confirmed by performing the initial experiments and it was perceived that 85% is the average rate of germination of all plant seeds. The seeds were placed in dark, dry place at room temperature. Protocol of Abbasi et al. [15] was followed for surface sterilisation of seeds, i.e. seeds were soaked in 0.1% (w/v) mercuric chloride for 1 min to achieve sterilisation followed by three times washing with distilled water.
2.2 Bimetallic alloy nanoparticles
Nanoparticles were prepared and characterised by using environment friendly polyol process by Rahman et al. [3]. Chemical reduction method (polyol method) was preferred over other physical methods for the preparation of bimetallic alloy nanoparticles of very small dimensions. Physical methods are generally preferred, but not suitable for production of small nanoparticles with very narrow distribution of size. This method is very simple and less time consuming. Bimetallic alloy nanoparticles were synthesised chemically and data regarding the ratios of nanoparticles and its transmission electron microscope and energy dispersive X‐ray spectrum graphs were reported by Rahman et al. [3, 16, 17, 18]. Sizes of the nanoparticles were calculated by Debye–Scherrer equation. Scanning electron microscope graphs of bimetallic nanoparticles are reported by Khan et al. [14] earlier.
2.3 Seed germination protocol
For the preparation of suspension of nanoparticles, 30 µg/ml nanoparticles of bimetallic alloys were directly suspended in distilled water and ultrasonic vibration (100 W and 40 kHz) was used for dispersion for about 30 min following the protocol of Savithramma et al. [10]. Magnetic bar was placed into the suspension of nanoparticles in distilled water and was stirred for 5–10 min on a magnetic stirrer to escape clumping of particles.
A total of 3 ml of nanoparticles suspension was added in 30 ml of MS0 medium with the aid of micropipette. Seeds were inoculated on media after the autoclaving and solidification of medium. Seeds were germinated after ∼2–4 days and after 14 days initial data about seed germination was recorded. After the time interval of every 2 weeks the experimental data was collected and the experiment was prolonged up to 42 days, i.e. 6 weeks.
2.4 Seed germination parameters
2.4.1 Percentage germination frequency
After every 14 days the germination frequency of seeds was recorded. Emergence of radicle was the parameter for seeds to be considered as geminated [19].
2.4.2 Root and shoot length
At 14 days’ time interval root and shoot length of plantlets were documented starting from date of inoculation. Average root and shoot lengths were tabulated and compared.
2.4.3 Seedling vigor index (VI)
Protocol suggested by Abdul‐Baki and Anderson [20] was used to calculate the seedling VI and expressed as an index numbers [19]
2.5 Analytical methods
Method of Nayyar and Gupta [21] was followed with some modifications to prepare extracts for enzyme activities. A 1 g of plant tissue was homogenised with 10 ml of extraction buffer (50 mM potassium phosphate buffer containing 1% PVPP, buffer pH 7). The homogenate thus obtained was centrifuged at 15,000g at 4°C for 30 min and the supernatant was either directly used for analysis or stored at 4°C. The corresponding absorbance was attuned with a regression curve of standard solutions of several concentrations.
Dry samples for antioxidant activity were prepared by pulverising dried plant material in chilled pestle and mortar. A 0.1 g of plant sample in powdered form was mixed with 1 ml of 100% pure methanol. The solution was retained for 5 min and then vortexed for 5 min, sonicated (30 min; Toshiba; Japan) and then centrifuged (13,000 rpm, 10 min). The supernatant was then collected for analysis either instantly or was kept at 4°C for future use.
The free radical scavenging assay (FRSA) of methanolic extracts of Eruca sativa was measured in terms of hydrogen donating or radical scavenging ability using the stable free radical of 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH). Protocol of Lee et al. [22] was followed with some modifications according to which the test extracts were prepared in methanol so the DPPH was also prepared in methanol. DPPH solution was added in sample solution according to the defined calculated concentrations separately. These solution mixtures were kept in dark for 30 min (incubation period) at room temperature. After 30 min, the absorbance was measured at 515 nm using micro‐plate reader. Lower absorbance of the reaction mixture indicated higher FRSA. Finally, the radical scavenging activity was calculated as percentage of DPPH discoloration using the equation
where AE is absorbance of the solution, when extract has been added at a particular level and AD is the absorbance of the DPPH solution with nothing added (blank, without extract).
Low molecular weight, chain breaking antioxidants excluding the role of antioxidant enzymes and metal binding proteins are measured in terms of total antioxidant capacity (TAC). Oxidative stress widely evaluated by quantification of many compounds which comprise of ascorbate, thiols, carotenoids, flavonoids, urate and so on. Quantifying these compounds is difficult, costly and takes much more time. Some of the compounds which are normally not measured as antioxidants have some chain breaking antioxidant activity also so TAC assay is helpful to measure all these compounds collectively including complex interactions occurring during chain breaking antioxidants. Usually TAC is diminished under oxidative stress condition and management of chain breaking antioxidants upsurges antioxidant capacity [23].
The TAC of the methanol extract was estimated by the phosphomolybdenum method according to the method defined by Prieto et al. [24]. According to this assay Mo (VI) is reduced to Mo (V) by the extract and green phosphate/Mo (V) complex is formed at acidic pH. A 50 µl of the sample was combined with 450 µl of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The sample was then placed in a water bath at 95°C for 90 min and then samples were cooled at room temperature. The absorbance of the reaction mixture was measured at 695 nm using a spectrophotometer against the blank. Methanol was used as the blank. The antioxidant activity is expressed as the number of gram equivalent of ascorbic acid [25].
According to the procedure called Folin–Ciocalteu (FC) method by Singleton and Rossi, total phenolic content (TPC) was estimated [26]. Concisely, three solutions were prepared for TPC activity. Ten times diluted solution of FC reagent in distilled water, 6% sodium carbonate solution in distilled water, and 4 mg/ml solution of gallic acid in methanol was prepared. 25, 20, 15, 10 and 5 µg/ml concentrations of gallic acid were used as a positive control and 20 µl of dimethylsulfoxide (DMSO) as a negative control. Absorbance was measured at 630 nm by using a microplate reader.
Total flavonoid content (TFC) was determined by following the procedure of Haq et al. [27]. Stock solutions of 10% aluminium chloride in distilled water, 1 M potassium acetate in distilled water, and 4 mg/ml quercitin in methanol were prepared. A 20 µl of methanol was used as a negative control and 40, 20, 10, 5 and 2.5 µg/ml of final concentration of quercitin as a positive control. Absorbance was recorded at 415 nm using a microplate reader.
For total protein content estimation technique of Lowry et al. [28] was used. Three reagents, i.e. A (1 g Na‐K tartrate × 4 H2 O, 50 g Na2 CO3, 250 ml 1 N NaOH), B (2 g Na‐K tartrate × 4H2 O, 1 g CuSO4. 5H2 O, 0.1 N NaOH) and C (FC reagent ten times diluted with distilled water) were used. A 40 µl of protein extract was taken and 36 µl of reagent A was added and then incubated for 10 min at 50°C and after cooling the reaction mixture 4 µl of reagent B was added and again incubated under same conditions. After cooling the reaction mixture at 25°C 120 µl of reagent C was added. The absorbance was then recorded at 650 nm using a microplate reader. The standard curve of bovine serum albumin (BSA) was prepared at a series of 25, 50, 75, 100 and 125 µg/ml used.
Superoxide dismutase (SOD) activity was performed according to method of Ullah et al. [29] with minor changes. Inhibition of photochemical reduction of nitro blue tetrazolium (NBT) by SOD is the principle of assay. 1 mM EDTA, 130 mM methionine, 0.02 mM riboflavin, 0.75 mM NBT, and 50 mM phosphate buffer (pH 7) was used. Blank was prepared by mixing all the chemicals except enzyme extracts in the same quantity. The reaction mixture was exposed to fluorescent light for 7 min. OD was taken at 560 nm.
Activity of enzyme was calculated applying Lambert–Beer law
where A is the absorbance, Ɛ is the extinction coefficient (6.39 mM−1 cm−1), l is the length of each wall (0.25 cm), C is the concentration of enzyme (value of C measured in nM/min/mg FW), and FW is the fresh weight of the sample.
For peroxidase (POD) activity method of Lagrimini [30] was used. 27.5 mM H2 O2 (10×), 100 mM guaiacol (10×), distilled water, 1% polyvinyl‐pyrrolidone (PVP) and 50 mM potassium‐phosphate buffer of pH 7 were used as chemicals. A reaction mixture of 200 µl was prepared using these chemicals. Blank was prepared by mixing of 60 µl potassium‐phosphate buffer, 20 µl guaiacol, 100 µl distilled water, and 20 µl H2 O2. Absorbance was recorded at 470 nm with a gap of 20 s and activity was calculated according to the formula used for SOD activity.
3 Results and discussion
3.1 Seed germination frequency
Seed germination frequency and root length are the most common parameters evaluated for estimation of phytotoxicity and unstable chemicals or samples [31, 32]. This process starts with inhibition of water and ends in appearance of roots [33]. In our study, seeds were considered to be germinated upon emergence of radicles coming out of their seed coats.
Seed germination frequency was calculated at different time intervals, i.e. 14, 28, and 42 days (Table 1). Among all combinations used germination frequency was reduced as compared to control seeds after 2 weeks. After 4 weeks, all nanoparticles showed increased germination frequency as compared to control seeds except AuCu 3:1 and AgCu 1:3 which showed 27 and 20% germination frequencies, respectively. After 6 weeks of inoculation AuCu 3:1, AgCu 1:3, and AgAu 1:3 showed comparatively reduced response.
Table 1.
Effect of bimetallic alloy nanoparticles on in vitro seed germination of Eruca sativa
| Serial no. | Treatments | % seed germination after 14 days | % seed germination after 28 days | % seed germination after 42 days |
|---|---|---|---|---|
| 1 | control (MS0) | 76.6 | 40 | 100 |
| 2 | AgCu (1:3) | 43.3 | 20 | 80 |
| 3 | AgCu (3:1) | 53.3 | 53.3 | 100 |
| 4 | AuCu (1:3) | 60 | 66.6 | 100 |
| 5 | AuCu (3:1) | 40 | 26.6 | 80 |
| 6 | AgAu (1:3) | 50 | 73.3 | 60 |
| 7 | AgAu (3:1) | 60 | 60 | 100 |
Higher plants strongly interact with their environment and supposed to be affected by nanoparticles existing in nature. Previously, Lin and Xing [8] analysed the effect of five different types of nanoparticles on seed germination and root growth of six higher species including Raphanus sativus, Brassica napus, Lolium multiflorum, Lactuca sativa, Zea mays, and Cucumis sativus.
In previous reports, it is concluded that the toxicity of nanoparticles depends on two different actions, i.e. chemical composition and release of toxic ions and stress caused by surface size or shape of nanoparticles [34]. Minimum germination frequency was observed by seeds treated with AgCu 1:3 nanoparticles, which were smaller in size among all the nanoparticles used, i.e. 18 nm of size so it is confirmed that size and surface area are also stronger indicators of phytotoxicity than concentration of nanoparticles as reported by Barrena et al. [35] and Yang and Watts [12].
As reported by Lee et al. [9] copper nanoparticles have a toxic effect on two species mung bean (Phaseolus radiatus) and wheat (Triticum aestivum). Similarly, in this study as in AgCu 1:3 nanoparticles copper has a greater contribution so it has a more toxic effect in the bimetallic form on E. sativa specie.
3.2 Root and shoot elongation and seed VI
Mean root and shoot lengths were recorded after 2, 4 and 6 weeks of time intervals (Table 2). After 14 days of inoculation three flasks were harvested and root and shoot lengths were measured and according to the data control seeds showed root length of 1.9 cm and shoot length 1.5 cm. Minimum mean root and shoot lengths were shown by AgCu 3:1, which is 0.8 cm. After 28 days of experiment, root and shoot lengths were increased by AgCu 3:1, i.e. 3.1 and 3.8 cm, respectively, and minimum root and shoot lengths were recorded for AgCu 1:3, i.e. 0.2 and 1.1 cm, respectively.
Table 2.
Effect of metal nanoparticles on root length, shoot length and seed VI of Eruca sativa
| Germination period | Treatments | Root length, cm | Shoot length, cm | VI |
|---|---|---|---|---|
| after 2 weeks | MS0 | 1.9 | 1.5 | 340 |
| AgCu (1:3) | 1 | 1.6 | 156 | |
| AgCu (3:1) | 0.8 | 0.8 | 96 | |
| AuCu (1:3) | 1.8 | 1.5 | 264 | |
| AuCu (3:1) | 1.4 | 1.5 | 290 | |
| AgAu (1:3) | 1.4 | 1.2 | 208 | |
| AgAu (3:1) | 1.3 | 1.2 | 150 | |
| after 4 weeks | MS0 | 1.5 | 2.5 | 164 |
| AgCu (1:3) | 0.2 | 1.1 | 27.3 | |
| AgCu (3:1) | 3.1 | 3.8 | 368 | |
| AuCu (1:3) | 1.3 | 3.3 | 313.3 | |
| AuCu (3:1) | 0.8 | 1 | 51 | |
| AgAu (1:3) | 1.9 | 3.4 | 393.5 | |
| AgAu (3:1) | 2.3 | 3.6 | 361.2 | |
| after 6 weeks | MS0 | 5 | 9.8 | 1480 |
| AgCu (1:3) | 2.1 | 5.1 | 576 | |
| AgCu (3:1) | 6.4 | 6.9 | 1330 | |
| AuCu (1:3) | 4.2 | 6.6 | 1080 | |
| AuCu (3:1) | 2.9 | 6.5 | 752 | |
| AgAu (1:3) | 2.6 | 3.5 | 366 | |
| AgAu (3:1) | 5.1 | 6.6 | 1170 |
In previous reports, it is concluded that toxicity of nanoparticles depends on two different actions, i.e. chemical composition and release of toxic ions and stress caused by surface size or shape of nanoparticles [34]. So root and shoot elongation or inhibition in plantlets is depending directly on these two actions. Smaller the particle size more toxic the nanoparticle will be in its action and more stress it will induce on plants [36, 37]. Copper in pure as well as in bimetallic form in this study has reduced the root and shoot length of Eruca sativa and showed more stress than other nanoparticles. These results are similar to the previously reported results of Lee et al. [9]. After 42 days of inoculation mostly root and shoot lengths were inhibited as compared to control which can be justified by the reason that with the passage of time more ions from particles were released and aggregated or accumulated in roots and shoots more surface ions were exposed and they were more toxic to plantlets.
Like the root and shoot lengths of the plantlets at different treatment of nanoparticles, seed VI is also a parameter calculated to check the vigour of the seed at different time intervals applying different treatments. According to data collected at different time intervals, it is hence shown that seed is more vigorous after 42 days of treatment and it was less vigorous in early days after inoculation may be because of seed dormancy.
3.3 % DPPH radical scavenging activity and TAC
After exposing the Eruca sativa seeds to different nanoparticles, significant radical scavenging activity was noticed in plantlets (Fig. 1). Increased antioxidant capacity was shown by plantlets treated with AgCu 3:1, AuCu 3:1, AgAu 1:3, and AgAu 3:1 which was then drastically reduced in AgCu 1:3. In response to toxicity and stress DPPH and antioxidant capacity in plantlets treated with bimetallic nanoparticles having copper was expected to be enhanced as shown by AgCu 1:3. In AgCu 3:1, DPPH activity was reduced and it was considered that this was due to the more ratio of Ag in the nanoparticles as Ag is less stress inducing and shows positive impact so no defence mechanisms were activated against them. DPPH and TAC are dependent on both size of the nanoparticles and chemical composition and surface area of the nanoparticles. More surface area provides the more exposed nanoparticles and more ions to interact with the plant cells and in response more stress induced.
Fig. 1.
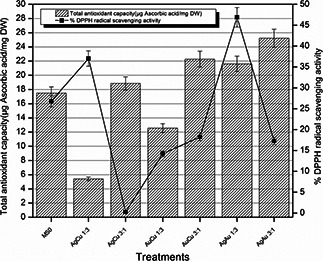
% DPPH radical scavenging activity and TAC of Eruca sativa plantlets against different treatments of nanoparticles
In Fig. 1, TAC and DPPH radical scavenging activity of the plantlets treated with nanoparticles is shown. According to the data shown, it is clear that control seeds showed approximately more radical scavenging activity as well as TAC, bimetallic nanoparticles containing Au in a larger ratio contributed to increase the capacity up to maximum. Some of the differences observed between DPPH radical scavenging activity and TAC in AgCu 3:1 and AgAu 3:1 treatment can have the reason that TAC is the collective value of antioxidants and numerous other compounds which are involved in some chain breaking antioxidant activity [23]. As we are applying metal nanoparticles and after a long period of inoculation accumulation of these particles occurs and ions are released in cellular compartments so DPPH radical scavenging activity can be lesser in some treatments which have released more ions and these ions already had scavenged the radicals.
3.4 Total flavonoid and TPC
According to the results obtained after performing total flavonoid and total phenolic activities trend showed increased phenolics and flavonoids in plantlets treated with AgCu 1:3 nanoparticles (Fig. 2). Plantlets treated with bimetallic nanoparticles AuCu 1:3, AuCu 3:1, and AgAu 1:3 showed the reduced phenolic content. Similarly, TFC was also reduced in plantlets treated with AuCu 1:3, AuCu 3:1, and AgAu 1:3. Total flavonoid and total phenolics also depend upon both the surface area of the nanoparticles used and the size of the nanoparticles; smaller the size more stress will be induced and in response more flavonoids and phenolics will be biosynthesised. Similarly, more toxic the nanoparticle is because of its chemical composition, more stress it will induce and in response more flavonoids and phenolics will be produced. As confirmed from previous studies Cu nanoparticles are inducing more stress and thus under stress conditions more secondary metabolites are released to maintain the defence mechanism and resistance of plants against these stresses.
Fig. 2.
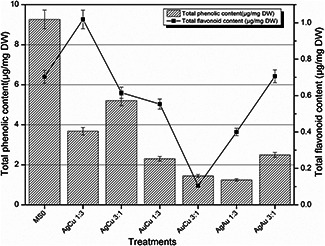
Total flavonoid and TPC after 6 weeks of time interval
3.5 Total protein content, SOD and POD
Fresh weight enzymatic activities were performed on 42 days old plantlets material of Eruca sativa. By analysing the readings of these activities, it is evident that maximum total protein content was accumulated in plantlets which were treated by AgCu 1:3 bimetallic nanoparticles, which was 718.3928571 µg BSAE/20 mg FW. Under stress conditions induced by bimetallic nanoparticles of smallest size more protein was produced in response by the defence mechanism of plant cells. Similarly, SOD and POD activities were also enhanced in the plantlets treated with AgCu 1:3 which were 0.31925 and 0.31174 nM/min/mg FW, respectively. Fig. 3 shows that total protein content is comparatively less in plantlets treated with AuCu 3:1 and AuCu 1:3 nanoparticles. POD activity showed almost the same trend like SOD activity and it is also maximum at AgCu 1:3, which is 0.311737089 nM/min/mg FW.
Fig. 3.
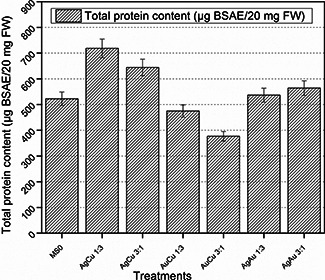
Total protein content of fresh matter after 6 weeks of Eruca sativa treated with bimetallic alloy nanoparticles
In variants with nanoparticles SOD activity was higher as compared to control and POD activity also showed stimulation in response to nanoparticles and this was reduced in concentrations of copper ions used as compared to nanoparticles [38]. SOD and POD activities play an important role in protecting plants against oxidative damage and so it is triggered in plants treated with nanoparticles like AgNPs [39]. In this study, SOD and POD activities were also increased as compared to control as indicated in Fig. 4. SOD and POD activities followed the same trend for all the combinations applied and also the total protein content was higher in those plants in which SOD and POD activities were high. Reason of this increased protein content may be under stressful conditions cells released some of the secondary proteins to balance the oxidative damage exerted as a result of stress.
Fig. 4.
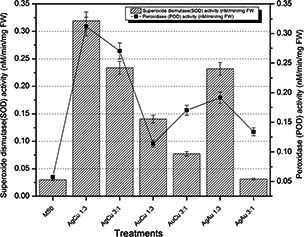
SOD and POD activities of fresh matter after 6 weeks of Eruca sativa treated with bimetallic alloy nanoparticles
4 Conclusions
In this study, it is inferred that metals at nanoscale have different behaviour than metals in bulk form. Chemical composition of compounds and size and shape both matters for nanoparticles to exert effect on plants. Currently, there are very few reports on the application of bimetallic alloys on medicinal plants. So for the first time effects of bimetallic nanoparticles were observed. Small sized and more toxic metal contributed more to inhibit the growth and seed germination frequency of Eruca sativa and it induced more stress. Consequently, more antioxidant activities and enzyme activities were observed in plantlets treated with those nanoparticles. These results can be helpful for future studies on phytotoxicity of various nanomaterials especially engineered nanoparticles under certain conditions.
5 References
- 1. Zhang X. Karn B.: ‘Nanoscale environmental science and technology: challenges and opportunities’, Environ. Sci. Technol., 2005, 39, pp. 94A –95A [PubMed] [Google Scholar]
- 2. Biswas P. Wu C.Y.: ‘Critical review: nanoparticles and the environment’, J. Air Waste Manage. Assoc., 2005, 55, pp. 708 –746 [DOI] [PubMed] [Google Scholar]
- 3. Rahman L.U. Qureshi R. Yasinzai M.M. et al.: ‘Synthesis and spectroscopic characterization of Ag‐Cu alloy nanoparticles prepared in various ratios’, C. R. Chim., 2012, 15, pp. 533 –538 [Google Scholar]
- 4. Shah A. Rahman L.U. Qureshi R. et al.: ‘Synthesis, characterization and applications of bimetallic (Au‐Ag, Au‐Pt, Au‐Ru) alloy nanoparticles’, Rev. Adv. Mater. Sci., 2012, 30, pp. 133 –149 [Google Scholar]
- 5. Rao S.R. Ravishankar G.A.: ‘Plant cell cultures: chemical factories of secondary metabolites’, Biotechnol. Adv., 2002, 20, pp. 101 –153 [DOI] [PubMed] [Google Scholar]
- 6. Pitta‐Alvarez S.I. Spollansky T.C. Giulietti A.M.: ‘The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida ‘, Enzyme Microb. Technol., 2000, 26, pp. 491 –504 [DOI] [PubMed] [Google Scholar]
- 7. Filová A.: ‘Production of secondary metabolites in plant tissue cultures’, Res. J. Agri. Sci., 2014, 46, pp. 236 –245 [Google Scholar]
- 8. Lin D. Xing B.: ‘Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth’, Environ. Pollut., 2007, 150, pp. 243 –250 [DOI] [PubMed] [Google Scholar]
- 9. Lee W. An Y. Yoon H. et al.: ‘Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant uptake for water insoluble nanoparticles’, Environ. Toxicol. Chem., 2008, 27, pp. 1915 –1921 [DOI] [PubMed] [Google Scholar]
- 10. Savithramma N. Ankanna S. Bhumi G.: ‘Effect of nanoparticles on seed germination and seedling growth of Boswellia ovalifoliolata – an endemic and endangered medicinal tree taxon’, Nano Vis., 2012, 2, pp. 61 –68 [Google Scholar]
- 11. Zaka M. Abbasi B.H. Rahman L.U. et al.: ‘Synthesis and characterisation of metal nanoparticles and their effects on seed germination and seedling growth in commercially important Eruca sativa ’, IET Nanobiotechnol., 2016, 10, pp. 134 –140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang L. Watts D.J.: ‘Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles’, Toxicol. Lett., 2005, 158, pp. 122 –132 [DOI] [PubMed] [Google Scholar]
- 13. Vannini C. Domingo G. Onelli E. et al.: ‘Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate’, PLOS ONE, 2013, 8, p. e68752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan M.S. Zaka M. Abbasi B.H. et al.: ‘Seed germination and biochemical profile of Silybum marianum exposed to monometallic and bimetallic alloy nanoparticles’, IET Nanobiotechnol., 2016, doi: 10.1049/iet‐nbt.2015.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbasi B.H. Khan M.A. Mahmood T. et al.: ‘Shoot regeneration and free‐radical scavenging activity in Silybum marianum L’, Plant Cells Tissues Organs, 2010, 101, pp. 371 –376 [Google Scholar]
- 16. Rahman L.U. Shah A. Lunsford S.K. et al.: ‘Monitoring of 2‐butanone using a Ag–Cu bimetallic alloy nanoscale electrochemical sensor’, RSC Adv., 2015, 5, pp. 44427 –44434 [Google Scholar]
- 17. Rahman L.U. Shah A. Qureshi R. et al.: ‘Spectroscopic analysis of Au–Cu alloy nanoparticles of various compositions synthesized by a chemical reduction method’, Adv. Mater. Sci. Eng., 2015, doi: 10.1155/2015/638629 [Google Scholar]
- 18. Rahman L.U. Shah A. Khan S.B. et al.: ‘Synthesis, characterization, and application of Au–Ag alloy nanoparticles for the sensing of an environmental toxin, pyrene’, J. Appl. Electrochem., 2015, 45, pp. 463 –472 [Google Scholar]
- 19. Ushahra J. Malik C.P.: ‘Putrescine and ascorbic acid mediated enhancement in growth and antioxidant status of Eruca sativa varieties’, CIBTech J. Biotechnol., 2013, 2, pp. 53 –64 [Google Scholar]
- 20. Abdul‐Baki A.A. Anderson J.D.: ‘Vigor determination in soybean and seed multiple criteria’, Crop. Sci., 1973, 13, pp. 630 –633 [Google Scholar]
- 21. Nayyar H. Gupta D.: ‘Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants’, Environ. Exp. Bot., 2006, 58, pp. 106 –113 [Google Scholar]
- 22. Lee S.K. Zakaria H.M. Cheng H.S. et al.: ‘Evaluation of antioxidant potential of natural products’, Comb. Chem. High. Throughput Screen, 1998, 1, pp. 35 –46 [PubMed] [Google Scholar]
- 23. Young I.S.: ‘Measurement of total antioxidant capacity’, J. Clin. Pathol., 2001, 54, p. 339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prieto P. Pineda M. Aguilar M.: ‘Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E’, Anal. Biochem., 1999, 269, pp. 337 –341 [DOI] [PubMed] [Google Scholar]
- 25. Aliyu A.B. Ibrahim H. Ibrahim M.A. et al.: ‘Free radical scavenging and total antioxidant capacity of methanol extract of Ethulia conyzoides growing in Nigeria’, Rom. Biotechnol. Lett., 2012, 17, pp. 7458 –7465 [Google Scholar]
- 26. Singleton V.L. Rossi J.A.: ‘Colorimetry of total phenolics with phosphomolybdic‐phosphotungstic acid reagents’, Am. J. Enol. Viticult., 1965, 16, pp. 144 –153 [Google Scholar]
- 27. Haq I.U. Ullah N. Bibi G. et al.: ‘Antioxidant and cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions’, Iran. J. Pharm. Res., 2012, 11, pp. 241 –249 [PMC free article] [PubMed] [Google Scholar]
- 28. Lowry O.H. Rosebrough N.J. Farr A.L. et al.: ‘Protein measurement with the folin phenol reagent’, J. Biol. Chem., 1951, 193, pp. 265 –275 [PubMed] [Google Scholar]
- 29. Ullah N. Haq I.U. Safdar N. et al.: ‘Physiological and biochemical mechanisms of allelopathy mediated by the allelochemical extracts of Phytolacca latbenia (Moq.) H. Walter’, Toxicol. Ind. Health., 2015, 31, pp. 931 –937 [DOI] [PubMed] [Google Scholar]
- 30. Lagrimini L.M.: ‘Wound‐induced deposition of polyphenols in transgenic plants overexpressing peroxidase’, Plant Physiol., 1991, 96, pp. 577 –583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Munzuroglu O. Geckil H.: ‘Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus ’, Arch. Environ. Contam. Toxicol., 2002, 43, pp. 203 –213 [DOI] [PubMed] [Google Scholar]
- 32. Wang X. Sun C. Gao S. et al.: ‘Validation of germination rate and root elongation as an indicator to assess phytotoxicity with Cucumis sativus ’, Chemosphere, 2001, 44, pp. 1711 –1721 [DOI] [PubMed] [Google Scholar]
- 33. Kordan H.A.: ‘Seed viability and germination: a multi‐purpose experimental system’, J. Biol. Educ., 1992, 26, pp. 247 –251 [Google Scholar]
- 34. Brunner T.J. Wick P. Manser P. et al.: ‘In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility’, Environ. Sci. Technol., 2006, 40, pp. 4374 –4381 [DOI] [PubMed] [Google Scholar]
- 35. Barrena R. Casals E. Colan J. et al.: ‘Evaluation of the ecotoxicity of model nanoparticles’, Chemosphere, 2009, 75, pp. 850 –857 [DOI] [PubMed] [Google Scholar]
- 36. Ma X. Geiser‐Lee J. Deng Y. et al.: ‘Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation’, Sci. Total Environ., 2010, 408, pp. 3053 –3061 [DOI] [PubMed] [Google Scholar]
- 37. Yin L. Cheng Y. Espinasse B. et al.: ‘More than the ions: the effects of silver nanoparticles on Lolium multiflorum ’, Environ. Sci. Technol., 2011, 45, pp. 2360 –2367 [DOI] [PubMed] [Google Scholar]
- 38. Nekrasovaa G.F. Ushakova O.S. Ermakov A.E. et al.: ‘Effects of copper (II) ions and copper oxide nanoparticles on Elodea densa planch’, Russ. J. Ecol., 2011, 42, pp. 458 –463 [Google Scholar]
- 39. Krishnaraj C. Jagan E.G. Ramachandran R. et al.: ‘Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) wettst plant growth metabolism’, Process. Biochem., 2012, 47, pp. 651 –658 [Google Scholar]


