Abstract
In the present study, high purity copper oxide nanoparticles (NPs) were synthesised using Tridax procumbens leaf extract. Green syntheses of nano‐mosquitocides rely on plant compounds as reducing and stabilising agents. Copper oxide NPs were characterised using X‐ray diffraction (XRD) analysis, Fourier transform infrared (FT‐IR), Field‐emission scanning electron microscopy with energy dispersive spectroscopy, Ultraviolet–visible spectrophotometry and fluorescence spectroscopy. XRD studies of the NPs indicate crystalline nature which was perfectly matching with a monoclinic structure of bulk CuO with an average crystallite size of 16 nm. Formation of copper oxide NPs was confirmed by FT‐IR studies and photoluminescence spectra with emission peaks at 331, 411 and 433 nm were assigned to a near‐band‐edge emission band of CuO in the UV, violet and blue region. Gas chromatography–mass spectrometry studies inferred the phytochemical constituents of the leaf extract. Larvicidal activity of synthesised NPs using T. procumbens leaf extract was tested against Aedes aegypti species (dengue, chikungunya, zika and yellow fever transmit vector).
Inspec keywords: photoluminescence, spectrophotometry, thermal analysis, chromatography, nanoparticles, antibacterial activity, field emission electron microscopy, microorganisms, wide band gap semiconductors, scanning electron microscopy, X‐ray diffraction, copper compounds, ultraviolet spectra, nanofabrication, X‐ray chemical analysis, crystallites, visible spectra, field emission scanning electron microscopy, nanobiotechnology, semiconductor materials, semiconductor growth, fluorescence, mass spectra
Other keywords: energy dispersive spectroscopy, ultraviolet–visual spectrophotometry, fluorescence spectroscopy, chikungunya, green synthesis, mosquito larvicidal activity, zika, X‐ray diffraction analysis, field‐emission scanning electron microscopy, XRD, gas chromatography–mass spectrometry, copper oxide nanoparticles, dengue, tridax procumben leaf extract, nanomosquitocides, FTIR, monoclinic structure, crystallite size, photoluminescence spectra, near‐band‐edge emission band, phytochemical constituents, Aedes aegypti species, yellow fever transmit vector, CuO
1 Introduction
Chemical insecticides are used to control mosquitoes but they are harmful to non‐target organisms and cause human health problems. Hence, effective and eco‐friendly control strategies have to be focused to control this issue effectively. Developing biopesticides with multiple mechanisms may be successful for effective mosquito control. Mosquitoes are responsible for the transmitting dreadful diseases and parasites worldwide including malaria, dengue, filariasis etc., mosquito‐borne diseases are endemic and cause mortality of nearly two million people every year [1].
Aedes aegypti (A. aegypti) transmits viral pathogens to human which causes yellow fever and dengue [2]. Dengue fever is increasing and the human population is now at risk due to this. Effective measures are being taken for mosquito control but still, there are tough challenges including increased mosquito resistance to insecticides [3]. Larviciding is used in many vector control programs around the world and this research work aims towards the discovery of cost effective alternatives for effective control of mosquito vector. The search for cost effective and abundant sources of plant extract as mosquito larvicides and the use of plant extract is promising due to the availability and cost‐effectiveness [4].
Nanoscale materials have gained research interest, due to their potential to revolutionise a wide array of applications, facing important and timely challenges in parasitology and integrated pest management. In the last few years, nano‐synthesis routes have been improved using botanical and algal extracts as sources of reducing and stabilizing agents to produce metal and metal oxide nanoparticles (NPs) with a wide range of biological activities [5, 6]. The so‐called ‘green fabrication’ of NPs and nano‐composites has been reported as advantageous if compared with classic chemical and physical methods, since green synthesis is usually cheap, quick, and does not require high pressure, energy, temperature nor the use of highly toxic chemicals. Many studies have shown the potential of NPs for the control of arthropod pests of agricultural and medical‐veterinary importance (mainly mosquitoes and ticks) [7]. Green‐synthesised NPs are usually fabricated in aqueous suspensions; following bio‐reduction of metal ions by plant extract constituents soluble in water, therefore the final product is an ideal formulation to treat insect young instars living in the aquatic environment [8]. Metal oxide NPs have a major role in pharmaceutical, industrial and biotechnological applications. Copper and copper‐based nanostructured materials have efficient biocidal properties which are generally used in catalysis, pesticidal formulations and several health‐related applications [9, 10].
Tridax procumbens L. of family Asteraceae is a common medicinal herb used by ethno‐medical practitioners [11]. It has been used in treating various ailments such as wound healing, as anti‐coagulant, anti‐fungal agent and in the treatment of various infectious skin diseases [12]. Hence, the usage of this plant extract for the preparation of copper oxide NPs could have great potential for various applications. Therefore, in the present study, leaf extract of T. procumbens was used for the preparation of copper oxide NPs for mosquito A. aegypti larvicidal activity.
2 Materials and methods
2.1 Materials
The material used for the preparation of copper oxide NPs was copper sulphate (Sigma‐Aldrich) and was used without further purification. T. procumbens leaves were collected from in and around Sathyabama University, Chennai, India (latitude 12.8731°N longitude 80.2219°E).
2.2 Preparation of leaf extract and phytochemical analysis using gas chromatography–mass spectrometry (GC–MS)
The leaves of T. procumbens were thoroughly washed with deionised water for removing the dust particles. The preparation of leaf aqueous extract, taking 10 g of chopped leaves in a 250 ml Erlenmeyer flask with 100 mL of deionised water and boiled at 60°C for 10 min. This extract was filtered and stored for further experiments. Phytochemical constituents of T. procumbens leaf extract were determined using GC–MS (Shimadzu‐GCMS QP2010 Ultra).
2.3 Preparation of CuO NPs
The copper sulphate solution was prepared using 1 mM of copper sulphate in 100 ml of de‐ionised water. For the synthesis of CuO NPs, an optimum amount of leaf extract (40 ml) was added to 90 ml of aqueous copper sulphate solution and was kept under vigorous stirring for 4 h at 80°C until it reduced to a deep brown coloured solution. This solution was centrifuged at 5000g for 10 min. The pellet was collected and calcined in a box furnace at 400°C for 5 h.
2.4 Characterisation of CuO NPs
An X‐ray diffraction (XRD) study was performed using an X‐ray powder diffractometer SMART LAB Rigaku, Japan. The functional groups in the copper oxide NPs were analysed by using a Fourier transform infrared (FT‐IR) spectrometer (Perkin‐ Elmer RX1). An energy dispersive X‐ray spectrometer (SUPRA55 ZEISS with an energy dispersive X‐ray spectroscopy–wavelength dispersive X‐ray spectroscopy apparatus) was used for elemental analysis of copper oxide NPs. An Ultraviolet–visible (UV–vis) spectrophotometer (UV‐2101, Shimadzu) was used to study the optical property of CuO NPs in the wavelength range of 200–800 nm. Photoluminescence (PL) spectra of CuO NPs were recorded using a Shimadzu fluorospectrophotometer.
2.5 Parasites rearing and collection
Larvae of A. aegypti were collected from the water tank, broken bottles and small water courses at Velachery, Chennai and identified in Zonal Entomological Research Centre, Vellore. The larvae were kept in plastic trays containing tap water. They were maintained and nurture in the laboratory condition as per the reported protocol [13].
2.6 Mosquito larvicidal bioassay of plant extract and copper oxide NPs
The A. aegypti mosquito larvicidal experiment was performed by following the procedure of World Health Organisation. For larval bioassay analysis, A. aegypti larvae were taken in five groups of 20 in 249 ml of tap water and 1 ml of T. procumbens aqueous extract and copper oxide NPs concentration. The control group was maintained with dechlorinated tap water. The numbers of larval death were counted after 24 h of treatment and mortality % was calculated from the average of five replicates. The experimental results in which 100% mortality of larvae occurs alone were selected for further dose response larval bioassay.
2.7 Larvicidal bioassay by dose‐response
Based on the preliminary screening results, T. procumbens aqueous extract and T. procumbens mediated synthesised copper oxide NPs were subjected to dose response for larvicidal bioassay against the larvae of A. aegypti. Different concentrations ranging from 10, 25, 50, 75 and 100 mg/l (for T. procumbens extracts) and 1, 2.5, 5, 7.5, and 10 mg/l (for copper oxide NPs for larvicidal activity) were used. The numbers of dead A. aegypti larvae were calculated after 24 h of exposure and mortality % was calculated from the average of five replicates.
2.8 Statistical analysis
Average larval mortality data were subjected to probit analysis for calculating LC50. Here, LC50 was the lethal concentration (LC) that 50% kills. The fiducial limits of upper confidence limit (UCL) and lower confidence limit (LCL) were calculated by using the software developed by Reddy et al. [14]. There was a relationship between the experimental doses and death rate of A. aegypti larvae showed in the r 2 and all differences were considered significant P ≤ 0.05.
3 Results and discussion
The identification and composition of the main bioactive compounds present in the leaf extract of T. procumbens are shown in Table 1. Nine compounds were identified by GC–MS and the main compounds were 2,6,10‐trimethyl,14‐ethylene‐14‐pentadecne; 3,7,11,15‐tetramethyl‐2‐hexadecen‐1‐ol; 2‐hexadecen‐1‐ol, 3,7,11,15‐tetramethyl; l‐( + )‐ascorbic acid 2,6‐dihexadecanoate; 3,7,11,15‐tetramethyl‐2‐hexadecen‐1‐ol; 9,12,15‐octadecatrienoic acid; pentadecane, 8‐hexyl; tetrapentacontane and squalene (Fig. 1).
Table 1.
Phytocompounds identified from the T. procumbens leaf extract by GC–MS
| Retention Time (RT) | Name of the compound | Molecular formula | Mol. weight (g/mol) |
|---|---|---|---|
| 24.500 | 2,6,10‐Trimethyl,14‐ethylene‐14‐pentadecne | C20 H38 | 278 |
| 25.000 | 3,7,11,15‐Tetramethyl‐2‐hexadecen‐1‐ol | C20 H40 O | 296 |
| 25.379 | 2‐Hexadecen‐1‐ol, 3,7,11,15‐tetramethyl | C20 H40 O | 296 |
| 26.872 | l‐(+)‐Ascorbic acid 2,6‐dihexadecanoate | C38 H68 O8 | 652 |
| 29.152 | 3,7,11,15‐Tetramethyl‐2‐hexadecen‐1‐ol | C20 H40 O | 296 |
| 29.454 | 9,12,15‐Octadecatrienoic acid | C18 H30 O2 | 278 |
| 30.505 | Pentadecane, 8‐hexyl‐ | C21 H44 | 296 |
| 35.252 | Tetrapentacontane | C54 H110 | 758 |
| 36.065 | Squalene | C30 H50 | 410 |
Fig. 1.
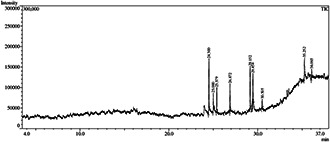
GC–MS chromatogram of T. procumbens leaf extract
The crystallinity and crystal phase of synthesised CuO NPs were studied by XRD and is shown in Fig. 2 i. XRD analysis show intense peaks at 32.6°, 35.5°, 38.7°, 48.6°, 53.5°, 58.2°, 61.4°, 66.2°, 67.9°, 72.5° and 75.0° corresponds to (1 1 0), (0 0 2), (1 1 1), (2 0 2), (0 2 0), (2 0 2), (1 1 3), (3 1 1), (1 1 3), (3 1 1) and (2 2 2), respectively. The observed diffraction reflections are comparable with JCPDS No. 45–0937 and are attributed to bulk CuO materials [15, 16]. All diffraction peaks can be indexed as the typical monoclinic structure and no extra diffraction peaks of other phases are observed, which indicates the high purity of the synthesised CuO NPs. Moreover, the well‐defined, sharp CuO reflections observed in the XRD patterns verify the well‐crystalline nature of CuO NPs. The average grain size of CuO NPs is determined to be 16 nm from the full‐width at half maximum (FWHM) of the most intense peak using Debye–Scherrer's equation:
where λ is the wavelength of the X‐ray radiation (for CuKα radiation, λ = 1.5418 Å), β is the FWHM in radians of the XRD peak and θ is the angle of diffraction.
Fig. 2.
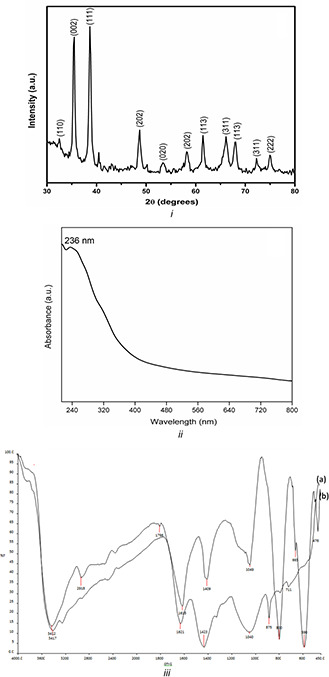
Characterisation of CuO NPs
(i) XRD pattern, (ii) UV−Vis absorption spectrum, (iii) FT‐IR spectrum of (a) T. procumbens leaf extract and (b) synthesised CuO NPs
The UV–visible absorption spectrum of green synthesised CuO NPs was obtained using T. procumbens leaf extracts (Fig. 2ii). CuO NPs have an optical absorbance range around 236 nm suggesting the formation of CuO NPs and are in good agreement with the previous reports [17, 18]. The bioactive molecules present in the T. procumbens leaf extract reduce precursor and formation of CuO NPs were confirmed by FT‐IR. Fig. 2iii shows the FT‐IR spectra of (a) T. procumbens leaf extract and (b) CuO NPs using T. procumbens leaf extract. The relative shifts in position and intensity were identified using FT‐IR (Fig. 2 iii(a)) recorded for T. procumbens leaf extract, where the peaks were observed at 3417 cm−1 (amide N–H stretching), 2921 cm−1 (alkane C–H stretching), 1799 cm−1 (anhydride C=O bending) and 1049 cm−1 (C–O stretching). From Fig. 2 iii(b), the interaction of CuO NPs with biomolecules of T. procumbens leaf showed intense peaks at 3412 cm−1 (amide N–H stretching), 2918 cm−1 (alkane C–H stretching), 1796 cm−1 (anhydride C=O bending) and 1040 cm−1 (C–O stretching). The peak at 471 cm−1 corresponds to vibrations of Cu–O, confirms the formation of CuO NPs. Moreover, it is confirmed that the peak corresponds to cuprous oxide (Cu2 O) at around 605–660 cm−1 is absent [15, 19].
The size and shape of green synthesised CuO NPs using T. procumbens leaf extract were analysed using field‐emission scanning electron microscopy (FESEM), which is shown in Fig. 3 a. The energy dispersive spectroscopy (EDAX) spectrum was used to analyse the elemental composition of the CuO NPs synthesised using T. procumbens leaf extract. In the EDAX spectrum, the presence of strong signals of Cu and O was observed (Fig. 3 b) which confirms the formation of CuO NPs.
Fig. 3.
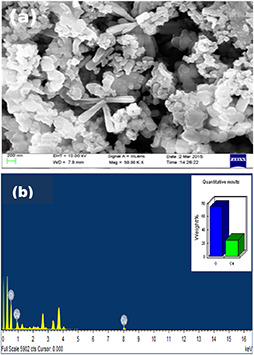
Surface morphological characterisation of CuO NPs
(a) FESEM, (B) EDAX spectrum of CuO NPs
In general, emission spectra of metal oxides can be divided into two broad categories namely the near‐band‐edge (NBE) UV emission and deep‐level (DL) defect‐related visible emissions [20, 21]. Fig. 4 shows the PL spectrum of the CuO NPs recorded in the wavelength range of 300–700 nm with an excitation wavelength of 300 nm at room temperature. The PL spectrum exhibits a UV emission peak at 331 nm and visible emission peaks in the violet region (411 nm), blue region (433 nm) and green region (605 nm). The sharp emission peaks appear at 331, 411 and 433 nm are assigned to the NBE emission band of the CuO in the UV, violet and blue region [9]. These results are in good agreement with the previous reports [22, 23, 24]. The sharp DL green emission peak at around 605 nm is assigned to the singly ionised oxygen vacancies [25]. The visible luminescence originates from the radioactive recombination of a photo‐generated hole with an electron occupying the oxygen vacancy [26, 27].
Fig. 4.
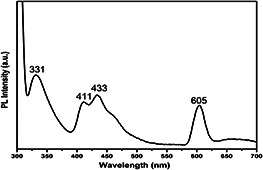
PL spectrum of CuO NPs
The larvicidal property of T. procumbens leaf extract and synthesised CuO NPs against A. aegypti was studied (Table 2). However, the highest activity was found in T. procumbens leaf extract and CuO NPs against the larvae of A. aegypti (LC50 = 60.965 and 4.209 mg/l; r2 = 0.977 and 0.993).
Table 2.
Dose‐dependent larvicidal activity against A. aegypti species
| Materials | Species | Conc., mg/l | % Mortality, mean ± SD | LC50, mg/l | UCL–LCL, mg/l | r2 | χ2 |
|---|---|---|---|---|---|---|---|
| Control (distilled H2 O) | A. aegypti | 25 | — | — | — | — | — |
| T. procumbens leaf extract | 100 | 80 ± 0.680 | 60.965 | 66.431–55.855 | 0.977 | 2.205 | |
| 75 | 69 ± 0.320 | ||||||
| 50 | 37 ± 0.190 | ||||||
| 25 | 19 ± 1.270 | ||||||
| 10 | 11 ± 1.020 | ||||||
| CuSO4 | 25 | — | — | — | — | — | |
| CuO NPs | 10 | 100 ± 0.00 | 4.209 | 5.622–2.651 | 0.993 | 10.101 | |
| 7.5 | 78 ± 0.230 | ||||||
| 5 | 53 ± 0.140 | ||||||
| 2.5 | 37 ± 0.280 | ||||||
| 1 | 17 ± 0.280 |
–, Nil mortality; LC50, lethal concentration that kills 50% of the exposed larvae; UCL, upper confidence limit; LCL, lower confidence limit; r 2, regression coefficient; χ 2, chi square; Significant at P < 0.05 level.
The CuO NPs, which are likely to cause ecological damage, have been identified as a potential replacement for chemical synthetic insecticides and hence, the need to use the bio‐based preparation of CuO NPs for the control of disease vectors. Biological‐mediated preparation of copper oxide NPs using Escherichia coli has been reported and the results indicated the presence of both Cu2 O and CuO phases [28]. Fungi can also be used to synthesise metallic oxide NPs. The biogenic synthesis of copper oxides was reported using Penicillium aurantiogriseum, Penicillium citrinum and Penicillium waksmanii isolated from soil [29]. Gopalakrishnan et al. reported the synthesis of Cu2 O NPs using T. procumbens leaf extract and studied their antibacterial activity against E. coli [30]. Monodispersed, versatile and highly stable CuO NPs were synthesised using Aloe vera extract. This method is both eco‐friendly and inexpensive [27]. Mageshwari et al. reported the synthesis, characterisation and antimicrobial activity of flower‐shaped CuO nanostructures [15]. Sankar et al. reported the green synthesis of colloidal copper oxide NPs using Carica papaya for photocatalytic dye degradation [19]. Green synthesis of CuO NPs by aqueous extract of Gundelia tournefortii and its evaluation of catalytic activity for reduction of 4‐nitrophenol has been reported [31]. Nasrollahzadeh et al. reported the Tamarix gallica leaf extract‐mediated novel route for the green synthesis of CuO NPs and their application to N ‐arylation of nitrogen‐containing heterocycles under ligand‐free conditions [32]. Furthermore, CuO NPs synthesised using Calotropis gigantea leaf extract is used for dye‐sensitised solar cells applications [16].
Recently, a green chemistry approach for the preparation of NPs using microbes, plants and plant based bioactive compounds showed potent mosquito larvicidal and pupicidal activity against a number of mosquito vectors [33].
4 Conclusions
Advanced technology for effective mosquito vector control is a need of the hour. The present study emphasises the cost effective and eco‐friendly approach for the synthesis of CuO NPs using leaf extract of T. procumbens is reported. The larvicidal activity against dengue, chikungunya and yellow fever transmit vector A. aegypti species was studied using CuO NPs and the results were discussed.
5 Acknowledgments
We thank the Centre for Nanoscience and Nanotechnology, Sathyabama Institute of Science and Technology, Chennai for providing X‐ray diffraction (XRD), Energy dispersive X‐ray analysis (EDAX) facilities. The author (KVA) thanks Prof. R. Jayavel, Centre for Research, Anna University, Chennai and Prof. R. Mohan, Department of Physics, Presidency College, Chennai for their constant support and guidance.
6 References
- 1. Benelli G.: ‘Research in mosquito control: current challenges for a brighter future’, Parasitol. Res., 2015, 114, pp. 2801 –2805 [DOI] [PubMed] [Google Scholar]
- 2. Cantrell C.L. Ali A. Duke S.O. et al.: ‘Identification of mosquito biting deterrent constituents from the Indian folk remedy plant Jatropha curcas ’, J. Med. Entomol., 2011, 48, pp. 836 –845 [DOI] [PubMed] [Google Scholar]
- 3. Velu K. Elumalai D. Hemalatha P. et al.: ‘Evaluation of silver nanoparticles toxicity of Arachis hypogaea peel extracts and its larvicidal activity against malaria and dengue vectors’, Environ. Sci. Pollut. Res., 2015, 22, pp. 17769 –17779 [DOI] [PubMed] [Google Scholar]
- 4. Yasoubi P.M. Berzegar M. Sahari A. et al.: ‘Total phenolic contents and antioxidant activity of pomegranate (Punica granatum L.) peel extracts’, J. Agric. Sci. Technol., 2007, 9, pp. 35 –42 [Google Scholar]
- 5. Stalin Dhas T. Ganesh Kumar V. Karthick V. et al.: ‘Facile synthesis of silver chloride nanoparticles using marine alga and its antibacterial efficacy’, Spectrochim. Acta A: Mol. Biomol. Spec., 2014, 120, pp. 416 –420 [DOI] [PubMed] [Google Scholar]
- 6. Benelli G.: ‘Green synthesized nanoparticles in the fight against mosquito‐borne diseases and cancer – a brief review’, Enzyme Microb. Technol., 2016, 95, pp. 58 –68 [DOI] [PubMed] [Google Scholar]
- 7. Benelli G. Pavela R. Maggi F. et al.: ‘Commentary: making green pesticides greener? The potential of plant products for nanosynthesis and pest control’, J. Clust. Sci., 2016, 28, pp. 3 –10 [Google Scholar]
- 8. Benelli G.: ‘Plant‐mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review’, Parasitol. Res., 2016, 115, pp. 23 –34 [DOI] [PubMed] [Google Scholar]
- 9. Rubilar O. Rai M. Tortella G. et al.: ‘Biogenic nanoparticles: copper, copper oxides, copper sulphides, complex copper nanostructures and their applications’, Biotechnol. Lett., 2013, 35, pp. 1365 –1375 [DOI] [PubMed] [Google Scholar]
- 10. Kung M.L. Tai M.H. Lin P.Y. et al.: ‘Silver decorated copper oxide (Ag@CuO) nanocomposite enhances ROS‐mediated bacterial architecture collapse’, Colloids Surf. B: Biointerfaces, 2017, 155, pp. 399 –407 [DOI] [PubMed] [Google Scholar]
- 11. Sahoo M. Chand P.K.: ‘ In vitro multiplication of a medicinal herb Tridax procumbens L. (Mexican daisy, coat button): influence of explanting season, growth regulator synergy, culture passage and planting substrate’, Phytomorphology, 1998, 48, pp. 195 –206 [Google Scholar]
- 12. Saini A. Soni H.K. Gupta P.: ‘A review on Tridax Procumbens ’, Imperial J. Interdiscip. Res., 2016, 2, pp. 308 –319 [Google Scholar]
- 13. Velayutham K. Rahuman A.A. Rajakumar G. et al.: ‘Larvicidal activity of green synthesized silver nanoparticles using bark aqueous extract of Ficus racemosa against Culex quinquefasciatus and Culex gelidus ’, Asian Pac. J. Trop. Med., 2013, 6, pp. 95 –101 [DOI] [PubMed] [Google Scholar]
- 14. Reddy P.J. Krishna D. Murthy U.S. et al.: ‘A microcomputer FORTRAN program for rapid determination of lethal concentration of biocides in mosquito control’, Comput. Appl. Biosci., 1992, 8, pp. 209 –213 [DOI] [PubMed] [Google Scholar]
- 15. Mageshwari K. Sathyamoorthy R.: ‘Flower‐shaped CuO nanostructures: synthesis, characterization and antimicrobial activity’, J. Mater. Sci. Tech., 2013, 29, pp. 909 –914 [Google Scholar]
- 16. Sharma J.K. Akhtar M.S. Ameen S. et al.: ‘Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye‐sensitized solar cells applications’, J. Alloys Compounds, 2015, 632, pp. 321 –325 [Google Scholar]
- 17. Ghidan A.Y. Al‐Antary T.M. Awwad A.M.: ‘Green synthesis of copper oxide nanoparticles using Punica granatum peels extract: effect on green peach aphid’, Environ. Nanotechnol. Monit. Manage., 2016, 6, pp. 95 –98 [Google Scholar]
- 18. Suman S. Kumar N. Kumar M.: ‘Electrochemical sensing and remediation of 4‐nitrophenol using bio‐synthesized copper oxide nanoparticles’, Chem. Eng. J., 2017, 313, pp. 283 –292 [Google Scholar]
- 19. Sankar R. Manikandan P. Malarvizhi V. et al.: ‘Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation’, Spectrochim. Acta A: Mol. Biomol. Spectrosc., 2014, 121, pp. 746 –750 [DOI] [PubMed] [Google Scholar]
- 20. Cao H.Q. Qiu X.Q. Luo B. et al.: ‘Synthesis and room‐temperature ultraviolet photoluminescence properties of zirconia nanowires’, J. Adv. Funct. Mater., 2004, 14, pp. 243 –246 [Google Scholar]
- 21. Kumari L. Li W.Z. Xu J.M. et al.: ‘Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties’, J. Cryst. Growth Des., 2009, 9, pp. 3874 –3880 [Google Scholar]
- 22. Aslani A. Oroojpour V.: ‘CO gas sensing of CuO nanostructures, synthesized by an assisted solvothermal wet chemical route’, Physica B, 2011, 406, pp. 150 –154 [Google Scholar]
- 23. Mukherjee N. Show B. Maji S.K. et al.: ‘CuO nano‐whiskers: electrodeposition, Raman analysis, photoluminescence study and photocatalytic activity’, Mater. Lett., 2011, 65, pp. 3248 –3250 [Google Scholar]
- 24. Erdogan I.Y. Gullu O.: ‘Optical and structural properties of CuO nanofilm: its diode application’, J. Alloys Compounds, 2010, 492, pp. 378 –383 [Google Scholar]
- 25. Ningthoujam R.S. Gajbhiye N.S. Ahmed A. et al.: ‘Re‐dispersible Li+ and Eu3+ co‐doped nanocrystalline ZnO: luminescence and EPR studies’, J. Nanosci. Nanotechnol., 2008, 8, pp. 3059 –3062 [DOI] [PubMed] [Google Scholar]
- 26. Vanheusden K. Warren W.L. Seager C.H. et al.: ‘Mechanisms behind green photoluminescence in ZnO phosphor powders’, J. Appl. Phys., 1996, 79, pp. 7983 –7990 [Google Scholar]
- 27. Gunalan S. Sivaraj R. Venckatesh R.: ‘ Aloe barbadensis miller mediated green synthesis of mono‐disperse copper oxide nanoparticles: optical properties’, Spectrochim. Acta A: Mol. Biomol. Spectrosc., 2012, 97, pp. 1140 –1144 [DOI] [PubMed] [Google Scholar]
- 28. Singh A.V. Patil R. Anand A. et al.: ‘Biological synthesis of copper oxide nano particles using Escherichia coli ’, Curr. Nanosci., 2010, 6, pp. 365 –369 [Google Scholar]
- 29. Honary S. Barabadi H. Gharaeifathabad E. et al.: ‘Green synthesis of copper oxide nanoparticles using Penicillium aurantiogriseum, Penicillium citrinum and Penicillium waksmanii ’, Dig. J. Nanomat. Biostruct., 2012, 7, pp. 999 –1005 [Google Scholar]
- 30. Gopalakrishnan K. Ramesh C. Ragunathan V. et al.: ‘Antibacterial activity of Cu2 O nanoparticles on E. coli synthesized from Tridax procumbens leaf extract and surface coating with polyaniline’, Dig. J. Nanomat. Biostruct., 2012, 7, pp. 833 –839 [Google Scholar]
- 31. Nasrollahzadeh M. Maham M. Sajadi S.M.: ‘Green synthesis of CuO nanoparticles by aqueous extract of Gundelia tournefortii and evaluation of their catalytic activity for the synthesis of N ‐monosubstituted ureas and reduction of 4‐nitrophenol’, J. Colloid Interface Sci., 2015, 455, pp. 245 –253 [DOI] [PubMed] [Google Scholar]
- 32. Nasrollahzadeh M. Sajadi S.M. Maham M.: ‘ Tamarix gallica leaf extract mediated novel route for green synthesis of CuO nanoparticles and their application for N ‐arylation of nitrogen‐containing heterocycles under ligand‐free conditions’, RSC Adv., 2015, 5, pp. 40628 –40635 [Google Scholar]
- 33. Ga'al H. Fouad H. Mao G. et al.: ‘Larvicidal and pupicidal evaluation of silver nanoparticles synthesized using Aquilaria sinensis and Pogostemon cablin essential oils against dengue and zika viruses vector Aedes albopictus mosquito and its histopathological analysis’, Artif. Cells Nanomed. Biotechnol., 2017, 31, pp. 1 –9 [DOI] [PubMed] [Google Scholar]


