Abstract
In this study, the bacterial strain CEES 33 was isolated from the coastal area of the Red Sea, Jeddah, Kingdom of Saudi Arabia. The bacterium isolate was identified and characterized by using biochemical and molecular methods. The isolate CEES 33 has been identified as Gram‐negative rod shaped and cream pigmented spherical colonies. It also demonstrated a positive result for nitrate reduction, oxidase, catalase, citrate utilization, lipase and exopolysaccharide production. Strain CEES 33 was characterized at the molecular level by partial 16S rRNA sequencing and it has been identified as Marinobacter lipolyticus (EMBL|LN835275.1). The lipolytic activity of the isolate was also observed 2.105 nkatml−1. Furthermore, the bacterial aqueous extract was used for green synthesis of silver nanoparticles (AgNPs), which was further confirmed by UV‐visible spectra (430 nm), XRD and SEM analysis. Moreover, the biological functional group that involved in AgNPs synthesis was confirmed by FTIR spectra. The biological activities of AgNPs were also investigated, which showed a significant growth inhibition of Candida albicans with 16 ± 2 mm zone of inhibition at 10 μg dose/wells. Therefore, bacterium Marinobacter lipolyticus might be used in future for lipase production and nanoparticles fabrication for biomedical application, to control fungal diseases caused by C. albicans.
Inspec keywords: enzymes, molecular biophysics, biochemistry, silver, nanoparticles, nanofabrication, nanomedicine, antibacterial activity, biomedical materials, ultraviolet spectra, visible spectra, Fourier transform infrared spectra, microorganisms, reduction (chemical), RNA, molecular configurations, X‐ray diffraction, scanning electron microscopy, diseases
Other keywords: lipase production, silver nanomaterial modulation, anticandidal activities, bacterial strain CEES 33, bacterial isolate, biochemical method, molecular method, gram‐negative rod shaped colonies, cream pigmented spherical colonies, nitrate reduction, oxidase, catalase, citrate utilisation, exopolysaccharide production, molecular level, partial 16S rRNA sequencing, Marinobacter lipolyticus strain EMBL|LN835275.1, lipolytic activity, bacterial aqueous extract, green synthesis, UV‐visible spectra, X‐ray diffraction, scanning electron microscopy, biological functional group, AgNPs synthesis, Fourier transform infrared spectroscopy, Candida albicans, media plate, industrial lipase production, biomedical application, fungal diseases, wavelength 430 nm, Ag
1 Introduction
Screening of halophiles and thermophiles is an important step for discovery of thermostable proteins and enzymes. Marine‐derived microorganisms of the Red Sea are stable at hyper‐saline and thermophilic conditions because they have unique thermostable enzymes and proteins. These thermostable microbial contents can be extracted to be used for a number of biotechnological applications at high temperature and hyper‐saline conditions [1, 2]. Moreover, lipase (EC 3.1.1.3) is one of the most important thermostable enzymes that could be extracted from marine‐derived microbial isolates of the Red Sea. Recently, lipase activity has gained more attention because of its catalytic reaction that depends on water availability, and it also has the ability to synthesise and hydrolyse esters of long chain fatty acid [3]. There is a growing demand for using thermostable lipases for production of cosmetics, detergents and agro‐industries [4, 5]. In addition, lipase can be used in various biotechnological applications such as bio‐polymer/bio‐diesel production, synthesis of fine chemicals for cosmetic ingredients and medically important drug production [6, 7]. A number of thermostable microorganisms that are able to produce thermostable enzymes, particularly lipase, have been isolated from several wild organisms [8, 9, 10]. Interestingly, some scientists genetically modified the organisms in order to express lipases for a huge production and wider applications [11, 12]. Screening methods for potential lipase‐producing bacteria are still in progress with variable activities. Isolation, identification and characterisation of extracellular hydrolytic enzyme producing marine‐bacteria have shown optimum enzymatic activities at hyper‐saline and thermostable condition, which opens a new avenue for research and technology development. Most of halophiles are able to tolerate a wide range of salt concentrations and these bacterial cell metabolisms are regulated by specific enzymes and factors, which in turn will support the microorganisms to adapt and survive under extreme conditions [13]. A huge number of bacterial isolates derived from different sources has been reported with positive lipolytic activity [14, 15]; however, marine microbial diversity of the Red Sea with lipolytic activity remains largely unexplored. Furthermore, bacterial extract has also been used for silver nanomaterials synthesis through bottom up bio‐fabrication process. This approach was cheap, eco‐friendly, and effective in controlling pathogenic organisms. There is an urgent need to develop novel strategies of synthetic control of nanoparticles to generate nanocrystals that possess well‐controlled surfaces, size, morphology and composition, and without hazardous material generation to be used for biomedical, optical and electronic applications. However, this objective is still a massive challenge for the researchers to overcome [16, 17]. Therefore, understanding the importance of developing innovative techniques for nanomaterials synthesis would result in less detrimental effects to human health and the ecosystem. In addition, a number of researchers and institutional bodies are increasing the focus to search for novel bacteria and develop innovative materials that can be used for new drug development. Recently, the research field of nanomaterials has received a great attention due to development of newly synthesised nanomaterials of well‐defined size and shape. Previous studies have observed some natural properties of nanoparticles such as high surface to volume ratios when their size is reduced [18, 19]. Several methods for nanomaterials synthesis have been reported in different studies such as sol–gel, ignition, pyro‐synthesis and many other physico‐chemical methods. However, the researchers have faced some limitations to use these methods because they cost a lot and not eco‐friendly [20, 21, 22, 23]. Therefore, there is a growing demand to develop innovative methods for nanomaterial synthesis to produce more environmentally safe and inexpensive nanomaterials. This will help to improve the development of nanoparticles based drugs to be used for different biomedical applications [24]. In this regard, applying nanomaterials with novel biological activities that have been synthesised by innovative strategies, has provided the best solution to overcome the environmental problems. For instance, synthesised nanomaterials can be applied in an antibacterial agent [25, 26, 27], bio‐control agent [28], cancer therapy [29], catalysts in chemical reactions [30], biosensor, etc. [31]. Nowadays, innovative strategies for nanomaterials synthesis have been applied, which include biological agents/systems such as bacteria, fungi and plant extracts. Recently, microorganisms‐based synthesised silver nanoparticles from bacteria, such as Ochrobactrum sp. [32], Streptomyces albidoflavus [33], Pseudomonas aeruginosa [34], Alteromonas macleodii [35], and Stenotrophomonas sp. [36]. In our study, the impact of biological nanomaterials was considered, thereby we aimed to explore marine bacterial isolates with lipolytic activities derived from extreme environmental conditions in the coast of the Red Sea. We also designed the study to do further characterisation by biochemical tests and identification by molecular techniques. The bacterial aqueous extract was applied for nanoparticles synthesis at room temperature without using any reducing agents. The resulted synthesised materials were consequently characterised by using modern spectroscopy such as UV‐visible, Fourier transform infrared spectroscopy (FTIR, X‐ray diffraction (XRD), nano particle size analyser and scanning electron microscopy (SEM). In addition, the antimicrobial activity has been assessed against pathogenic organisms such as Candida albicans.
2 Materials and methods
2.1 Materials
Marine media and chemical reagents, such as Zobell Marine Broth and agar, dextrose, gelatin, glycerol, glucose, maltose, sucrose and other salt were all purchased from Hi‐Media, Mumbai, India and from Scharlab. Silver nitrate was obtained from La Pine, Scientific Company, Berkeley, Calif, 94710, Chicago.
2.2 Marine water sample collection
Marine water samples were collected in Niskin bottles that are sterilised and thoroughly rinsed by acid. On 15 September 2015, sampling was carried out during a cruise trip within 5 Km away from the coast of the Red Sea. The samples were collected from three different sites at depths of 9 m from surface of water with the help of rosette sampler assembled with Niskin bottles. The collected samples were instantly processed and filtered through polycarbonate filters (EMD Millipore, Germany) to remove living macro‐organisms and suspended particles. All collected samples from the shore were immediately saved in the ice bag to be delivered for lab investigation and screening of microbial diversity. The location of sample collection was the coast of the Red Sea near Jeddah, Kingdom of Saudi Arabia.
2.3 Isolation of marine bacteria
The collected water samples from the coastal area of the Red Sea were stored in sterile bottles. Historically, the coastal area was hyper‐saline and consistently loaded with industrial and sewage wastewater from Jeddah city. To isolate marine‐derived bacteria, water samples were serially diluted with artificial seawater. Then, 1 µl of the diluted sample was inoculated onto Zobell marine media plate. The inoculated media were incubated at 35°C for four days, then the isolated colonies were further purified in new Zobell marine media. During our investigations, 39 bacterial isolates were selected, screened out, and identified at the molecular level. The morphological and biochemical properties were analysed through different biochemical tests such as catalase, oxidase test, citrate utilisation, methyl red test, nitrate reduction, indole production, starch hydrolysis, sugar fermentation, acid production and gelatin liquefaction test [37].
2.4 Molecular characterisation and phylogenetic tree construction
Out of total 12 marine bacterial isolates, isolate number CEES 33 has been identified as thermostable, lipase producer, and more stable at hyper‐saline. Therefore, CEES 33 has been selected for further assessments at the molecular level and the sample was submitted to Macrogen centre, Seoul, South Korea to be examined. The molecular sequencing of 16S rDNA partial chain of CEES 33 isolate has been done commercially by online sequencing service. During the molecular process, specific universal primers were used for sequencing: for forward sequence 518F, 5″CCAGCAGCCGCGGTAATACG3″, and for reverse sequence 800R, 5″TACCAGGGTATCTAATCC3″. After obtaining the results, the nucleotide sequence data arranged in FASTA format and deposited into the EMBL sequence database. Nucleotide BLASTn is an online program can be found on NCBI website, which was run to determine the homology, maximum likelihood and to identify the most similar sequences (http://www.ncbi.nlm.nih.gov/BLAST). Furthermore, genetic sequencing of bacterial isolate was also applied for phylogenetic tree construction by a neighbour‐joining method of MEGA 6.1 software program [38, 39].
2.5 Lipase activity assay
An assay was conducted to investigate Lipase activity of the isolated bacterial strain CEES 33 on solid media (agar plates). Marine agar media amended with 10 gl−1 of olive oil before pouring the sterilised media into plates. After an overnight incubation, the plates were inoculated with 10 µl of standard inoculums in the centre of plate surface and further incubated at 36°C for 3 days. Lipase production was assessed by observing a clear zone around the colony. Quantitative assay was carried out by preparing liquid broth media in 250 ml of Erlenmeyer flasks with 50 ml liquid volume. The media contain (gl−1): CaCl2, 0.1; K2HPO4, 1; KCl, 0.5; MgSO4⋅7H2O, 0.5; NaNO3, 3; NaCl, 15; meat extract, 1; trace element solution, 1 mll−1, amended with olive oil (20 gl−1) as sole energy and carbon source. Bacterial culture was inoculated into triplicate flasks with 0.1% standard inoculum of total volume, and all flasks were incubated in an orbital shaker (IKA, KS 4000i control, Korea) at 40°C and 160 rpm. Evaporation was properly checked and the level was balanced with the addition of sterile water. Lipase production in growing media was determined by Vorderwülbecke et al. enzyme assays [40].
2.6 Bacterial extracts preparation
The bacterial isolate CEES 33 was cultured in sterile marine broth media and incubated before the stationary phase of bacterial growth. Bacterial stain CEES 33 was allowed to grow at 36°C for 24 h in a 1 L conical flask with 500 ml working volume, and further incubated in an orbital shaker at 160 rpm. After an overnight incubation, grown culture media was centrifuged at 9000 rpm to obtain cell pellets and remove supernatant [41]. Furthermore, bacterial pellets (1 g) dissolved in 10 ml Mili Q water to be sonicated at 50°C for 1 h. After sonication, 10 ml aliquots were centrifuged at 10,000 rpm, and the obtained supernatans used for further application in material preparation and the pellets were discarded.
2.7 Silver nanoparticles synthesis and characterisation
Marine bacterial extract (10 ml) from the log growth phase before stationary phase was mixed in a drop wise manner with 90 ml of 1 mM of the chilled AgNO3 solution and the mixture was agitated at 100 rpm. Then, the reaction mixture was incubated for overnight at room temperature in dark room. As a result, the colour of the reaction mixture became dark brown and analysed by UV‐visible spectroscopy, which refers to absorption spectroscopy. Thereafter, this reaction was terminated in the oven at 80°C and centrifuged at 10,000 rpm for 1 h and subsequently washed three times with Milli Q water. For purification, particle suspension was transferred into a 12 kDa cutoff dialysis bag. Following that step, the suspensions of synthesised particles were re‐suspended in a buffer solution (HEPES, 20 mm mM, pH 7.4) with sucrose to optimise density of 2.5 gml−1. The solution containing particles was centrifuged with the concentration gradient of sucrose (optimised density ranged from 0.25 to 10 M) and consequently obtained a suspension with a layer of nanoparticles at 10,000 rpm for 2 h at 4°C. Finally, the resulted nanoparticles in the solution were determined by ICPE‐9000, Plasma Atomic Emission Spectrophotometer (UV‐1800, Shimadzu, Japan) [42].
2.8 Characterisation
The physical properties of synthesised AgNPs were assessed and characterised by UV‐visible, in which the reaction mixture was analysed after sample dilution with Milli Q water and incubation for overnight [43]. Purified silver nanomaterials are strongly water soluble and the colour change from brown to dark brown was observed in the solution. The colour change was observed after the formation of particles in the reaction mixture. The spectra of AgNPs containing solution were measured by using UV‐visible spectrophotometer (UV‐1800, UV Spectrophotometer, Shimadzu, Japan) between 300 and 1000 nm wavelength. Quartz cuvette of 10‐mm‐optical‐path‐length was used for this measurement. Furthermore, the other characteristics of synthesised nanoparticles such as the size, distribution and stability in the reaction mixture were identified by the Nanophox particle size analyser (Sympatec GmbH, Germany) [44]. The functional group and composition of nanoparticles were determined by FTIR spectrophotometer. Synthesised nanomaterials were grind with KBR because these particles act as a beam splitter during analysis. Nanoparticles spectra were recorded at a resolution of 4 cm−1 in a wave number region from zero to 3500 cm−1 [45]. The crystalline nature of the synthesised materials was also observed by XRD analysis. The analytical procedure was carried out by using Rigaku Miniflex X‐ray diffractometer in 2θ and range from 20° to 80° with Cu‐KA radiation (1 = 0.15406). In addition, further morphological analysis of the synthesised nanomaterials was carried out by SEM and transmission electron microscopy (TEM) analysis.
2.9 Antimicrobial activity
The antifungal activity of AgNPs synthesised from the bacterial extract of CEES 33 strain of M. lipolyticus was investigated against multi‐drug resistant human pathogens like C. albicans, which was obtained from King Fahad Medical Research Center, King Abdulaziz University, Jeddah. Stock cultures were maintained in 70% glycerol at 4°C. C. albicans was isolated from patients who suffer from urinary tract infections (UTI). Luria Bertani agar (LBA) plates were prepared through pouring the autoclaved melted media into sterile disposable Petri plates. Consequently, these plates were solidified and inoculated with C. albicans by a sterile glass spreader. After 15 min incubation, small wells of 8 mm diameter were made on the plates to be loaded with our testing material. Testing material was suspended in 50 µl aqueous suspension that contains 10 µg AgNPs, and then the suspension of testing material was loaded into each well. All plates were incubated at 35°C for 24 h in an incubator and subsequently checked for sensitivity against the pathogenic organism. In addition, antimicrobial activity was determined by measuring the diameter of the zone of inhibition around the well. Fluconazole (10 µg) is an antifungal agent used as a positive control against C. albicans. The study of antimicrobial activity has been done according to the standard method of the National Committee for Clinical and Laboratory Standards and the Clinical and Laboratory Standards Institute [46, 47]. Furthermore, the effect of AgNPs on the biofilm of C. albicans was also observed by electron microscopy.
3 Results and discussion
3.1 Characterisation of bacterial strain
Marine‐derived bacteria isolated from the coast of the Red Sea near Jeddah, Saudi Arabia, where the aquatic atmosphere was severely contaminated since a long time by oil refineries and sewage. Among 39 bacterial isolates, CEES 33 strain was screened out because of its significant lipolytic activity, high tolerance to hyper‐saline and stability at high temperatures. The marine isolate CEES 33 was identified morphologically and biochemically as follow: Gram‐negative rod shaped and cream pigmented spherical colonies on ZMA plates; also showed positive results for nitrate reduction, oxidase, catalase, citrate utilisation, starch and gelatin hydrolisation at room temperature (Table 1). Therefore, the isolate CEES 33 was hypothetically identified as Marinobacter sp. based on the biochemical and morphological features and comparison to the previously reported strain from Bergey's Manual of Determinative Bacteriology. Furthermore, the bacterial strain was also characterised at the molecular level by 16S rRNA gene sequencing, in which the data was submitted to EMBL database and thus obtained a unique accession number LN835275.1. The BLASTn sequence analysis tool also confirmed the identification of the bacterial strain as M. lipolyticus. The BLASTn analysis is a sequence similarity search tool that provides the primary information about a newly discovered nucleotide sequence. A neighbour‐joining tree with MEGA 6 program was used for creating a phylogenetic tree after sequence alignment with the related sequence (Fig. 1). During BLASTn analysis, CEES 33 bacterial strain has shown 99% sequence similarity with bacterial strain SM‐19 of Marinobacter lipolyticus (NR025671.1). In previous reports, halophilc and lipolytic bacterium Marinobacter sp. was isolated from different geographical locations like Sudan underground mine [48], coastal surface sea water of Visakhapatnam, India [49], salt lake of Xiaochaidan, China [50] and Sea sediments of South China [51].
Table 1.
Strain CEES 33 of M. lipolyticus morphological and biochemical properties
| Characteristics | Results |
|---|---|
| colony morphology | cream yellow, circular, convex |
| gram staining | − |
| shape | rod‐shaped |
| salt tolerance | 5–15% (w/v) optimum growth at 10% |
| temperature | 10–55°C |
| pH | 5–8 |
| spore formation | absence |
| nature | aerobic |
| catalase | + |
| oxidase | + |
| sugar utilisation and acid production from glucose, maltose, mannitol | + |
| sugar utilisation and without acid production from Glycerol and D‐lactose | − |
| gelatin hydrolysis | − |
| starch hydrolysis | − |
| indole | − |
| methyl red | − |
| voges‐proskauer | − |
| phosphatase | − |
| simmons citrate | + |
Fig. 1.
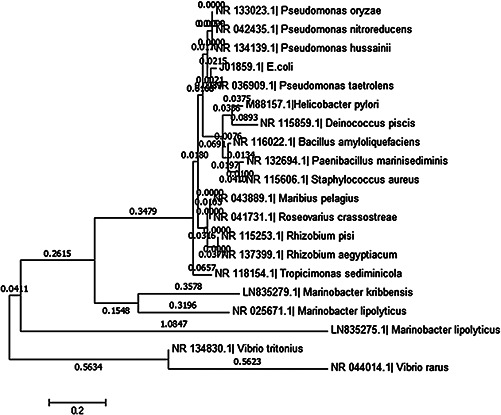
Phylogenetic tree of M. lipolyticus (embl|LN835275.1) constructed by the neighbour joining method after ClustalW alignment of all sequences with the help of MEGA 6 software
3.2 Lipase production
The lipolytic activity of marine‐derived bacterial isolate CEES33 was assessed qualitatively on solid media and quantitatively in a liquid culture broth using olive oil as a sole energy and carbon source. The lipolytic activity was observed as clear halos on solid media (Fig. 2). Previous studies demonstrated a positive lipolytic activity of Marinobacter confluentis, which was isolated from the junction between fresh water and marine water [50, 52]. In our study, the bacterial strain grown in a liquid culture medium and a significant lipolytic activity −2.105 nkatml−1 was detected in the supernatant. Furthermore, the supernatant lipolytic activity was stable at 6–8 pH and 70°C. In another study, the lipolytic enzymes of bacterial strain in 96‐well plates were determined by turbidity assay [53]. In our investigation, the lipolytic activity in the supernatant was the same in both liquid and solid agar media. In previous reports, a bacterial strain of Aneurinibacillu s sp. has shown a significant variable activity in different screening methods on different media supplemented with olive oil as a carbon source [52].
Fig. 2.

M. lipolyticus growth on olive oil containing media plate showing clear zone of lipolytic activity
3.3 Nanomaterials characterisation
3.3.1 UV‐visible spectra analysis of nanoparticles
The biosynthesis of AgNPs were a result of the Ag+ ion reduction to Ag0 in AgNO3 solution at room temperature by using an aqueous bacterial extract. After an overnight incubation, the colour changed from light to dark brown, which indicates the biogenic reduction [53]. The reduction was further confirmed by observing the optimal absorption peak around 430 nm (Fig. 3 A). The optical spectra of AgNPs identified by the oscillation that also known as surface plasmon resonance (SPR) [54]. This oscillation is a shift of band patterns, which depends on nanoparticle size and shape, particle aggregation and their distribution. Moreover, the shift in band patterns demonstrated the electrons movement of AgNPs in the reaction mixture was at the highest. This could be observed by the proximity between the conduction band and valence band. Therefore, the collective oscillations of electrons movement on AgNPs surface may result in a band of SPR absorption in a visible range [55]. The UV‐visible SPR results are compatible with the recent findings of organic coated green synthesised silver nanomaterials [56].
Fig. 3.
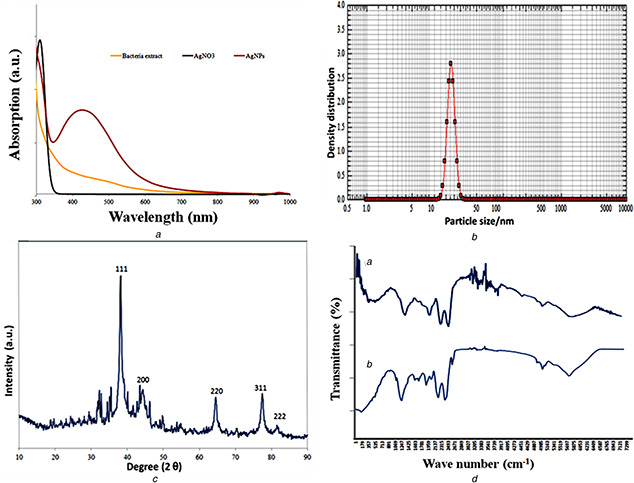
Characterisation of silver nanoparticles synthesised from the extract of M. lipolyticus
(A) UV‐visible spectrum a 423 nm, (B) Nanophox particle size analysis −15.6 nm, (C) XRD graph showing crystalline of silver particles, (D) FTIR spectra showing the involvement of functional group in particles formation
3.3.2 FTIR spectroscopy
The FTIR spectroscopy was also used to detect the biomolecules that exist as capping and reducing agents for AgNPs derived from log‐phase bacterial extract following their reaction with AgNO3 as shown in Fig. 3 D. The spectral analysis identified a marginal shift in the peak position of spectra. The obtained findings of FTIR spectra are in accordance with those of Khan et al. [57] and Basu et al. [58], who identified a similar trend in the FTIR spectra of the synthesised AgNPs from natural sources. The infrared region of bacterial extracts revealed different absorption bands at 3205, 3383, 2493, 2137, 1959, 1781, 1603, 1425, 1247, 1059, 891, 713 and 535 cm−1. In case of AgNPs, a shift in the absorbance peak with decreased band intensity was observed at 3310 cm−1. This indicates the binding of Ag with N‐H and/or O‐H group of the bacterial extract. These findings are also consistent with other studies, in which spectral peak was observed between 3205 to 3383 cm−1 and correspond to N‐H, and O‐H stretching vibrations of 1°, and 2° amines, amides, alcohol and H–bonded to phenols [58, 59]. The absorbance peak of AgNPs spectra at 1399 to 1671 cm−1 indicates C‐H stretching vibrations of alkene whereas the peak at 1059 cm−1 represents the C‐H in‐plane bending of alkenes, alcohols, carboxylic acids, esters, and ethers [60, 61]. Ankamwar et al. [62]. have also investigated the bio‐molecu involved the core particle matter. In our study, the spectral peak at 1603 cm−1 is predominant, which indicates the involvement of amide‐I bond (‐C = O) of proteins as capping agent and involved in the stabilisation of AgNPs. Therefore, we found such strong indication that the reduction of Ag+ took place through some hydroxyl groups, which are oxidised at the expense of Ag reduction.
3.3.3 XRD pattern
The XRD pattern of synthesised AgNPs confirmed that silver nanoparticles are crystalline in nature. In our study, we have shown the crystalline structure of dried AgNPs by analysing the XRD pattern. The distinct XRD peak at 2θ degrees of 38.09°, 44.04°, 64.49° and 77.39° (Fig. 3 C) can be attributed to the reflections of lattice planes 111, 200, 220 and 311. The obtained results showed a perfect matching with spherical shape and the crystalline structure of silver (JCPDS file no. 04‐0783). These reflections demonstrated a good correlation with Bragg's reflections. Moreover, the peak appears at 29.60° near 31.9° indicated the presence of Ag2 O [62, 63]. The unassigned peak at AgNPs spectrum showed the involvement of biological moieties of bacterial extract in material synthesis. Analysed data file number PDF#04‐0783 showed particles formation with crystallite size 15.6 nm. Similarly, Swanson and Tatge [64] have observed for the first time the XRD patterns of chemical synthesised silver materials. In general, the phase width of materials is directly proportional to the mean of material crystallite size. The broader peak is indicating the crystallite size is small [65]. In our results, broader peak indicates the bacterial extract is involved in crystal nuclei growth and particle formation. This result is also agreeing with the XRD pattern that was observed by Becheri et al. [66].
3.3.4 FESEM and EDX
FESEM analysis was used to investigate the surface morphology and size of biogenic AgNPs [67]. Using this tool, we observed the biogenic AgNPs mainly in uniform spherical shape with size ranging from 13.56 to 18.33 nm (Fig. 4 a and b). Moreover, the silver metal distribution beginners confirmed in our biogenic AgNPs by energy dispersive X‐ray [68]. Furthermore, synthesised nanoparticles shape and size confirmed by TEM depicted in Fig. 5. The data obtained at a particular point of gold‐coated sample using this analysis showed the involvement of silver in particle formation, whereas non‐metallic residue confirmed the involvement of biological moieties from bacterial extract.
Fig. 4.
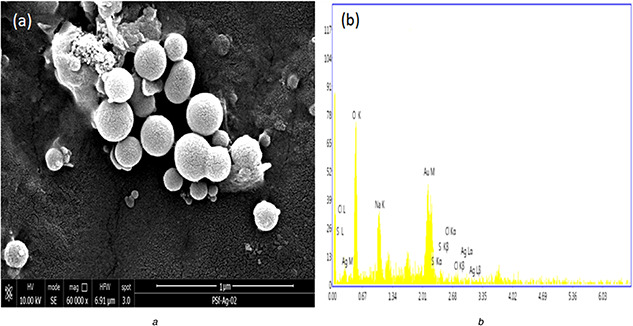
SEM of
(a) Biogenic silver nanoparticles, (b) EDX analysis
Fig. 5.
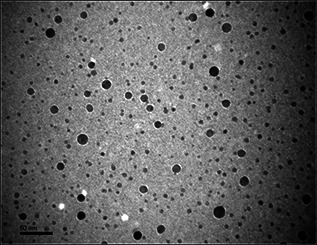
TEM of biogenic silver nanoparticles
3.4 Antimicrobial activity
The synthesised AgNPs were tested against the fungal strain, C. albicans to investigate the effect of synthesised nanomaterials on microbial growth inhibition. Therefore, we observed the zones of microbial growth inhibition against C. albicans on PDA plates (Fig. 6 b). In our study, we have found that the diameters of inhibition zones for both organisms were 16 ± 2 mm. The difference in zone diameter in our study and in the previously reported literature could be explained by the variation in the concentrations of the exposed AgNPs or because of the high diffusion of nanomaterials in the nutrient media. Similarly, the application of AgNPs as antimicrobial agents against several bacterial strains and C. albicans resulted in zone formation on solid agar media [69]. To improve our understanding of this interaction, a growth kinetic study was performed to investigate the effect of AgNPs in a dose‐dependent manner (10, 20, 40, 50, 80 and 100 μgml−1) on microbial growth. This effect was determined by comparing the optical density in the growing liquid broth media, and we found a significant reduction in microbial counts at 100 μgmL−1 (Fig. 6 a). Furthermore, the effect of synthesised AgNPs on biofilm was examined by SEM (Fig. 7). When C. albicans biofilm treated with 50 μgml−1 of AgNPs and incubated for overnight at 35°C temperature, distorted morphology was observed in the treated culture (Fig. 7 b), which was fixed in 2% glutaraldehyde and washed with distilled water for examination. Similarly, when C. albicans exposed to silver nanomaterials, detrimental effects were observed on cell morphology and metabolism [70]. Similarly, Raisuddin et al. demonstrated the antifungal activity of biogenic silver nanoparticles particularly against C. albicans [71].
Fig. 6.
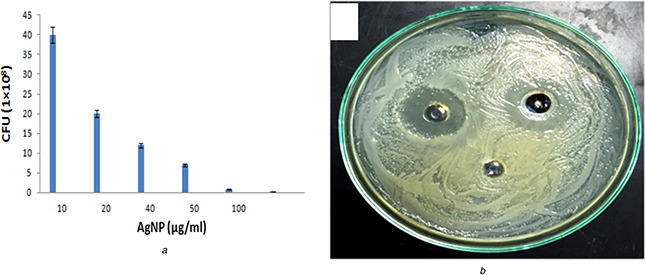
Anti candidal activity of biogenic nanoparticles
(A) Showing the effect of AgNps on colony forming unit in growing media, (B) Zone inhibition activity of C. albicans after overnight incubation on a plate
Fig. 7.
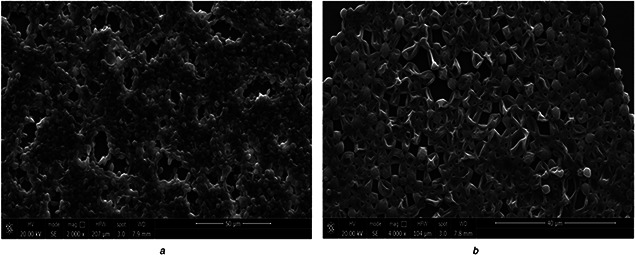
Effect of silver nanoparticles on C. albicans biofilm
(A) Untreated and, (B) Treated with 50 µg/ml for overnight at 35°C
4 Conclusion
In this study, we have demonstrated innovative strategies to explore marine‐derived bacteria with different biological activities of the coastal region of the Red Sea, Jeddah, Kingdom of Saudi Arabia. This study revealed marine‐derived microorganisms that ability to survive under extreme conditions such as hyper saline and variable range of temperature, and demonstrated a significant lipolytic activity. Furthermore, we have successfully demonstrated the application of bacterial extracts in green synthesis of AgNPs. Moreover, our findings indicated that a biological functional group from bacterial extract can play a significant role in the reduction of the Ag+ ion to Ag0 and their stabilisation in the formation of nanoparticles. Therefore, the proposed method for AgNPs synthesis is rapid and eco‐friendly, which provides a better solution to control the environmental problems. In addition, the produced AgNPs have spherical shape and size 15.6 nm. The green synthesised AgNPs showed a significant anti‐fungal activity, when investigated against medically important pathogenic microorganism like C. albicans. The present study demonstrated that the, biogenic AgNPs have a potential application as anti‐infective agents for controlling various human diseases. Therefore, the current research opens a new avenue for the green synthesis of nanoparticles, which could be easily applied for various industrial purposes.
5 Acknowledgments
This research was financially supported by the Centre of Excellence in Environmental Studies (CEES), King Abdulaziz University, Jeddah and Ministry of Higher Education of Kingdom of Saudi Arabia.
6 References
- 1. Alnahdi H.S.: ‘Isolation and screening of extracellular proteases produced by new Isolated Bacillus sp.’, J. App. Pharma. Sci., 2012, 2, (9), pp. 071 –074 [Google Scholar]
- 2. Nadeen F. Oves M. Qari H.A. et al.: ‘Red Sea microbial diversity for antimicrobial and anticancer agents’, J Mol. Biom. Diag., 2015, 7, p. 267 [Google Scholar]
- 3. Villeneuve P. Muderhwa J.M. Graille J. et al.: ‘Customizing lipases for biocatalysis: a survey of chemical, physical and molecular biological approaches’, J. Mol. Cata B Enz., 2012, 9, pp. 113 –148 [Google Scholar]
- 4. Ansorge‐Schumacher M.B. Thum O.: ‘immobilized lipases in the cosmetics industry’, Chem, Soci. Rev., 2013, 42, pp. 6475 –6490 [DOI] [PubMed] [Google Scholar]
- 5. Hasan F. Shah A.A. Javed S. et al.: ‘Enzymes used in detergents: Lipases’, Afr. J Biotechnol., 2010, 9, (31), pp. 4836 –4844 [Google Scholar]
- 6. Jaeger K.E. Dijkstra B.W. Reetz M.T.: ‘Bacterial biocatalysts: molecular biology, three‐dimensional structures, and biotechnological applications of lipases’, Annu. Rev. Microbiol., 1999, 53, pp. 315 –351 [DOI] [PubMed] [Google Scholar]
- 7. Pandey A. Benjamin S. Soccol C.R. et al.: ‘The realm of microbial lipases in biotechnology’, Biotechnol. Appl. Biochem., 1999, 29, pp. 119 –131 [PubMed] [Google Scholar]
- 8. Turner P. Mamo G. Karlsson E.N.: ‘Potential and utilization of thermophiles and thermostable enzymes in biorefining’, Microb. Cell Factories, 2007, 6, p. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salihu A. Alam M.Z.: ‘Thermostable lipases: an overview of production, purification and characterization’, Biosci. Biotechnol. Res. ASIA, 2014, 11, (3), pp. 1095 –1107 [Google Scholar]
- 10. Abusham R.A. Rahman R.N.Z.R. Salleh A.B. et al.: ‘Optimization of physical factors affecting the production of thermo‐stable organic solvent‐tolerant protease from a newly isolated halo tolerant Bacillus subtilis strain’, Microb. Cell Factories, 2009, 8, pp. 8 –20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quintana‐Castro R. Dıaz P. Valerio‐Alfaro G. et al.: ‘Gene cloning, expression, and characterization of the Geobacillus thermoleovorans CCR11 thermoalkaliphilic lipase’, Mol. Biotechnol., 2009, 42, (1), pp. 75 –83 [DOI] [PubMed] [Google Scholar]
- 12. Jiang Y. Zhou X. Chen Z.: ‘Cloning, expression, and biochemical characterization of a thermostable lipase from Geobacillus stearothermophilus JC’, World J. Microbiol. Biotechnol., 2010, 26, (4), pp. 747 –751 [Google Scholar]
- 13. Ventosa A. Nieto J.J. Oren A.: ‘Biology of moderately halophilic aerobic bacteria’, Microbiol. Mol. Biol. Rev., 1998, 62, pp. 504 –544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh P. Singh S.M. Roy U.: ‘Taxonomic characterization and the bio‐potential of bacteria isolated from glacier ice cores in the High Arctic’, J. Basic Microbiol., 2016, 56, (3), pp. 275 –285 [DOI] [PubMed] [Google Scholar]
- 15. Singh P. Singh S.M. Dhakephalkar P.: ‘Diversity, cold active enzymes and adaptation strategies of bacteria inhabiting glacier cryoconite holes of High Arctic’, Extremophiles, 2014, 18, (2), pp. 229 –242 [DOI] [PubMed] [Google Scholar]
- 16. Gericke M. Pinches A.: ‘Biological synthesis of metal nanoparticles’, Hydrometallurgy, 2006, 83, pp. 132 –140 [Google Scholar]
- 17. Tourinho P.S. vanGestel C.A. Lofts S. et al.: ‘Metal‐based nanoparticles in soil: fate, behavior, and effects on soil invertebrates’, Environ. Toxicol. Chem., 2012, 31, (8), pp. 1679 –1692 [DOI] [PubMed] [Google Scholar]
- 18. EI‐Sayed M.A.: ‘Some interesting properties of metals confined in time and nanometer space of different shapes’, Acc. Chem. Res., 2001, 34, pp. 257 –264 [DOI] [PubMed] [Google Scholar]
- 19. Chimene D. Alge D.L. Gaharwar A.K.: ‘Two‐dimensional nanomaterials for biomedical applications: emerging trends and future prospects’, Adv. Mater., 2015, 27, (45), pp. 7261 –7284 [DOI] [PubMed] [Google Scholar]
- 20. Smetana A.B. Klabunde K.J. Sorensen C.M.: ‘Synthesis of spherical silver nanoparticles by digestive ripening, stabilization with various agents, and their 3‐ D and 2‐D superlattice formation’, J. Colloid Inter. Sci., 2005, 284, pp. 521 –526 [DOI] [PubMed] [Google Scholar]
- 21. Yu D.G.: ‘Formation of colloidal silver nanoparticles stabilized by Na+–poly(c‐glutamic acid)–silver nitrate complex via chemical reduction process’, Colloids Surf B Biointerfaces, 2007, 59, pp. 171 –178 [DOI] [PubMed] [Google Scholar]
- 22. Kang J. Mathew V. Gim J. et al.: ‘Pyro‐synthesis of a high rate nano‐Li3 V2 (PO4)3 /C cathode with mixed morphology for advanced Li‐ion batteries’, Sci. Rep., 2014, 4, pp. 40 –47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oves M. Arshad M. Khan M.S. et al.: ‘Anti‐microbial activity of cobalt doped zinc oxide nanoparticles: Targeting water borne bacteria’, J. Saudi Chem. Soc., 2015, 19, pp. 581 –588 [Google Scholar]
- 24. Akema R.: ‘Biomediated unmodified silver nanoparticles as a green prove for Cu2+ ion detection’, Sens. Lett., 2015, 13, pp. 953 –960 [Google Scholar]
- 25. Azam A. Ahmed A.S. Oves M. et al.: ‘Antimicrobial activity of metal oxide nanoparticles against Gram‐positive and Gram‐negative bacteria: a comparative study’, Int. J. Nanomed., 2012, 7, pp. 6003 –6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oves M. Khan M.S. Zaidi A. et al.: ‘Antibacterial and cytotoxic efficacy of extracellular silver nanoparticles biofabricated from chromium reducing novel OS4 strain of Stenotrophomonas maltophilia ’, PLoS ONE, 2013, 8, (3), p. e59140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rahisuddin A.: ‘Extracellular synthesis of silver dimer nanoparticles using Callistemon viminalis (bottlebrush) extract and evaluation of their antibacterial activity’, Spectrosc. Lett., 2016, 49, (4), pp. 268 –275 [Google Scholar]
- 28. Rangaraj S. Gopalu K. Muthusamy P. et al.: ‘Augmented biocontrol action of silica nanoparticles and Pseudomonas fluorescens bioformulant in maize (Zea mays L.)’, RSC Adv., 2014, 4, p. 8461 [Google Scholar]
- 29. Chauhan A. Zubair S. Tufail S. et al.: ‘Fungus‐mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer’, PLoS ONE., 2011, 6, pp. 2305 –2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J. Gu H.: ‘Novel metal nanomaterials and their catalytic applications’, Molecules, 2015, 20, pp. 17070 –17092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holzinger M. Goff L.A. Cosnier S.: ‘Nanomaterials for biosensing applications: a review’, Front. Chem., 2014, 2, p. 0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas R. Janardhanan A. Varghese R.T. et al.: ‘Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp.’, Braz. J. Microbiol., 2014, 45, p. 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prakasham R.S. Buddana S.K. Yannam S.K. et al.: ‘Characterization of silver nanoparticles synthesized by using marine isolate Streptomyces albidoflavus ’, J. Microbiol. Biotechnol., 2012, 22, (5), pp. 614 –621 [DOI] [PubMed] [Google Scholar]
- 34. Shivakrishna P. Prasad M.R. Krishna G. et al.: ‘Synthesis of silver nano particles from marine bacteria Pseudomonas aerogenosa ’, Octa. J. Biosci., 2013, 1, (2), pp. 108 –114 [Google Scholar]
- 35. Mehta A. Sidhu C. Pinnaka A.K. et al.: ‘Extracellular polysaccharide production by a novel osmotolerant marine strain of Alteromonas macleodii and its application towards biomineralization of silver’, PLoS One, 2014, 16, 9, (6), p. e98798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malhotra A. Dolma K. Kaur N. et al.: ‘Biosynthesis of gold and silver nanoparticles using a novel marine strain of Stenotrophomonas ’, Bioresour. Technol., 2013, 142, pp. 727 –731 [DOI] [PubMed] [Google Scholar]
- 37. Holt J.G. Krieg N.R. Sneath P.H.A. et al.: ‘Bergey's Manual of determinative Bacteriology’ (Williams and Wilkins, Lippincott, 1994, 9th edn.), pp. 307 –308 [Google Scholar]
- 38. Saitou N. Nei M.: ‘The neighbour‐joining method: a new method for constructing phylogenetic trees’, Mol. Biol. Evol., 1987, 4, pp. 406 –425 [DOI] [PubMed] [Google Scholar]
- 39. Kumar S. Nei M. Dudley J. et al.: ‘MEGA: A biologist‐centric software for evolutionary analysis of DNA and protein sequences’, Brief Bioinfo., 2008, 9, pp. 299 –306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vorderwülbecke T. Kieslich K. Erdmann H.: ‘Comparison of lipases by different assays’, Enzyme Microbial Technol., 1992, 14, (8), pp. 631 –639 [Google Scholar]
- 41. Wei X. Luo M. Li W. et al.: ‘Synthesis of silver nanoparticles by solar irradiation of cell‐free Bacillus amyloliquefaciens extracts and AgNO3’, Bioresour. Technol., 2012, 103, pp. 273 –278 [DOI] [PubMed] [Google Scholar]
- 42. Vasimalai N. Sheeba G. John S.A.: ‘Ultrasensitive fluorescence‐quenched chemosensor for Hg(II) in aqueous solution based on mercaptothiadiazole capped silvernanoparticles’, J. Hazard. Mater., 2012, 213‐214, pp. 193 –199 [DOI] [PubMed] [Google Scholar]
- 43. Loo Y.Y. Chieng B.W. Nishibuchi M. et al.: ‘Synthesis of silver nanoparticles by using tea leaf extract from Camellia sinensis’, Int. J. Nanomedicine, 2012, 7, pp. 4263 –4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hasan M. Iqbal J. Awan U. et al.: ‘Mechanistic study of silver nanoparticle's synthesis by dragon's blood resin ethanol extract and antiradiation activity’, J. Nanosci. Nanotechnol., 2015, 15, (2), pp. 1320 –1326 [DOI] [PubMed] [Google Scholar]
- 45. Bryaskova R. Pencheva D. Nikolov S. et al.: ‘Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP)’, J. Chem. Biol., 2011, 4, pp. 185 –191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. National Committee for Clinical Laboratory Standards : ‘Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27‐A’ (National Committee for Clinical Laboratory Standards, Wayne, PA, 1997) [Google Scholar]
- 47. CLSI : ‘Laboratory Quality Control Based on Risk Management; Approved Guideline. CLSI document EP23‐ATM’ (Clinical and Laboratory Standards Institute, Wayne, PA, 2011) [Google Scholar]
- 48. Bonis B.M. Gralnick J.A.: ‘ Marinobacter subterrani, a genetically tractable neutrophilic Fe(II)‐oxidizing strain isolated from the Soudan Iron Mine’, Front Microbiol., 2015, 16, (6), p. 719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vaidya B. Kumar R. Korpole S. et al.: ‘ Marinobacter nitratireducens sp. nov., a halophilic and lipolytic bacterium isolated from coastal surface sea water’, Int. J. Syst. Evol. Microbiol., 2015, 65, (7), pp. 2056 –2063 [DOI] [PubMed] [Google Scholar]
- 50. Zhong Z.P. Liu Y. Liu H.C. et al.: ‘ Marinobacter halophilus sp. nov., a halophilic bacterium isolated from a salt lake’, Int. J. Syst. Evol. Microbiol., 2015, 65, (9), pp. 2838 –2845 [DOI] [PubMed] [Google Scholar]
- 51. Cui Z. Gao W. Xu G. et al.: ‘ Marinobacter aromaticivorans sp. nov., a polycyclic aromatic hydrocarbon‐degrading bacterium isolated from sea sediment’, Int. J. Syst. Evol. Microbiol., 2016, 66, (1), pp. 353 –359 [DOI] [PubMed] [Google Scholar]
- 52. Park S. Kim S. Kang C.H. et al.: ‘ Marinobacter confluentis sp. nov., a lipolytic bacterium isolated from a junction between the ocean and a freshwater lake’, Int. J. Syst. Evol. Microbiol., 2015, 65, (12), pp. 4873 –4879 [DOI] [PubMed] [Google Scholar]
- 53. Barig S. Schiemann M. Mirsky V.M. et al.: ‘Quantitative turbidity assay for lipolytic enzymes in microtiter plates’, Anal. Bioanal. Chem., 2013, 405, (26), pp. 8539 –8547 [DOI] [PubMed] [Google Scholar]
- 54. Stathopoulou P.M. Savvides A.L. Karagouni A.D. et al.: ‘Unraveling the lipolytic activity of thermophilic bacteria isolated from a volcanic environment’, Biomed. Res. Int., 2013, p. 703130, doi:10.1155/2013/703130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jena J. Pradhan N. Dash B.P. et al.: ‘Pigment mediated biogenic synthesis of silver nanoparticles using diatom Amphora sp. and its antimicrobial activity’, J. Saudi Chem. Soc., 2015, 19, (6), pp. 661 –666 [Google Scholar]
- 56. Wiley B.J. Im S.H. Li Z.Y. et al.: ‘Maneuvering the surface plasmon resonance of silver nanostructures through shape‐controlled synthesis’, J. Phys. Chem. B., 2006, 110, (32), pp. 15666 –15675 [DOI] [PubMed] [Google Scholar]
- 57. Khan M. Khan M. Adil S.F. et al.: ‘Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract’, Int. J. Nanomed., 2013, 8, p. 1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Basu S. Maji J. Ganguly J.: ‘Rapid green synthesis of silver nanoparticles by aqueous extract of seed of Nyctanthes arbor‐tritis’, Appl. Nanosci, 2016, 6, (1), pp. 1 –5 [Google Scholar]
- 59. Yehia R.S. Al‐Sheikh H.: ‘Biosynthesis and characterization of silver nanoparticles produced by Pleurotus ostreatus and their anticandidal and anticancer activities’, World J. Microbiol. Biotechnol., 2014, 30, pp. 2797 –2803 [DOI] [PubMed] [Google Scholar]
- 60. Shankar S. Rai A. Ahmad A. et al.: ‘Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth’, J. Colloid Inter. Sci., 2004, 275, pp. 496 –502 [DOI] [PubMed] [Google Scholar]
- 61. Huang J. Li Q. Sun D. et al.: ‘Biosynthesis of silver and gold nanoparticles by novel sundried Cinna‐mommum camphora leaf’, Nanotechnology, 2007, 18, pp. 105104 –105114 [Google Scholar]
- 62. Ankamwar B. Chaudhary M. Sastry M.: ‘Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing’, Synth. React. Inorg. Metal‐Org. Nano‐Metal Chem., 2005, 35, pp. 19 –26 [Google Scholar]
- 63. Durai P. Chinnasamy A. Gajendran B. et al.: ‘Synthesis and characterization of silver nanoparticles using crystal compound of sodium para‐hydroxybenzoate tetrahydrate isolated from Vitex negundo. L leaves and its apoptotic effect on human colon cancer cell lines’, Eur. J. Med. Chem., 2014, 84, pp. 90 –99 [DOI] [PubMed] [Google Scholar]
- 64. Swanson H.E. Tatge E.: ‘Data for 54 inorganic substances, National Bureau of Standards’, Washington, D.C. LT. B. S. Circular, 1953, 539, pp. 1 –21 [Google Scholar]
- 65. Sun Q. Cai X. Li J. et al.: ‘Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity’, Colloids Surf. A Physicochem. Eng. Aspect., 2014, 444, pp. 226 –231 [Google Scholar]
- 66. Becheri A. Durr M. Nostro P.L. et al.: ‘Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV‐absorbers’, J. Nanopart. Res., 2008, 10, pp. 679 –689 [Google Scholar]
- 67. Bhuvaneswari T. Thiyagarajan M. Geetha N. et al.: ‘Bioactive compound loaded stable silver nanoparticle synthesis from microwave irradiated aqueous extracellular leaf extracts of Naringi crenulata and its wound healing activity in experimental rat model’, Acta Trop., 2014, 135, pp. 55 –61 [DOI] [PubMed] [Google Scholar]
- 68. Sathiya C.K. Akilandeswari S.: ‘Fabrication and characterization of silver nanoparticles using Delonix elata leaf broth’, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2014, 15, (128), pp. 337 –341 [DOI] [PubMed] [Google Scholar]
- 69. Aazam E.S. Zaheer Z.: ‘Growth of Ag‐nanoparticles in an aqueous solution and their antimicrobial activities against Gram positive, Gram negative bacterial strains and Candida fungus’, Bioprocess Biosyst. Eng., 2016, 39, (4), pp. 575 –584 [DOI] [PubMed] [Google Scholar]
- 70. Vazquez‐Muñoz R. Avalos‐Borja M. Castro‐Longoria E.: ‘Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles’, PLoS One, 2014, 7, 9, (10), p. 108876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rahisuddin Al‐Thabaiti S.A. Khan Z. et al.: ‘Biosynthesis of silver nanoparticles and its antibacterial and antifungal activities towards Gram‐positive, Gram‐negative bacterial strains and different species of Candida fungus’, Bioprocess Biosyst. Eng., 2015, 38, (9), pp. 1773 –1781 [DOI] [PubMed] [Google Scholar]


