Abstract
This study aimed to develop sorafenib loaded magnetic microspheres for the treatment of hepatocellular carcinoma. To achieve this goal, superparamagnetic iron oxide nanoparticles (SPIONs) were synthesised and encapsulated in alginate microspheres together with an antineoplastic agent, sorafenib. In the study, firstly SPIONs were synthesised and characterised by dynamic light scattering, energy‐dispersive X‐ray spectroscopy, and scanning electron microscopy. Then, alginate‐SPIONs microspheres were developed, and further characterised by electron spin resonance spectrometer and vibrating sample magnetometer. Besides the magnetic properties of SPIONs, alginate microspheres with SPIONs were also found to have magnetic properties. The potential use of microspheres in hyperthermia treatment was then investigated and an increase of about 4°C in the environment was found out. Drug release studies and cytotoxicity tests were performed after sorafenib was encapsulated into the magnetic microspheres. According to release studies, sorafenib has been released from microspheres for 8 h. Cytotoxicity tests showed that alginate‐SPION‐sorafenib microspheres were highly effective against cancerous cells and promising for cancer therapy.
Inspec keywords: drug delivery systems, drugs, nanofabrication, magnetic particles, iron compounds, scanning electron microscopy, hyperthermia, biomedical materials, encapsulation, nanoparticles, light scattering, nanomagnetics, cellular biophysics, toxicology, cancer, nanomedicine, superparamagnetism, nanocomposites, magnetometry, paramagnetic resonance, X‐ray chemical analysis
Other keywords: sorafenib loaded alginate microspheres, hepatocellular carcinoma treatment, sorafenib loaded magnetic microspheres, superparamagnetic iron oxide nanoparticles, dynamic light scattering, energy‐dispersive X‐ray spectroscopy, scanning electron microscopy, electron spin resonance spectrometer, vibrating sample magnetometer, hyperthermia treatment, drug release, alginate‐SPION‐sorafenib microspheres, antineoplastic agent, cytotoxicity tests, cancerous cells, time 8.0 hour, Fe3 O4
1 Introduction
Among the many different types of treatments, chemotherapy is one of the most common treatments for cancer. The disadvantage of chemotherapy is that it damages healthy cells as well as cancerous cells, which is a painful process for the patient. Targetted and controlled drug delivery methods have been developed to overcome this problem [1, 2, 3, 4].
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and it ranks fourth among the cancer types that cause death [5]. The common treatments currently applied for HCC are Trans‐Arterial Chemoembolisation (TACE) and Drug Eluting Bead – Trans‐Arterial Chemo Embolisation (DEB‐TACE) [6, 7]. DEB‐TACE is an endovascular treatment that uses microspheres to release chemotherapeutic agents in a controlled release manner within a target lesion [8, 9]. The main disadvantage of DEB‐TACE is that it cannot provide an image in treatment, so CT scans are usually used for this purpose [10]. For this reason, it is uncomfortable for the patient to take images during the treatment.
Sorafenib is the first systemic agent approved for the treatment of advanced HCC [11]. It has been proved as a very effective multi‐kinase inhibitor for the treatment. Sorafenib, a bi‐aryl urea molecule, that can suppress several mechanisms of kinase proteins, plays an important role in the treatment of HCC. Sorafenib inhibits tumour growth and angiogenesis by targetting the Receptor Tyrosine and Serine/Threonine Kinases [12].
Biocompatible biopolymeric microspheres as drug delivery systems have numerous advantages in cancer treatment, especially [2, 13]. This study aims to develop magnetically responsive biopolymeric microspheres to deliver the chemotherapeutic agent, sorafenib by controlling with an external magnetic field. In this study, alginate which is one of the most biocompatible polymers was used as a carrier of sorafenib and also superparamagnetic iron oxide nanoparticles (SPIONs) were synthesised. By encapsulating SPIONs into the alginate microspheres, the microspheres can be captured in the vessels and targetted to the tumour region accurately by an external magnetic field. In our work, besides the magnetic and morphological features, the physicochemical and cytotoxic properties were also investigated to show the effectiveness of the microspheres in cancer treatment. Results from this study will provide important information on the potential application in the HCC treatment.
2 Materials and methods
2.1 Materials
Sodium alginate, calcium chloride (CaCl2 anhydrous), Tween‐80, sodium hydroxide (NaOH) and hydroxypropyl methylcellulose (HPMC) were purchased from Sigma‐Aldrich (MO, USA). Iron (II) chloride tetrahydrate, iron (III) chloride hexahydrate, and hydrochloric acid (HCl) were supplied from Merck (Germany). Sorafenib tosylate was obtained from Novartis (Switzerland). In cell culture studies, media (Dulbecco's modified Eagle's medium (DMEM)/F‐12, penicillin‐streptomycin, fetal calf serum (FCS), trypsin‐EDTA and 3‐(4,5‐dimethyldiazol‐2‐yl)‐2,5 diphenyltetrazolium bromide (MTT) were ordered from Sigma‐Aldrich (MO, USA). DI (deionised) water was also used throughout all the related procedures.
2.2 Synthesis of SPIONs
The co‐precipitation method was utilised to synthesise SPIONs [14]. Briefly, 60 ml 0.5% HCl solution was placed in a flask and nitrogen gas was purged through the solution for 15 min to remove any oxygen. 5.2 g of iron(III) chloride hexahydrate and 2.0 g of iron(II) chloride tetrahydrate were then added. The temperature was increased to 65°C under vigorous stirring and nitrogen gas flow for 30 min. Later, 1.0 M 100 ml NaOH solution was mixed dropwise with a pre‐prepared solution using a needle. The colour of the solution turned into black that indicated the co‐precipitation reaction had started. Then, the mixture was stirred vigorously for 1 h and subsequently was cooled to room temperature while still stirring for another 1 h. The black precipitate was washed four times with DI water and SPIONs were collected using a neodymium magnet. Next, SPION suspension was poured into a Petri dish and dried at 50°C for two days so that SPION powder was obtained.
2.3 Synthesis of alginate‐SPION microspheres
A total of 25 ml 1.2% alginate solution was prepared and vigorously stirred using a magnetic stirrer. 0.03 g HPMC was added as a stabilising agent and the solution was continuously stirred until a homogenous solution was obtained. A total of 125 mg of the SPION was then added into the alginate solution and the mixture was sonicated for 40 min until the SPIONs were fully dispersed in the alginate solution. To be used as a crosslinking solution, 2.5, 5.0, or 10% CaCl2 solutions were prepared and 1.0 ml of Tween‐80 was then added as a surfactant aid to achieve more homogeneous microspheres. The alginate‐SPION solution was added into various concentrations of CaCl2 solutions through an encapsulator (B‐395 pro, Büchi, Essen, Germany) attached with a nozzle with a diameter of 80 µm. Alginate‐SPION microspheres were obtained at a fixed electrode tension (600 V) and various vibration frequencies of encapsulator [1].
2.4 Synthesis of alginate‐SPION‐sorafenib microspheres
The sorafenib solution was prepared by dissolving 200 mg of sorafenib tosylate drug (one capsule) in 20 ml methanol at 65°C. Then, the drug‐methanol mixture was centrifuged at 5000 rpm for 5 min and cooled to room temperature. The supernatant was then taken and filtered using a 0.22 µm syringe filter to remove undissolved substances. Various amounts of sorafenib solution were added to the CaCl2 solution that the final concentrations of sorafenib were 0.1, 0.25, or 1.0 mg/ml, and the total volume was 100 ml. Then, the alginate‐SPION solution was added into the crosslinking solution containing the sorafenib utilising the encapsulator as described previously.
2.5 Morphological and physicochemical analysis
2.5.1 Electron microscopy and energy‐dispersive X‐ray spectroscopy
The morphological characterisations of the SPIONs were evaluated with scanning and transmission electron microscopies (SEM; FEI Quanta200, Japan and TEM; FEI Tecnai G2, Japan) and chemical composition of the SPIONs were investigated by an energy‐dispersive X‐ray spectrometer (EDX, FEI Quanta200, Japan) operating at 15 keV. To prepare SEM and EDX samples, powder samples were fixed on double‐sided carbon adhesive tape which was mounted on sample stubs. Each sample was coated with gold using a precision etching coating system (PECS, Gatan 682) before characterisation. For TEM analysis, SPIONs solution was dropped on the copper grid and dried at room temperature. The images were collected operating 120 keV. Elemental mapping analysis of sorafenib loaded alginate‐SPION microspheres was carried out with an EDX attached SEM (Tescan GAIA3, Czech Republic) without gold coating.
2.5.2 Dynamic light scattering (DLS) and zeta potential
The size distribution and zeta potential of the synthesised SPIONs were analysed with a DLS instrument (Zetasizer 3000 HSA, Malvern Instruments, UK) in phosphate‐buffered saline (PBS) solution at 25°C. Each sample was sonicated in an ultrasonic bath for 2 min prior to analysis.
2.5.3 Electron spin resonance (ESR) spectroscopy and vibrating sample magnetometry
Prepared SPIONs and alginate‐SPION microspheres were characterised by an ESR spectrometer (Bruker EMX 113 X‐Band ESR, Germany). The instrumental parameters were as follows: Central magnetic field: 270.0 mT, sweep time: 83.89 s, modulation frequency: 100 kHz and amplitude: 0.1 mT, microwave frequency: 9.78 GHz and power: 0.125 mW. Magnetic properties of prepared SPIONs, alginate‐SPION microspheres and alginate microspheres were characterised with a vibrating sample magnetometer (VSM, LDJ 9600, Lake Shore, USA).
2.5.4 In vitro magnetic hyperthermia
To determine the heating potential of the samples with magnetic hyperthermia system, specific absorption rate (SAR) measurements were performed. An easy set‐up consisting of a power generator, a fibre optic thermometer and a data acquisition system was used. Prior to analysis, the samples were suspended in isopropyl alcohol reaching a concentration of 5 mg/ml. Experiments were conducted under the magnetic field and frequency. The temperature of the samples was recorded through the optical fibre system, initiating from 25°C.
2.5.5 Optical imaging
The images of the synthesised alginate microspheres were taken by a light microscope (Olympus CH40) at the end of each synthesis. Microspheres were dispersed on a microscope cover glass and overall size was calculated by measuring the microspheres using ImageJ software. For the calculation, the average of five different images for each synthesis was taken.
2.5.6 Entrapment efficiency (EE) and drug release
Three different concentrations of sorafenib (0.1, 0.25, or 1.0 mg/ml) were used in the synthesis of alginate‐SPION‐sorafenib microspheres and the entrapment efficiencies of the microspheres were assessed. The prepared microspheres were centrifuged at 3000 rpm for 5 min and the drug concentration in the supernatant was determined by a UV–visible spectrophotometer (Nanodrop1000, Thermo USA) at a wavelength of maximum (λ max) 263 nm. Drug EE of microspheres was calculated by the following formula [15]:
The release profile of sorafenib from microspheres was examined in PBS solution (pH 7.4) [16]. In brief, 0.25 mg/ml sorafenib was loaded into the prepared microspheres and microsphere‐sorafenib suspension was centrifuged at 3000 rpm for 5 min. After removing the supernatant, sorafenib‐loaded microspheres were resuspended in 20 ml of PBS and the suspension was incubated in a water bath under stable shaking at 100 rpm at 37°C. Finally, the amount of released sorafenib in the medium was quantified by the UV–visible spectrophotometer at 263 nm in different time intervals.
2.5.7 In vitro cytotoxicity test
Cytotoxicity effects of the alginate and alginate‐SPION microspheres in HEPG2 liver cancer and L929 fibroblast cell lines were evaluated by MTT test [17]. Cells were cultured in a medium of DMEM/F‐12 supplemented with 10% FCS and 100‐U/ml penicillin‐streptomycin at 37°C in a 5% CO2 atmosphere. Cells were seeded in 96‐well plates at a density of 1 × 104 cells per well and incubated overnight. The microspheres at various concentrations were diluted using the medium, added to the cells and the cells were then incubated for 24 h. Next day, the medium in each well was aspirated and 200 µl of medium containing 13 µl MTT solution (5 mg/ml, dissolved in RPMI 1640 without phenol red) was added onto the cells. At the fourth hour of incubation at 37°C, 100 µl of isopropanol–HCl was added to solubilise formed formazan crystals. Then, the absorbance of each well was measured at 570 nm using a microplate spectrophotometer (ASYS Biochrome, UK). The cell viability was determined as a percentage of control.
3 Result and discussion
3.1 Morphological and physicochemical analysis
Synthesised SPIONs were analysed by SEM and TEM and the obtained photographs revealed that nanoparticles were spherical. According to the SEM and TEM images, nanoparticles have a size of ∼10–15 nm in diameter that is favourable to obtain superparamagnetic behaviour. In addition, EDX analysis showed that synthesised SPIONs consisted of iron and oxygen elements as expected. The carbon peak in the spectrum corresponded to tape used as a substrate, and the peak in the gold (Au) area corresponded to the coating (Fig. 1).
Fig. 1.
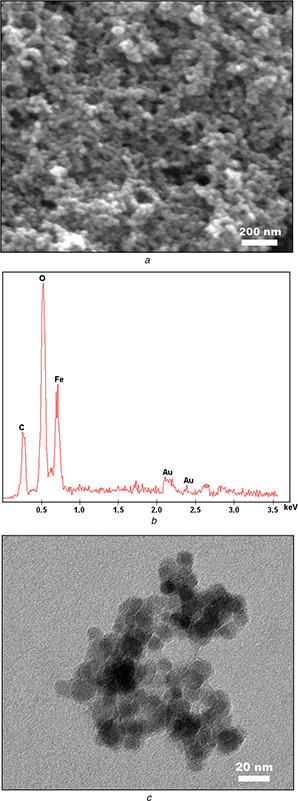
Electron microscope analyses of SPIONs
(a) SEM image, (b) EDX spectrum, (c) TEM image of SPIONs
In the elemental mapping of Sorafenib loaded alginate‐SPION microspheres, carbon and fluorine were investigated which came from alginate and sorafenib, respectively. It was found that fluorine atoms and thus the sorafenib molecules are homogeneously dispersed in the microsphere according to Fig. 2.
Fig. 2.

Elemental analysis of Sorafenib loaded alginate‐SPION microsphere
(a) SEM image, (b) Carbon, (c) Fluorine
The average size of SPIONs was obtained with DLS. The hydrodynamic radius was 61 nm with a PDI of 0.27 showing that SPIONs have relatively narrow size distribution. The obtained size value was inherently larger than the one that obtained with SEM and TEM due to the difference in measurement method [18]. That is because of the superparamagnetic property of the SPION caused to aggregation. Besides, the zeta potential of SPIONs was found out as −27.0 mV that means SPIONs were moderately stable (Fig. 3) [19].
Fig. 3.
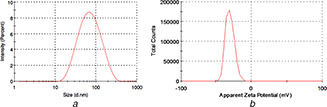
Particle size and zeta potential analysis of SPIONs
(a) Dynamic light scattering, (b) Zeta potential graph of SPIONs
The effect of CaCl2 concentration and applied frequency on the size and morphology of the alginate‐SPION microspheres was determined by an optical microscope Fig. 4. The most spherical shape and narrow size distribution were obtained by optimising synthesis parameters. The optimal production condition was a frequency of 1600 Hz and CaCl2 concentration of 5% (Table 1).
Fig. 4.
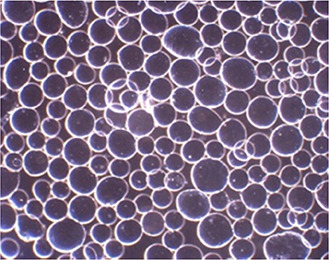
Optical microscope image of alginate‐SPION microspheres (magnification: x4)
Table 1.
Effect of CaCl2 concentration and frequency to microsphere size
| CaCl2 concentration, % | Frequency, Hz | Mean diameter, µm | Standard deviation |
|---|---|---|---|
| 1.5 | 1400 | 154.8 | 34.0 |
| 1600 | 136.6 | 17.0 | |
| 1800 | 149.0 | 9.4 | |
| 5 | 1400 | 135.9 | 18.5 |
| 1600 | 109.3 | 5.1 | |
| 1800 | 112.7 | 3.3 | |
| 10 | 1400 | 135.7 | 11.5 |
| 1600 | 132.0 | 6.0 | |
| 1800 | 121.0 | 11.7 |
The ESR spectra of SPION and alginate‐SPION microsphere were presented in Fig. 5. The characteristic ESR spectrum of SPIONs was changed expectedly when encapsulated within alginate microspheres. The resonance lines were shifted to higher fields with rising intensity. Also, the spectral line width was increased from 100 to 120 mT that was compatible with the previous studies [20, 21].
Fig. 5.
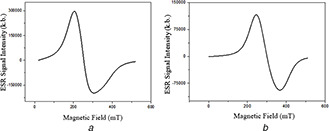
Electron spin resonance studies
(a) SPION, (b) Alginate‐SPION microsphere with g values 2.759 and 2.240, respectively
As seen in Fig. 6, maximum saturation magnetisation (about 70 emu/g) was determined for SPION while it decreased to 4.0 emu/g for alginate‐SPION microspheres, and to almost 0.0 emu/g for alginate microspheres. This reduction in magnetisation can be explained with the non‐magnetic property of alginate material.
Fig. 6.
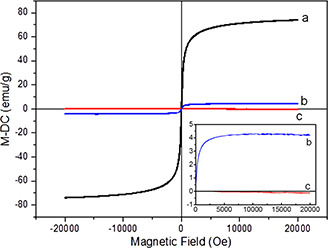
Magnetic field response of particles
(a) SPIONs, (b) Alginate‐SPION microspheres, (c) Alginate microspheres
In the hyperthermia study, SPIONs exhibited the highest temperature rise, while alginate‐SPION microspheres also achieved a temperature increase of 4°C after 6 min. SAR values of the SPIONs and alginate‐SPION microspheres were calculated as 99.3 and 22.7 J/gs, respectively (Fig. 7). The results indicated that SPIONs and alginate‐SPION microsphere samples can heat the environment and can be used in hyperthermia area, which is one of the scopes of this study. Due to the coating of SPIONs with alginate, alginate‐SPION microspheres showed a lower temperature difference as expected. On the other hand, alginate microspheres samples did not provide almost any heating performance confirming the results recorded with the VSM studies. The combined application of hyperthermia and chemotherapy has attracted increasing interest with its potency to overcome the disadvantages of monotherapies [22]. When hyperthermia is integrated with chemotherapy involving drug‐loaded micro‐/nano‐sized delivery systems, the heat generated not only affects cancer cells but also triggers drug release [23, 24]. Thus, the chemotherapeutic agent can be released in the designated region without damaging normal cells so that only cancerous cells can be specifically destroyed.
Fig. 7.
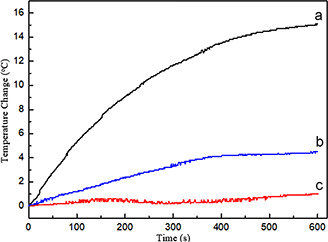
Temperature increment of particles under magnetic field
(a) SPIONs, (b) Alginate‐SPION microspheres, (c) Alginate microspheres
3.2 Drug loading efficiency and release profile
Alginate and alginate‐SPION microspheres showed a loading efficiency of 65.6 and 58.8%, respectively, when 0.25 mg/ml sorafenib was used. The difference in drug encapsulation amount for microspheres can be explained with contained iron oxide core in alginate‐SPION which does not participate in the drug encapsulation. Besides, when the amount of sorafenib used was 0.1 and 1.0 mg/ml for alginate‐SPION microspheres, the loading efficiency was determined to be 86.2 and 42.2%, respectively. This attributes to loading of sorafenib had reached its saturation point and no more drugs can be encapsulated in microspheres (Table 2) [25].
Table 2.
Sorafenib encapsulation efficiency (%)
| Sorafenib, mg/mL | Alginate microsphere | Alginate‐SPION microsphere |
|---|---|---|
| 0.10 | — | 86.2 |
| 0.25 | 65.6 | 58.8 |
| 1.00 | — | 42.2 |
Fig. 8 showed in vitro drug release profile of sorafenib‐loaded alginate‐SPION microspheres in PBS medium for 8 h. It was observed that alginate‐SPION‐sorafenib microspheres exhibited biphasic drug release behaviour with an initial burst release and a slower release afterwards. In the first 1 h, the percentage of released sorafenib was found to be as 54% whereas in the second phase sorafenib released in a sustained manner by maintaining its concentration in the release medium. At the end of 8 h, almost 81% of sorafenib was released from the microspheres. The burst release of sorafenib may be attributed to the fact that some of the drugs were adsorbed onto the surface of the alginate‐SPION microspheres. The biodegradable characteristics of alginate maintained the microspheres consequently allowed sorafenib to detach and released to the environment [26]. To prevent the burst release as well as assure a long‐term sustained release pattern, drug delivery systems can be engineered by using layer‐by‐layer approaches [27]. However, importantly, the initial rapid release phenomenon of drugs may also be useful to quickly reach the effective treatment concentration [28].
Fig. 8.
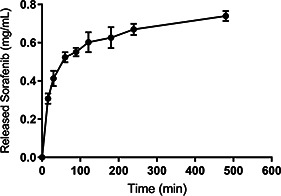
Release profile of sorafenib encapsulated alginate‐SPION microspheres
3.3 In vitro cytotoxicity
Cytotoxicity effects of sorafenib encapsulated alginate‐SPION microspheres were examined in L929 fibroblast and HEPG2 liver cancer cell lines. The cell lines were incubated with the microspheres at various concentrations (10, 100, 500 µg/ml) with different encapsulated sorafenib amounts. The result of the MTT assay showed that bare alginate‐SPION microspheres did not exhibit any toxicity on L929 and significant toxicity on HEPG2 cells. When microspheres produced by encapsulation using 0.25 mg/ml drug were used at 500 µg/ml concentration on L929 cells, viability decreased to 90%. When L929 cells were exposed to 100 and 500 µg/ml microsphere concentrations that 1.0 mg/ml drug was used in the production step, the viability was decreased to 82 and 73%, respectively. In addition, when we examined the effect of microspheres on HEPG2 cells, it was seen that the viability significantly decreased as the microsphere and drug concentration increased. Comparing the viability values obtained after using 100 µg/ml of microspheres produced using 10 mg/ml drug, HEPG2 cell viability decreased to 33%, while L929 cell viability remained at 85% (Fig. 9).
Fig. 9.
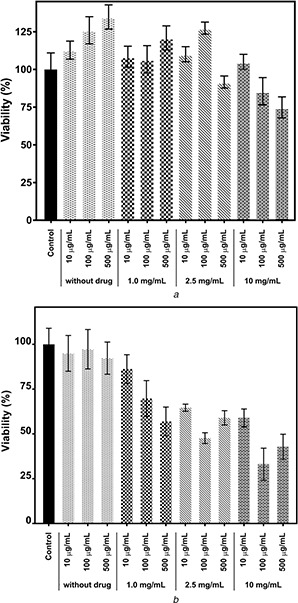
Effects of microspheres on cell viability of
(a) L929, (b) HEPG2 cells as determined by MTT assay
4 Conclusion
This study represented the preparation and characterisation of alginate‐SPION‐sorafenib microspheres that were a potential candidate in the treatment of HCC. SPIONs were synthesised by co‐precipitation method and encapsulated with the chemotherapy agent, sorafenib, within alginate microspheres. ESR and VSM results showed that microspheres had magnetic properties and could be used in targetting. According to hyperthermia results, microspheres tended to increase temperature almost 4°C that improved their effectiveness in therapeutic applications. In drug release studies, it was determined that the microspheres released almost 81% of the encapsulated sorafenib in a controlled manner for 8 h in vitro. Cytotoxicity tests carried out in both healthy and cancerous cells revealed that the sorafenib loaded microspheres were highly toxic to cancerous cells depending on the concentration but caused minimal damage to healthy cells. In conclusion, alginate‐SPION‐sorafenib microspheres can be recommended as a promising chemotherapy and hyperthermia agent to be used and developed for further in vivo studies.
5 References
- 1. Olivares A., Silva P.: ‘Viability dataset on microencapsulated probiotics: sodium alginate viscosity effect’, Data Briefs, 2019, 27, p. 104735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jyoti K., Bhatia R.K., Martis E.A.F. et al.: ‘Soluble curcumin amalgamated chitosan microspheres augmented drug delivery and cytotoxicity in colon cancer cells: in vitro and in vivo study’, Colloids Surf. B, Biointerfaces, 2016, 148, pp. 674–683 [DOI] [PubMed] [Google Scholar]
- 3. Bhatnagar D., Tuteja S.K., Rastogi R. et al.: ‘Label‐free detection of hemoglobin using MWNT‐embedded screen‐printed electrode’, Bionanoscience, 2013, 3, (3), pp. 223–231 [Google Scholar]
- 4. Demirbilek M.E., Demirbilek M., Karahaliloǧlu Z. et al.: ‘Oxidative stress parameters of L929 cells cultured on plasma‐modified PDLLA scaffolds’, Appl. Biochem. Biotechnol., 2011, 164, (6), pp. 780–792 [DOI] [PubMed] [Google Scholar]
- 5. Christina F.: ‘Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐ adjusted life‐years for 32 cancer groups, 1990 to 2015’, JAMA Oncol., 2018, 4, (11), pp. 1553–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roma S., Amato D.D., Ranalli T. et al.: ‘Vascular anomalies of the celiac trunk and implications in treatment of HCC with TACE’, Radiol. Case Rep., 2019, 14, pp. 1221–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Z., Chapiro J., Schernthaner R. et al.: ‘Multimodality 3D tumor segmentation in HCC patients treated with TACE’, Acad. Radiol., 2015, 22, (7), pp. 840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Melchiorre F., Patella F., Pescatori L. et al.: ‘DEB‐TACE: a standard review’, Future Oncol., 2018, 14, (28), pp. 2969–2984 [DOI] [PubMed] [Google Scholar]
- 9. Lencioni R., Llovet J.M., Han G. et al.: ‘Sorafenib or placebo plus TACE with doxorubicin‐eluting beads for intermediate stage HCC: the SPACE trial’, J. Hepatol., 2016, 64, (5), pp. 1090–1098 [DOI] [PubMed] [Google Scholar]
- 10. Song J.E., Kim D.Y.: ‘Conventional vs drug‐eluting beads transarterial chemoembolization for hepatocellular carcinoma’, World J. Hepatol., 2017, 9, (18), pp. 808–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Llovet J.: ‘Sorafenib in advanced hepatocellular carcinoma’, N. Engl. J. Med., 2008, 359, (4), pp. 378–390 [DOI] [PubMed] [Google Scholar]
- 12. Ma R., Chen J., Liang Y. et al.: ‘Sorafenib: a potential therapeutic drug for hepatic fibrosis and its outcomes’, Biomed. Pharmacother., 2017, 88, pp. 459–468 [DOI] [PubMed] [Google Scholar]
- 13. Pundir A.R., Wankhade R.P., Bhalerao S.S.: ‘Microspheres: novel approach for cancer targeting’, Int. J. Pharm. Technol., 2012, 4, (2), pp. 2034–2054 [Google Scholar]
- 14. Erdal E., Demirbilek M., Yeh Y. et al.: ‘A comparative study of receptor‐targeted magnetosome and HSA‐coated iron oxide nanoparticles as MRI contrast‐enhancing agent in animal cancer model’, Appl. Biochem. Biotechnol., 2018, 185, (1), pp. 91–113 [DOI] [PubMed] [Google Scholar]
- 15. Alp E., Çirak T., Demirbilek M. et al.: ‘Targeted delivery of etoposide to osteosarcoma cells using poly(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate) (PHBV) nanoparticles’, Turkish J. Biol., 2017, 41, (5), pp. 719–733 [Google Scholar]
- 16. Akbal Ö., Erdal E., Vural T. et al.: ‘Comparison of protein‐ and polysaccharide‐based nanoparticles for cancer therapy: synthesis, characterization, drug release, and interaction with a breast cancer cell line’, Artif. Cells Nanomed. Biotechnol., 2017, 45, (2), pp. 193–203 [DOI] [PubMed] [Google Scholar]
- 17. Akbal O., Bolat G., Yaman Y.T. et al.: ‘Folic acid conjugated Prussian blue nanoparticles: synthesis, physicochemical characterization and targeted cancer cell sensing’, Colloids Surf. B, Biointerfaces, 2020, 187, (August 2019), p. 110655 [DOI] [PubMed] [Google Scholar]
- 18. Bhattacharjee S.: ‘DLS and zeta potential – what they are and what they are not?’, J. Controlled Release, 2016, 235, pp. 337–351 [DOI] [PubMed] [Google Scholar]
- 19. Rost N.C.V., Sen K., Savliwala S. et al.: ‘Magnetic particle imaging performance of liposomes encapsulating iron oxide nanoparticles’, J. Magn. Magn. Mater., 2020, 504, (February), p. 166675 [Google Scholar]
- 20. Noginova N., Chen F., Weaver T. et al.: ‘Magnetic resonance in nanoparticles: between ferro‐ and paramagnetism’, J. Phys. Condens. Matter, 2007, 19, (24), p. 246208 [DOI] [PubMed] [Google Scholar]
- 21. Schaer M., Crittin M., Kasmi L. et al.: ‘Multi‐functional magnetic photoluminescent photocatalytic polystyrene‐based micro‐ and nano‐fibers obtained by electrospinning’, Fibers, 2014, 2, (1), pp. 75–91 [Google Scholar]
- 22. Phung D.C., Nguyen H.T., Phuong Tran T.T. et al.: ‘Combined hyperthermia and chemotherapy as a synergistic anticancer treatment’, J. Pharm. Invest., 2019, 49, (5), pp. 519–526 [Google Scholar]
- 23. Hayashi K., Ono K., Suzuki H. et al.: ‘High‐frequency, magnetic‐field‐responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect’, ACS Appl. Mater. Interfaces, 2010, 2, (7), pp. 1903–1911 [DOI] [PubMed] [Google Scholar]
- 24. Liang Y.J., Wang H., Yu H. et al.: ‘Magnetic navigation helps PLGA drug loaded magnetic microspheres achieve precise chemoembolization and hyperthermia’, Colloids Surf. A, Physicochem. Eng. Aspects, 2020, 588, (December 2019), p. 124364 [Google Scholar]
- 25. Ling D.C.C.: ‘Effects of synthesis parameters on drug loading capacity of starch nanoparticles’, Universiti Malaysia Sarawak, 2015. [Google Scholar]
- 26. Devalapally H., Chakilam A., Amiji M.M.: ‘Role of nanotechnology in pharmaceutical product development’, J. Pharm. Sci., 2007, 96, (10), pp. 2547–2565 [DOI] [PubMed] [Google Scholar]
- 27. Severino P., da Silva C.F., Andrade L.N. et al.: ‘Alginate nanoparticles for drug delivery and targeting’, Curr. Pharm. Des., 2019, 25, pp. 1312–1334 [DOI] [PubMed] [Google Scholar]
- 28. Jia L., Shen J., Li Z. et al.: ‘In vitro and in vivo evaluation of paclitaxel‐loaded mesoporous silica nanoparticles with three pore sizes’, Int. J. Pharm., 2013, 445, (1–2), pp. 12–19 [DOI] [PubMed] [Google Scholar]


