Abstract
While cancer is the leading cause of human's deaths worldwide, finding an imaging agent which can detect cancer tumours is needed for cancer diagnosis. In the present study, PEG‐citrate dendrimer‐G2 was used as a nano‐carrier of FITC dye and Iohexol to help passive targeting and uptake of both imaging agents in cancer cells/tumour in vitro and in vivo. Dendrimer was synthesisedand the product characterised using LC‐MS, FT‐IR, DLS, ELS, AFM, and 1 HNMR. After FITC loading into dendrimer, MTT was performed to determine the cytotoxicity of formulation on HEK‐293 and MCF‐7 cells. In vitro imaging using dendrimer‐FITC was done via fluorescent microscope thereafter. Moreover, CT imaging using Iohexol was employed to show the targeting nature and ability of the complex to use as imaging agent in vivo. Data yielded in this study corroborate the notion that the promised dendrimer was synthesised properly and had no toxicity along with FITC on normal cell. Furthermore, CT and fluorescent images showed the targeting nature and imaging ability of Iohexol/FITC loaded dendrimer in vitro and in vivo. Overall, results showed promising characteristics of the novel complexes using dendrimer‐G2 both in vitro and in vivo.
Inspec keywords: drug delivery systems, cellular biophysics, molecular biophysics, fluorescence, cancer, tumours, drugs, nanomedicine, biomedical materials, dyes, toxicology
Other keywords: imaging agent, cancer tumours, cancer diagnosis, PEG‐citrate dendrimer‐G, FITC dye, cancer cells, FITC loading, vitro imaging, dendrimer‐FITC, CT imaging, targeting nature, promised dendrimer, fluorescent images, imaging ability, Iohexol/FITC
1 Introduction
Breast cancer has been the second leading cause of deaths among cancers and after cardiovascular diseases worldwide [1, 2]. While, finding a suitable remedy for cancer has still been remained unsuccessful, diagnosis of cancer seems more necessary. Cancer diagnosis is performed using various types of methods including tissue biopsy and imaging. However, biopsy (liquid biopsy, needle biopsy etc.) uses invasive methods to examine the existence of malignant tissues in an individuals' body in which human's health condition risks are inevitable. Therefore, using a non‐invasive method to diagnose cancer is very important [3]. Imaging techniques (SPECT, MRI, CT etc.) are non‐invasive, cost‐effective, and really fast methods which are now in centre of attention of many researches to develop novel imaging agents/equipment [4]. In this regard, nanoparticles' usage for constructing a novel imaging agent is very common [5]. Moreover, type of the nanoparticle is crucial to achieve the better success. Among all nanoparticles, dendrimers have diverse advantages which can draw the researchers' attention more than others [6]. Dendrimers are consisting of a core part and a peripheral part which includes the branches. The number of branches specifies the generation of the dendrimer. Thus, dendrimers are ideal carriers in nuclear medical imaging [7] owing to their high mono‐dispersity, 3D globular shapes, and their lots of branches [8]. Usage of PAMAM dendrimer as a new radiotracer for SPECT imaging and radiotherapy of gliomas was introduced for the first time by Zhao, L., et al. [9]. PAMAM is a positively charged dendrimer which is cytotoxic because of the unwanted interactions with the cell membrane of the cells. Positively charged nanoparticles can interfere with the natural mechanism of cell membrane [10]. Hence, exploiting a negatively charged dendrimer is considered more appropriate for the medical applications. PEG‐citrate dendrimer‐G2 (dendrimer‐G2) is prepared and modified previously as a suitable carrier of different conventional imaging agents for cancer diagnosis [11]. The valuable properties of this dendrimer are its biocompatibility, biodegradability [12], easy and low‐cost synthesis, adequate water solubility [10]. Its PEG‐based core can facilitate the passive delivery of the loaded agent in the dendrimer to the cancer cells [13, 14, 15]. Moreover, its citric acid branches facilitate the degradation and safe elimination of dendrimer by entering the citric acid branches in the TCA cycle and produce energy for the cells [16]. More importantly, by the negative charge of PEG‐citrate dendrimer‐G2 which is an anionic dendrimer, the problem of cell membrane interference has been fixed. Many studies has been done using PEG‐citrate dendrimer‐G2 [13, 17]. As an illustration, Haririan et al. in a research reported two different conjugates of this dendrimer (G1 and G2) with cisplatin in the aquatic environment. Based on the in vitro results, the G2 dendrimer‐cisplatin conjugate has more toxicity on cancer cells and has better therapeutic effect. Furthermore, haemolysis action was nearly similar for the both generations of the dendrimer. Conjugates had almost two times more apoptosis/necrosis effect. They clearly concluded that these conjugates with high potential and less haemolysis can be candidates as the novel anti‐tumour agents [17]. In another study, conjugation of dendrimer‐G2 to C595 monoclonal antibody labelled with Gd3+ for MRI imaging was carried out [17, 18]. Different more research were done using conjugated forms of this dendrimer such as being conjugated with aptamer [19], peptide [20] etc. and all were successful in diagnosis or therapy in vitro.
On the other hand, researchers face different problems using the conventional contrast agents. As a case in point, Iohexol (trade name: omnipaque™ or exypaque) as a commonly used iodinated, non‐ionic, water‐soluble CT‐imaging agent [21], has various undesirable effects for instance, mild‐to‐intense allergies and contrast‐induced nephropathy (CIN) [22]. Additionally, it cannot efficiently enter the cells and target specific cell type. Consequently, it cannot be used as tumour imaging agent in free mode. Thus, employing a proper nano‐carrier can lessen the amount of toxicity on healthy cells (subsequently, diminish the probability of CIN or allergies) and help targeting the cancer cells (even passive delivery) to use as a tumour imaging agent [19]. Moreover, by using this frequently contrast agent in nanoparticulated form, precision, and resolution of the CT images are expected to become more.
Another imaging agent which was used in this research along with the dendrimer is fluorescein isothiocyanate (FITC) dye. FITC is a fluorescent dye which is typically used for fluorescent imaging. Oral and intravenous use of fluorescein can cause adverse effects such as acute hypotension, hives, anaphylactic shock and cardiac arrest [23].
In the present research, based on the information given in the previous paragraphs, the goal was to construct the PEG‐citrate dendrimer‐G2 –Iohexol/FITC complex and evaluate its in vivo and in vitro characteristics to use as a suitable novel imaging agent for cancer tumours' diagnosis.
2 Materials and methods
2.1 Materials
Iohexol was purchased from Santa Cruz Biotechnology, Inc. (Texas, USA). Polyethylene glycol PEG‐600, citric acid, FITC dye, dimethyl sulphoxide (DMSO) and N, N'‐Dicyclohexylcarbodiimide (DCC) were supplied from Merck (Darmstadt, Germany). N‐hydroxysuccinimide (NHS) and also 1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide hydrochloride (EDC) were obtained from Sigma‐Aldrich Inc. (St. Louis, MO, USA). Infrared spectrum was measured by Perkin Elmer Spectrum BX‐II spectrometer. 1 HNMR spectra was measured in DMSO via Bruker 500 MHz instrument. LC‐MS analysis was done using Agilent 6410 Triple Quadrupole LC/MS. Atomic force microscopy (AFM) images were captured by JPK Nanowizard II. Dynamic light scattering (DLS) was measured by Malvern Nano‐ZS. MCF‐7 and HEK‐293 cell lines were purchased from Pasteur Institute of Iran. All the other chemical reagents exploited were of analytical grade and they were used without additional purification as received.
All animal experiments were approved by Tehran University of Medical Sciences’ Animal Experimental Committee and all procedures were performed in accordance with the National Institutes of Health Guidelines for the Use and Care of Laboratory Animals.
2.2 Methods
2.2.1 Synthesis of PEG‐citrate dendrimer‐G2
PEG‐citrate dendrimer‐G2 was synthesised and purified based on the method reported by Haririan, I., et al. [17] previously. In a Brief, 2 mL PEG‐600 diluted in sufficient amount of DMSO. Subsequently, 7.5 g DCC was added to the solution. The reaction continued stirring for 20 min at 25°C. 0.71 g citric acid was added and reaction remained stirred at room temperature for 1 h. After that, 2.2 mL DCC and 5 mL DMSO were added and the reaction was continued stirring for about 15 min. Subsequently, 2.1 g of citric acid was added and the reaction was continued for 1 week at room temperature while stirring. Then, dendrimer‐G2 was purified using dialysis (dialysis bag 500–1000 Da cut‐off) and Sephadex G‐50 column (GE Healthcare Life Sciences, UK). Finally, purified dendrimer‐G2 freeze dried for further experiments. Characterisations of dendrimer‐G2 were done using LC‐MS, FT‐IR, DLS, HNMR and AFM.
2.2.2 Iohexol and FITC loading into dendrimer‐G2
Iohexol and FITC were loaded in dendrimer‐G2 by mixing them (standard molar ratios: 1 mg Iohexol: 2 mg dendrimer‐G2) separately and incubated while shaking at 4°C for 30 min using an orbital shaker/incubator (unimax 1010/incubator 1000, Heighdolph Instruments, Germany). Entrapped Iohexol/FITC were removed using dialysis (dialysis bag 500–1000 Da cut‐off) in deionised water (1 × 1 L for 2 days).
2.2.3 Cell culture and anti‐proliferative assessment using MTT assay
The human breast cancer cell line (MCF‐7) and Human Embryonic Kidney cell line (HEK‐293) were acquired from national Cell Bank of Iran (Pasteur Institute, Tehran, Iran). The cells were cultured in DMEM medium supplemented with 10% foetal bovine serum (FBS) in 5% carbon dioxide (CO2) humidified incubator. The cytotoxicity of Dendrimer‐G2 ‐FITC and free FITC on MCF‐7 and HEK‐293 cells were assessed at 8, 20, 100 µM concentrations using MTT assay, 24 and 48 h after treatment using microplate reader at 570 nm. Data were analysed using Prism 5 statistical software and by one‐way ANOVA followed by the Tukey test. The P‐value for this test was <0.05, n = 3.
2.2.4 Biochemical analysis
Serum level of control and contrast agent injected groups were analysis for BUN, Creatinine, ALP, SGOT, and SGPT. The P ‐value for this test was <0.05, n = 3.
2.2.5 Fluorescent and CT imaging using dendrimer‐G2 ‐FITC
Fluorescent imaging of dendrimer‐G2 ‐FITC absorbed by MCF‐7 cells was captured using cell imaging multi‐mode microplate reader (Cytation 3, BioTek, USA). MCF‐7 cells were cultured in a 12‐well plate under the conditions mentioned in the previous section. After one day, cultured cells were treated with 20 µM Dendrimer‐G2 ‐FITC as the fluorescence imaging agent and incubated for 3 h at room temperature. Afterwards, cells were washed three times using hosphate‐buffered saline (PBS). Cells were then fixed with 4% paraformaldehyde for 15 min. Then, cells were washed three times with PBS again. Cultured cells were then stained using DAPI (0.5 mg/ml, 3 min) for nuclei staining and again were washed three to four times with PBS. The image of the sample was then recorded. For CT imaging 3 tumour mouse models (CT‐26 Mus musculus colon carcinoma) were injected intratumoural with Dendrimer‐G2 ‐Iohexol (0.25 cc at the concentration of 8 µM) and placed under the SPECT/CT system (Symbia T2; Siemens Medical Solutions USA, Inc.) to show that the new formulation has the ability to image the tumour site in the mice bodies. Subsequently, images were evaluated using SYNGO software and tumour size was obtained.
2.2.6 Statistical analysis
Data distribution (normality) was evaluated by Kolmogorov‐Smirnov test. Afterward, data analysed to cluster comparison and significant differences analysis using One‐way Annona test followed by Tukey test, data then analysed again one by one using T‐test to ensure the validation of analyses. Data were analysed and graphs were drawn using Prism 5 and Microsoft excel. Data were presented as mean ± SEM in graphs (p value <0.05, n = 3).
3 Results
3.1 Characterisations of dendrimer‐G2
LC/MS graph for the precise confirmation of dendrimer‐G2 synthesis and the LC‐MS fragment were shown in Figs. 1 a and b. The attained chemical structure of the dendrimer‐G2 is in agreement with our approximation. FT‐IR technique is well‐known as an applicable method to detect the organic molecule functional groups [24]. The FT‐IR spectrum of the dendrimer‐G2 is displayed in Fig. 1 c. The band at 1232 cm−1 can be assigned as CO in steric bonds between first generation and second generation dendrimer citric acids which are the representative of dendrimer‐G2 synthesis. (C = O) bond observed in 1706 cm−1 is assigned as terminal citric acids of dendrimer‐G2. Furthermore, (O–H) stretches of citric acid are shown in 3430 cm−1. Fig. 1 d shows the 1 HNMR spectrum characterisation of the dendrimer‐G2 structure. Appearance of the strong peak of (O–CH2 –CO) at the area of 3.8 indicates the PEG structure. The doublet peaks associated with the A–B hydrogen system in citric acid observed at the area of 2.8 ppm. In addition, the presence of triplet peaks of (O‐CH2 ‐CH2) at the region of 3.4 ppm approves the presence of PEG in dendrimer‐G2.
Fig. 1.
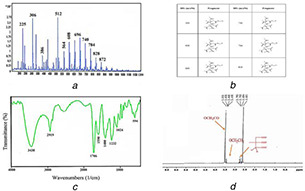
LC/MS graph for the precise confirmation of dendrimer‐G 2 synthesis and the LC‐MS fragment
(a) LC‐MS of PEG‐citrate dendrimer‐G2, (b) LC‐MS fragment of the PEG‐citrate dendrimer‐G2, (c) FT‐IR spectrum of PEG‐citrate dendrimer‐G2, (d) 1 HNMR spectrum of PEG‐citrate dendrimer‐G2
3.2 Hydrodynamic size and zeta potential of dendrimer‐G2 by DLS
The average size and zeta potential of the dendrimer‐G2 were determined by dynamic light scattering (DLS) technique which are 119 nm and −7.32 mV, respectively, and the graphs are shown in Fig. 2.
Fig. 2.
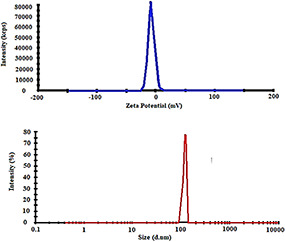
Zeta potential (above) and hydrodynamic size (below) of the PEG‐citrate dendrimer‐G2. As it clearly shows, dendrimer has negative charge (−7.32 mV) and the size of 117 nm
3.3 Morphological study of dendrimer‐G2 using AFM
Surface morphology investigation was characterised by AFM technique and the AFM images are presented in Fig. 3. Based on the AFM images, Dendrimer‐G2 has a smooth surface, globular structure, and narrow size distribution. Images were obtained using intermitted contact mode.
Fig. 3.
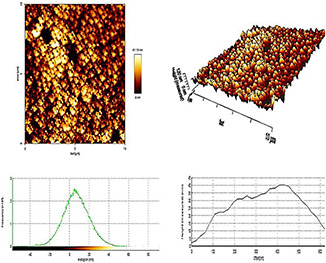
2D (above) and 3D (middle) AFM images and graphs (below) of PEG‐citrate dendrimer‐G2. Blue Plots compare the height (Y‐axis) to offset (X‐axis) measurements (nm) of the dendrimer. The Green Histograms show the frequency. The peak‐to‐valley roughness Rt of the dendrimer G2 is 14.5 nm and the physical size is 10 × 10 nm
3.4 Anti‐proliferative assessment using MTT assay
Anti‐proliferative effect of free FITC and nanoparticulated FITC (dendrimer‐G2 ‐FITC) were measured using MTT assay. Graphs (viability) are shown in Fig. 4. Based on the results, dendrimer‐G2 ‐FITC has no significant toxicity but free FITC at concentration 100 µM had toxicity on HEK‐293 cells after 24 and 48 h of treatment. There was no significant toxicity of free FITC and dendrimer‐G2 ‐FITC on MCF‐7 cells.
Fig. 4.
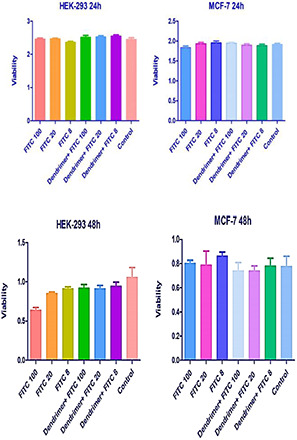
MTT diagrams of free FITC and dendrimer‐G2 ‐FITC complex on HEK‐293 and MCF‐7 cells. Data are presented as mean ± SEM. Based on statistical analyses, only 100 µM concentration of free FITC had significant differences in anti‐proliferation from the untreated control cells. The similar concentrations of dendrimer‐G2 ‐FITC showed no toxicity on HEK‐293 (p value <0.05, n = 3)
3.5 Biochemical analysis
Biochemical analysis showed that the serum levels of BUN, creatinine, ALP, SGOT, and SGPT were not significantly affected in contrast agent injected group compared to control group (Fig. 5).
Fig. 5.
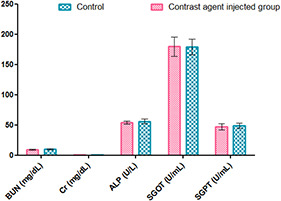
Some serum biochemical parameters in control and contrast agent injected group
3.6 Fluorescent and CT imaging using dendrimer‐G2 ‐FITC
Fluorescent image of MCF‐7 cells treated with Dendrimer‐G2 ‐FITC shows that FITC loaded dendrimer has very good uptake in MCF‐7 cells and provides great fluorescent images in this formulation (Fig. 6 A). CT images of mouse injected with dendrimer‐G2 ‐Iohexol show its passive targeting nature of the dendrimer‐G2 to cancer cells and its ability to use in tumour CT imaging. Images are shown in Fig. 6B.
Fig. 6.
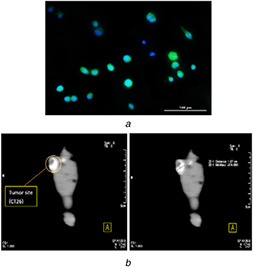
(a) Fluorescent microscopy images (20X) of dendrimer‐G2 ‐ FITC complex on MCF‐7 cells. Nuclei were stained with DAPI (blue), (b) CT images of the mice injected with dendrimer‐G2 ‐Iohexol complex 30 min after injection. The mice injected intravenously into their tail vein. Tumour size was found to be 1.07 cm
4 Discussion
Nano imaging agent is one of the great importance for diagnosis of the diseases by decreasing the cytotoxicity of the novel constructions on the cells or tissues compared to the conventional contrast agents. The main aim of this study was to construct a novel diagnostic agent using PEG‐citrate dendrimer‐G2 and an imaging agent (FITC or Iohexol) with low toxicity on normal cells and high affinity to cancer cells compared to the free imaging agent. Moreover, it was considered that this novel diagnostic agent should be sufficiently low‐cost and the synthesis should be easy enough to make the agent more than suitable for frequent usage in clinics. Additionally, biocompatibility and biodegradability, negative charge and water solubility of the PEG‐based dendrimer [25, 26] were the main advantages which could make this dendrimer more popular to use in clinical trials compared to the other nanoparticles. In this regard, PEG‐citrate dendrimer‐G2 was synthesised without purification of its first generation (on step protocol). The product was lyophilised and characterised using DLS, ELS, 1 HNMR, LC‐MS, FT‐IR, and AFM tests. The mentioned tests demonstrated that the dendrimer was constructed appropriately and lack of extra picks in each graphs was a confirmation. Moreover, as it was expected, the morphology of dendrimer was smooth and globular. The size was in harmony with the size assessed by the DLS technique. ELS clearly showed the negative charge of dendrimer. After characterisation, MTT assay was done using dendrimer‐G2 ‐FITC. Fluorescent imaging on MCF‐7 cells was done using dendrimer‐G2 ‐FITC thereafter. Using the dendrimer‐G2 ‐Iohexol complex, in vivo CT images were taken to show the imaging ability of the complex and passive targeting nature of dendrimer‐G2.
Based on the previous studies of this research group's member, PEG‐citrate dendrimer‐G2 was introduced for the imaging of cancer cells [15]. Other studies of the group used various forms of imaging agents using this dendrimer for precise detecting of the desired malignancies. In 2017, this group reported the synthesis and evaluation of a novel theranostics agent for cancer using PEG‐citrate dendrimer‐G2 conjugated with AS1411 aptamer as the targeting and therapeutic agent. This conjugate carried Iohexol as a contrast agent for imaging cancer cells. They reported that this novel agent can be a great candidate for cancer diagnosis and therapy without toxicity on normal cells [19]. Another study of the group in 2018 showed that using radiolabeled technetium‐99m‐PEG‐citrate dendrimer‐G2 conjugated glutamine (as the targeting agent), a promising molecular imaging radiopharmaceutical can be achieved for cancer diagnosis. This study made use of SPECT/CT to detect the tumour site. They reported that this dendrimer has high potential to form complex with technetium‐99 m using radiochemical purity and it can precisely detect the tumour using SPECT/CT [20].
This dendrimer consists of a biocompatible PEGylated core and biodegradable peripheral citric acid groups which its monomeric units are the chemical intermediates or products of metabolic pathways (such as, Krebs cycle) [27]. These compounds can be metabolised and removed by the cellular processes or used as cells' energy source step by step. Therefore, this dendrimer is non‐toxic and cannot be accumulated in the body. Using this dendrimer which has high potential to carry different agents/drugs, hydrophobic agents/drugs can become more soluble.
In this study using purification steps by dialysis, DMSO was removed completely so the acidic nature of the dendrimer and as a result the complex's acidity was diminished. Moreover, the anti‐proliferative assay showed that this complex has no toxicity on normal cells. As it was presented in the related section in results, after 24 and 48 h of treatment all the concentrations of dendrimer‐FITC showed less cytotoxicity compared to the identical concentrations of free FITC on HEK‐293 cell‐line. Data yield from biochemical analysis showed that the contrast agent were not affected on serum level of injected group, which indicated the safety of desired contrast agent.
CIN is the third regularly cause of acute kidney injury. Although some of disadvantages of CT imaging agents are short‐time blood preservation and renal cytotoxicity because of non‐specific biodistribution in the body. In a study, it was reported that Iohexol can cause direct dose dependent proximal tubule cytotoxicity and lessen the proliferation and viability of them simultaneously, so that a non‐ionic radiocontrast (for example, Iohexol) can cause acute renal failure [28, 29, 30, 31]. Therefore, it is crucial to invent an alternative to the conventional contrast medias. Oral, topical use, and intravenous injection of fluorescein dyes can cause life threatening side‐effects.
Surprisingly, in vivo images (CT images) displayed the tumour site and there was no sign of off‐target uptake in the pictures. The tumour CT number was an evident sign that the complex can contrast most of the tumour's area (about 78% based on thetumour diameter measurement using a Vernier calipers).
Taking everything into account, Iohexol/FITC loaded PEG‐citrate dendrimer‐G2 showed promising results in in vivo and in vitro evaluations. These complex were easily synthesised and had less cytotoxicity on normal cells. Thus, taking everything into consideration, this dendrimer can be a safe agent for use in the cancer imaging. Moreover, it demonstrated higher uptake in CT‐26 tumour cells compared to the normal cells of the mouse's body because of the PEGylated core of dendrimer which can be considered as a suitable agent with potential affinity to cancer cells. However, more studies need to be done using conjugated form of this dendrimer (with aptamers, antibodies, amino acids etc.) to show the differences between active targeted complex via a small molecule and non‐targeted complex. Furthermore, it can be used as a multimodal imaging (such as, SPECT/CT) agent by adding appropriate compounds to the complex. Certainly, more studies for bio‐safety check of this complex is mandatory in the future researches.
5 Conclusion
The obtained results demonstrated that the PEG‐citrate dendrimer‐G2 was synthesised properly. Toxicity of the FITC was negligible when loaded into the dendrimer. Finally, CT‐images showed promising results and contrast in the tumour site. Thus, this dendrimer can be a great candidate for imaging agents' delivery.
6 Acknowledgments
The authors wish to thanks all the technicians support during the experiments in the Tehran University of Medical Sciences.
7 References
- 1. Siegel R.L. Miller K.D. Jemal A.: ‘Cancer statistics, 2015’, CA Cancer J. Clin., 2015, 65, (1), pp. 5 –29 [DOI] [PubMed] [Google Scholar]
- 2. Fitzmaurice C. Dicker D. Pain A. et al.: ‘The global burden of cancer 2013’, JAMA Oncol., 2015, 1, (4), pp. 505 –527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruening W. Fontanarosa J. Tipton K. et al.: ‘Systematic review: comparative effectiveness of core‐needle and open surgical biopsy to diagnose breast lesions’, Ann. Intern. Med., 2010, 152, (4), pp. 238 –246 [DOI] [PubMed] [Google Scholar]
- 4. Lu F.‐M. Yuan Z.: ‘Pet/spect molecular imaging in clinical neuroscience: recent advances in the investigation of Cns diseases’, Quant. Imaging. Med. Surg., 2015, 5, (3), pp. 433 –447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nune S.K. Gunda P. Thallapally P.K. et al.: ‘Nanoparticles for biomedical imaging’, Expert Opin. Drug Delivery, 2009, 6, (11), pp. 1175 –1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Madaan K. Kumar S. Poonia N. et al.: ‘Dendrimers in drug delivery and targeting: drug‐dendrimer interactions and toxicity issues’, J. Pharm. Bioallied. Sci., 2014, 6, (3), pp. 139 –150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barrett T. Ravizzini G. Choyke P.L. et al.: ‘Dendrimers application related to bioimaging’, IEEE Eng. Med. Biol. Mag., Q. Mag. Eng. Med. Biol. Soc., 2009, 28, (1), pp. 12 –22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samad A. Alam M.I. Saxena K.: ‘Dendrimers: a class of polymers in the nanotechnology for the delivery of active pharmaceuticals’, Curr. Pharm. Des., 2009, 15, (25), pp. 2958 –2969 [DOI] [PubMed] [Google Scholar]
- 9. Zhao L. Zhu J. Cheng Y. et al.: ‘Chlorotoxin‐conjugated multifunctional dendrimers labeled with radionuclide 131 I for single photon emission computed tomography imaging and radiotherapy of gliomas’, ACS Appl. Mater. Interfaces, 2015, 7, (35), pp. 19798 –19808 [DOI] [PubMed] [Google Scholar]
- 10. Jain K. Kesharwani P. Gupta U. et al.: ‘Dendrimer toxicity: let's meet the challenge’, Int. J. Pharm., 2010, 394, (1–2), pp. 122 –142 [DOI] [PubMed] [Google Scholar]
- 11. Namazi H. Motamedi S. Namvari M.: ‘Synthesis of new functionalized citric acid‐based dendrimers as nanocarrier agents for drug delivery’, Bioimpacts, 2011, 1, (1), pp. 63 –69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naeini A.T. Adeli M. Vossoughi M.: ‘Poly(citric acid)‐block‐poly(ethylene glycol) copolymers‐‐new biocompatible hybrid materials for nanomedicine’, Nanomedicine, 2010, 6, (4), pp. 556 –562 [DOI] [PubMed] [Google Scholar]
- 13. Namazi H. Toomari Hamrahloo Y.: ‘Novel Ph sensitive nanocarrier agents based on citric acid dendrimers containing conjugated Β‐cyclodextrins’, Adv. Pharm. Bull., 2011, 1, (1), pp. 40 –47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ardestani M.S. Fordoei A.S. Abdoli A. et al.: ‘Nanosilver based anionic linear globular dendrimer with a special significant antiretroviral activity’, J. Mater. Sci. Mater. Med., 2015, 26, (5), p. 179 [DOI] [PubMed] [Google Scholar]
- 15. Alavidjeh M.S. Haririan I. Khorramizadeh M.R. et al.: ‘Anionic linear‐globular dendrimers: biocompatible hybrid materials with potential uses in nanomedicine’, J. Mater. Sci. Mater. Med., 2010, 21, (4), pp. 1121 –1133 [DOI] [PubMed] [Google Scholar]
- 16. Assadi A. Najafabadi V.S. Shandiz S.A.S. et al.: ‘Novel chlorambucil‐conjugated anionic linear‐globular peg‐based second‐generation dendrimer: in vitro/in vivo improved anticancer activity’, Onco. Targets Ther., 2016, 9, pp. 5531 –5543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haririan I. Alavidjeh M.S. Khorramizadeh M.R. et al.: ‘Anionic linear‐globular dendrimer‐cis‐platinum (Ii) conjugates promote cytotoxicity in vitro against different cancer cell lines’, Int. J. Nanomed., 2010, 5, pp. 63 –75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shahbazi‐Gahrouei D. Roufeh M. Tavakoli M.B.: ‘Gadolinium‐diethylenetriaminepenta‐acetic acid conjugated with monoclonal antibody C595 as new magnetic resonance imaging contrast agents for breast cancer (MCF‐7) detection’, Iran. Biomed. J., 2006, 10, (4), pp. 209 –213 [Google Scholar]
- 19. Mohammadzadeh P. Cohan R.A. Ghoreishi S.M. et al.: ‘AS1411 aptamer‐anionic linear globular dendrimer G2‐iohexol selective nano‐theranostics’, Sci. Rep., 2017, 7, (1), p. 11832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghoreishi S.M. Khalaj A. Sabzevari O. et al.: ‘Technetium‐99 m chelator‐free radiolabeling of specific glutamine tumor imaging nanoprobe: in vitro and in vivo evaluations’, Int. J. Nanomed., 2018, 13, pp. 4671 –4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.G, H.: ‘Omnipaque (Iohexol) Injection. Product Label. Dailymed US National Library of Medicine’
- 22. Mehran R. Nikolsky E.: ‘Contrast‐induced nephropathy: definition, epidemiology, and patients at risk’, Kidney Int. Suppl., 2006, 69, (100), pp. S11 –S15 [DOI] [PubMed] [Google Scholar]
- 23. Ziomek C.A.: ‘The use of fluorescein isothiocyanate (FITC) as a short‐term cell lineage marker in the peri‐implantation mouse embryo’, Wilhelm Roux’s Arch. Dev. Biol., 1982, 191, (1), pp. 37 –41 [DOI] [PubMed] [Google Scholar]
- 24. Mortazavi‐Derazkola S. Zinatloo‐Ajabshir S. Salavati‐Niasari M.: ‘New facile preparation of Ho2 O3 nanostructured material with improved photocatalytic performance’, J. Mater. Sci., Mater. Electron., 2017, 28, (2), pp. 1914 –1924 [Google Scholar]
- 25. Darvish Mohamadi T. Amanlou M. Ghalandarlaki N. et al.: ‘Gd(3+)‐DTPA‐meglumine‐anionic linear globular dendrimer G1: novel nanosized low toxic tumor molecular MR imaging agent’, ISRN. Pharm., 2013, 2013, p. 378452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hashempour Alamdari N. Alaei‐Beirami M. Sadat Shandiz S.A. et al.: ‘Gd3+‐asparagine‐anionic linear globular dendrimer second‐generation G2 complexes: novel nanobiohybrid theranostics’, Contrast Media Mol. Imaging, 2017, 2017, p. 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mirzaei M. Mehravi B. Ardestani M.S. et al.: ‘In vitro evaluation of Gd(3+)‐anionic linear globular dendrimer‐monoclonal antibody: potential magnetic resonance imaging contrast agents for prostate cancer cell imaging’, Mol. Imaging Biol., 2015, 17, (6), pp. 770 –776 [DOI] [PubMed] [Google Scholar]
- 28. Lee H.T. Jan M. Bae S.C. et al.: ‘A1 adenosine receptor knockout mice are protected against acute radiocontrast nephropathy in vivo’, Am. J. Physiol. Renal Physiol., 2006, 290, (6), pp. F1367 –F1375 [DOI] [PubMed] [Google Scholar]
- 29. Homma K.: ‘Contrast‐induced acute kidney injury’, Keio J. Med., 2016, 65, (4), pp. 67 –73 [DOI] [PubMed] [Google Scholar]
- 30. Liu Y. Ai K. Lu L.: ‘Nanoparticulate X‐ray computed tomography contrast agents: from design validation to in vivo applications’, Acc Chem. Res., 2012, 45, (10), pp. 1817 –1827 [DOI] [PubMed] [Google Scholar]
- 31. Cronin R.E.: ‘Contrast‐induced nephropathy: pathogenesis and prevention’, Pediatr. Nephrol., 2010, 25, (2), pp. 191 –204 [DOI] [PubMed] [Google Scholar]


