Abstract
A successful protocol was developed to aid in the reduction in dandruff‐causing fungi, namely Malassezia globasa and Malassezia furfur. Both the species were isolated from volunteers aged between 20 and 22 suffering from dandruff, cultured ex vivo, and tested against the presence of synthesised zinc oxide nanoparticles (ZnNP). Direct microscopy, scanning electron microscopy (SEM), and biochemical assays specific to Malassezia species were conducted to identify dandruff‐causing fungal species. Microwave‐mediated synthesis of ZnNP was performed and characterised by UV–vis, X‐ray diffraction, and SEM. The nanoparticles were tested against both Malassezia species and proved highly effective in inhibiting these fungi, although M. furfur was more susceptible than M. globosa. An optimum amount of 100 ppm was found to be sufficient to work as an antifungal agent. Synergistic effects of ZnNP with commercial shampoos were tested, and the result showed enhanced antifungal effects. To mimic the natural biofilm formed by these species on human skin, the formation of fungal biofilm was allowed on polystyrene coverslips. ZnNP was effective in eradication biofilm. Since zinc is an essential mineral for all living organism and is considered as biocompatible, the synthesised nanomaterials can be used in the formulation of antidandruff shampoos.
Inspec keywords: skin, X‐ray diffraction, antibacterial activity, scanning electron microscopy, biochemistry, biomedical materials, nanoparticles, nanofabrication, visible spectra, zinc compounds, microorganisms, ultraviolet spectra, nanomedicine
Other keywords: Malassezia furfur, Malassezia species, fungal species, microwave‐mediated synthesis, Malassezia globasa, zinc oxide nanoparticles, dandruff‐causing fungi, scanning electron microscopy, direct microscopy, biochemical assays, UV–visible spectroscopy, X‐ray diffraction, antifungal agent, natural biofilm, eradication biofilm, antidandruff shampoos, human skin, ZnO
1 Introduction
Since long, infections caused by fungus have notably contributed to the increasing health disorders and even death. Fungal infections can be localised or systemic. Yeasts or dermatophytes cause most common fungal infections on the body surface. Among yeasts, species of Candida and Malassezia are widespread that cause severe skin infections. Malassezia fungi are commonly found on human skin mostly on outer body parts which include trunks, face, and scalp [1, 2]. Molecular analysis has confirmed the genus Malassezia in seven different species; Malassezia furfur, Malassezia globosa, Malassezia pachydermatis, Malassezia slooffiae, Malassezia restricta, Malassezia sympodialis, and Malassezia obtuse [3]. Most of the species of this genus are lipid‐dependent which form colonies and biofilms on the part of the skin having seborrhoea in nature. These fungi are reported to cause dreadful skin infections like pityriasis vesicular and seborrhoeic dermatitis [2]. Dandruff is a common chronic condition of the scalp caused by Malassezia species. Symptoms include itching of scalp and shedding of superficial dead scalp skin [2, 4]. The dead skin of scalp contains spores of the fungus and gets transmitted to nearby individuals either by physical contacts or by clothes and personal belongings. It is reported that ∼5% of the population in age group 20–30 years get affected, and males are more prone to dandruff than females [5]. In humans, M. globosa and M. furfur are the most common fungus which causes dandruff and seborrhoeic dermatitis. M. globosa demonstrates good lipase activity which helps initiation of the dandruff formation. M. globosa cannot produce fatty acids, so it depends on the host body surface abundant in fatty acids secretions. M. furfur is lipophilic and belongs to dimorphic fungus. Surface adhesion molecules help Malassezia species to glue the epithelial layer of human skin. Metabolism by species of Malassezia on human body surface results in elevation of oleic acid levels that lead to the symptoms of dandruff. Though dandruff is a chronic condition; it can be prevented with the proper treatment. Proper use of skin ointments and shampoo either medicated or non‐medicated will help in eradication of dandruff from the infected scale. Presently, most effective antifungal agents are based on polyenes, triazoles, and echinocandins. Administration of these available antifungal compounds is often known to cause various complications [6, 7, 8], and these drugs are unable to prevent reoccurrence of dandruff which is the most prevalent problem [9]. Presently, shampoos are supplemented with antifungal agents like ketoconazole, salicylic acid, selenium sulphide, sulphur, coal tar, and having property to control and eradicate dandruff. Zinc pyrithione (ZPT) is noted to be a highly efficient antidandruff agent. Toxicological study on ZPT on animal models showed non‐specific and reversible tissue alterations to exposed organism [10]. Prolong use of it also cause allergic reaction, like rash, itching, blistered, and peeling of skin.
Among the metal oxide nanoparticles, zinc oxide is remarkable because it has broad applications in various areas such as optical, piezoelectric, magnetic, and gas sensing. It is widely reported that zinc oxide has antimicrobial properties without the inducing toxicity and environmental effects of other biocide agents like silver, copper, or the nanoparticles [11, 12, 13]. Besides these properties, zinc oxide nanostructure exhibits high catalytic efficiency, strong adsorption ability, and are used more and more frequently in the manufacture of sunscreens, baby powder, antidandruff shampoos and antiseptic ointments, rubber processing, wastewater treatment, and as a fungicide [14, 15]. Besides antimicrobial property, ZNP possess excellent durability and heat resistance [16]. These nanoparticles are an important source of zinc, which is involved in various essential biochemical reactions in the body since they function as enzyme co‐factors. Besides, co‐factor of many important enzymes, ZnO possesses high antioxidant property [17]. They also help in maintaining a healthy immune system [18]. The objective of this work was to evaluate zinc oxide nanoparticle (ZnNP) against M. globosa, and M. furfur isolates to selects these nanoparticles for future formulation of antidandruff shampoos.
2 Materials and methods
2.1 Materials
Zinc acetate dihydrate, Tris (hydroxymethyl) aminomethane (TRIS), and ethanol were purchased from Merck (India). Chloramphenicol was purchased from an authorised medical outlet at Haldia. Tween 20, 40, 60, 80, potassium hydroxide, Methylene Blue, hydrogen peroxide, bile esculin, agar, xylene, and all other reagents were purchased from HiMedia, India. All reagents used were of analytical grade. Fungal strains were isolated from volunteers and cultured under sterile conditions in culture media followed by its phenotypic characterisation in the laboratory.
2.2 ZnNPs synthesis and characterisation
A simple microwave‐assisted route was developed for the synthesis of ZnNP. In a glass beaker, 20 ml of the 20% aqueous TRIS solution was added to 25 ml of the 0.05 M zinc acetate dihydrate solution drop‐wise. The well‐stirred mixture was subjected to microwave irradiation at 300 W for 3 min. Then this product was centrifuged at 12,000 rpm for 15 min and washed twice with distilled water to remove excess TRIS buffer and two times with ethanol. Finally, the resultant product was kept overnight at 80°C in an incubator for drying. Synthesised ZnNP was characterised using Shimadzu UV spectrophotometer (PharmaSpac, UV‐1700), X‐ray diffraction (XRD) (PANalytical X‐PERT PRO), and scanning electron microscopy (SEM) (JEOL, JSM‐6360, UK).
2.3 Isolation and characterisation of Malassezia species
Volunteers were selected from students of age group 20 to 22 years, all undergraduate students of an educational institution in West Bengal, India. All volunteers had reported of having a high incidence of dandruff and skull itching problems. A questionnaire was used to getting informative data about the history of each person related to dandruff infection.
The study was conducted during the winter season. Malassezia specimens were isolated from dandruff scales of a volunteer using a sterile comb. The scales were inoculated into both Dixon agar, and Sabouraud Dextrose Agar media supplemented with coconut oil and chloramphenicol (0.5%) using sterile cotton swab and incubated at 32°C for 7 days. The fungal growth was examined using microscope after staining with lactophenol cotton blue stain. The yeasts isolated were identified by their morphological and physiological properties according to Guillot et al. [19]. Additional tests were performed for identification of individual Malassezia species which are described below.
2.4 Biochemical characterisation of Malassezia species
2.4.1 Catalase reaction
The presence of catalase was determined by using a drop of hydrogen peroxide (3% solution) on fungal growth, and production of gas bubbles was considered as a positive reaction [19].
2.4.2 Tween assimilation test
Tween assimilation test was conducted on the isolates because Malassezia species are reported to utilise different Tween compounds as a lipid supplement [19]. Briefly, freshly grown fungal isolates suspension (10 µl) was diluted to 1 ml in sterilised double‐distilled water and spread on Sabouraud dextrose agar medium. In each plate, four wells were made using cork borer, and the individual wells were filled with 5 μl of Tween 20 and 80, double‐distilled water, and phosphate buffer (pH 7.4). The plates were incubated for a week at 32°C, and the growth was assessed around the individual wells periodically.
2.4.3 Splitting of esculin
The β‐glucosidase activity of different Malassezia species was assayed using the method described by Guillot et al. [19]. Briefly, a loop of fresh yeast was inoculated deeply in the esculin agar tube and incubated for 5 days at 32°C, and following results were evaluated. The splitting of esculin is revealed by the darkening of the medium. The medium used was bile esculin agar slant; a nutrient agar‐based medium containing 0.1% esculin and 10% bile salts, and allowed to solidify at a slant. The bile salts inhibit some bacteria, and so the ability to grow in the presence of bile salts represents a second test use for the medium.
2.5 Antifungal activity of ZnNP along and with antidandruff shampoos on Malassezia species
ZnNP was tested against M. globasa and M. furfur to check the efficacy of these particles as antifungal agent. Minimum inhibitory concentration (MIC) was calculated by growing the fungal isolates in Dixon medium supplemented with different concentrations of ZnNP (0–200 ppm) according to standard method [20].
The antimycotic activity of the particles was further tested by agar well diffusion assay method using four different concentrations (25, 50, 100, and 150 ppm) of ZnNP. In brief, 100 µl of freshly grown each of Malassezia species was uniformly spread onto Dixon agar medium plate. Pores of 5 mm diameter were made on the agar plate, in which ZnNP of different concentrations and negative control (distilled water) was filled. Inhibition of fungal growth around each well onto the plate after incubation period at 32–35°C for 48 h was recorded.
The synergistic effect of ZnNP and antidandruff shampoos was studied following the above agar well diffusion assay. In brief, cultures of M. globasa and M. furfur were incubated and grown on Dixon plates at 32°C kept for 48 h. Next, under sterile conditions, ZnNP (50 ppm) along with 1:10 dilution of shampoo (50 µl) of each of the four brands, namely Head & Shoulders, Clinic Plus MEN, Clinic Plus WOMEN, and Clinic All Clear, were loaded in each of the four wells. Finally, growth inhibition around each well was recorded.
2.6 Malassezia yeasts biofilm eradication with ZnNP treatment
Eradication of biofilm was studied following the methods of Shrestha et al. [21]. In brief, biofilm formation was allowed on the surface of sterile glass coverslips by inoculating fungal isolates in Dixon liquid medium in multi‐well plates for 24 h at 32°C in an incubator. Each of M. globasa, M. furfur, and mixed culture of two isolates were checked against varying concentrations of ZnNP (25–200 ppm). The arrangement was further left at 32°C in an incubator for 48 h. Post‐formation of biofilm on each coverslip, each of them was washed with chilled deionised water to remove unbound cells. To check the eradication efficacy of nanomaterials by light microscope, cells were stained with 0.1% Safranin for 30 min. Excess stain was removed by gently dipping the coverslips in deionised water and finally dried at room temperature. The biofilm on coverslips was analysed by light microscope at 140× (IX 51 Olympus, Hamburg, Germany). For SEM analysis, biofilm samples which were developed on glass coverslips were washed with 4% formaldehyde in 0.1 M phosphate buffer and placed in 1% Osmium tetroxide for 1 h. After Osmium tetroxide treatment, samples were washed in deionised water, and subsequently dehydrated in a series of ethanol washes (60–100%) for 10 min. Lastly, coverslips were air‐dried and stored in a desiccator till further use. The samples were ready for SEM analysis.
2.7 Cytoplasmic leakage study
For cellular material release study, the procedure of Singh [22] was followed. Briefly, fungal cells were harvested from 5 ml of an overnight culture of two different strains of Malassezia by centrifugation at 5000 rpm, and the pellet was washed with PBS buffer (1×, pH 7.2) twice. Next, the pellet was suspended in 4 ml of PBS buffer. One millilitre from each cell suspension was taken in an eppendrof and treated with and without ZnNP (50 ppm) and incubated at room temperature for different time periods (0, 3, 6 h). Lowry's method was followed for estimation of protein leakage [23].
2.8 Microbial adhesion to hydrocarbons assay
The fungal adhesion to host surfaces is an important event for fungal colonisation and spread of skin infection. Also, adhesion is possible only because of cell surface hydrocarbon. So, to check the effects of ZnNP on hydrocarbon molecules of fungal cell surfaces, microbial adhesion to hydrocarbons (MATH) test was performed according to Rosenberg et al. [24] with slight modifications. Fungal cells were harvested from 5 ml of overnight grown Malassezia culture by centrifugation at low speed, and the pellet was washed with PBS buffer (1×, pH 7.2) twice. The cells were then suspended in PBS buffer, and 700 µl of cell suspension was distributed into four eppendorf tubes. ZnNP (0, 50, 100, and 150 ppm) was added to the four tube. After that, 300 µl of xylene was added in each set. The entire arrangement was conducted for both M. globasa and M. furfur species. The sets were incubated in the dark for 1 h at room temperature. Following this, each of the sets was subjected to slight vortexing for 10 min. The sets were then kept at equilibrium at 30°C for 20 m after which 200 of upper aqueous phase was removed and optical density recorded at 600 nm.
2.9 Statistical analysis
All experiments were performed at least thrice. The data in the text were presented as mean ± standard deviation for all the experiments and the Student T ‐test was performed.
3 Results and discussions
3.1 ZnNP synthesis and characterisation
Fig. 1 a shows UV–vis spectra of synthesised ZnNP. There is a definite hump at 355 nm which indicate synthesis of nanoparticles and its monodispersed nature [25]. Band gap energy value corresponds to the wavelength of 355 nm is nearly Eg = 3.49 eV following the formula Eg = 1240/λ [26]. The XRD profile shows typical peaks of ZnNPs (Fig. 1 b) which specifies crystalline nature of the synthesised nanomaterials. Scanning electron microscope was used to study the size and surface morphology of ZnNP. The surface morphology of ZnNP was studied using SEM. From the micrographs, it was observed that nanoparticles were almost spherical and well dispersed with few patches of agglomeration (Figs. 1 c and d). The occurrences of such aggregation are common due to the high surface energy of ZnNP that results in clump formation.
Fig. 1.
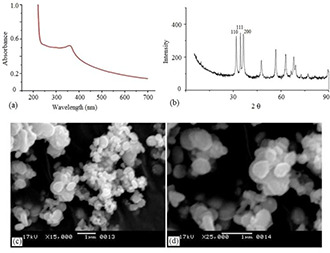
Characterisation of Zinc oxide nanoparticles
(a) UV–visible spectra, (b) XRD profile, (c, d) SEM image of ZnO nanoparticles (ZnNP)
3.2 Characterisation of isolated Malassezia species
The fungal samples collected from volunteers showed a positive result for the presence of Malassezia‐like species when grown in Dixon agar medium (Figs. 2 a and b). Microscopic analysis of isolates also confirmed yeast‐like colonies (Figs. 2 c –f). The visible colour of the colonies and microscopic images both confirmed the presence of two most severe dandruff‐causing Malassezia species viz., M. globosa and M. furfur. The colonies of M. globosa were spherical with a broad base. M. furfur colonies were smooth, opaque, and umbonate.
Fig. 2.
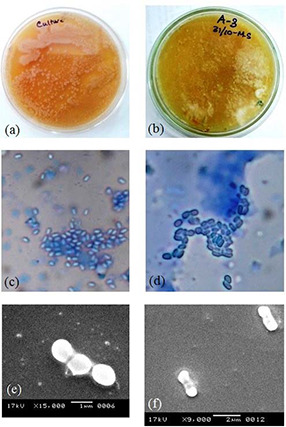
In vitro growth and morphological study of Malassezia species. Colonies grown on
(a, b) Dixon agar medium, (c, d) simple microscopic, (e, f) SEM images of M. globosa and M. furfur
Further, biochemical tests (Tween assimilation, esculin splitting, and catalase) confirmed these two species which are most prevalent among volunteers suffering from dandruff (Fig. 3). The catalase reaction was positive for all species (Figs. 3 a and b). The Tween diffusion test showed positive results for M. furfur (Fig. 3 d) and negative for M. globosa (Fig. 3 c). Both species showed negative β‐glucosidase activity, i.e. splitting of esculin (Figs. 3 e –f). The Tween test allows the differentiation of different Malassezia species. The phenomenon of precipitation may correspond to the hydrolysis of Tweens in the absence of fungal growth which corresponded to the insoluble fatty acid. However, when the Tweens allow growth, the precipitate is absent or remains weak likely because of the acidification of the medium. The pattern of growth which indicates assimilation of Tween 20 was a ring of growth inhibition at a distance of the well. M. furfur required fatty acid supplementation for growth in culture, and consequently, they are named lipid‐dependent species. Complex culture media, such as modified Dixon agar and Leeming and Notman agar, provide a variety of fatty acids, required by these particular lipid‐dependent species [27].
Fig. 3.
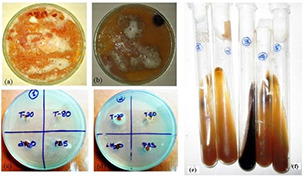
Characterisation of Malassezia yeasts
(a, b) Catalase test, (c, d) Tween assimilation test, (e, f) Splitting of esculin of M. globosa and M. furfur
3.3 Antifungal activity of ZnNP along and with antidandruff shampoos on Malassezia species
The Malassezia species demonstrated susceptibility towards the synthesised nanoparticles, although M. furfur was more susceptible than M. globosa. MIC values of ZnNP against both the species recorded were 200 ppm.
To further check the efficacy of ZnNP, we did well diffusion assay with varying concentrations (25–150 ppm) of particles. Zone of inhibition around the wells filled with ZnNP was observed in respective plates containing the Malassezia species (Fig. 4). The growth inhibition around the well loaded with ZnNP was positively correlated with the concentration of the materials loaded in each well (Fig. 4). Concurrent with the MIC result, M. furfur (Fig. 4 b) was more susceptible than M. globosa (Fig. 4 a).
Fig. 4.
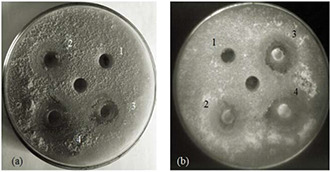
Zone of inhibition around the well loaded with ZnNP (1, 25 ppm; 2, 50 ppm; 3, 100 ppm; and 4, 150 ppm). The central well was filled with sterile deionised water. Plate a, M. globosa and plate b, M. furfur
Dandruff is often seen in the primary care setting in the dermatology domain. It is thought that ZPT heals the scalp by normalising epithelial keratinisation, sebum production, or both. Some studies have also shown a significant reduction in the numbers of yeast organisms after the application of ZPT. Thus, the innovative idea to use non‐toxic ZnNP with shampoo dilutions to eradicate maximum surface area of the fungal population causing dandruff with every instantaneous wash of scalp with water was thought to be a preventive care development. This observation was demonstrated in antifungal experiments that showed the synergistic effect of ZnNP (50 ppm) and commercially prescribed antidandruff shampoos (10 times dilution) be seen on Malassezia community. Commercial shampoos tested were Head & Shoulders, Clinic Plus MEN, Clinic Plus WOMEN, and Clinic All Clear. The combined effort of ZnNP and commercial shampoo was more pronounced in M. furfur culture than M. globasa. As opposed to control where fungal growth was prominent, the zone of inhibition in and/around our formulations was broad and overlapped (Fig. 5).
Fig. 5.
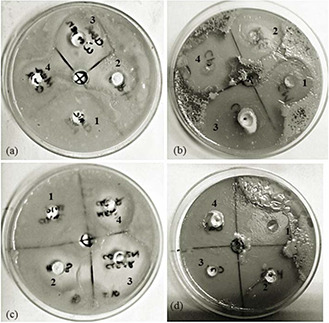
Agar well diffusion assay
(a, c) Inhibition of fungal growth on account of shampoos. (b, d) Synergistic effect of shampoo and ZnNP on fungal growth. Plate a and b, M. globosa and plate c and d, M. furfur. Wells 1–4 represent commercial shampoos tested (Head & Shoulders, Clinic Plus MEN, Clinic Plus WOMEN, and Clinic All Clear, respectively)
The exact mechanism of the cytotoxic effect of ZnNP on prokaryotes is not fully explained. He et al. [18] reported that ZnNP interfere with normal cell functions and causes increase in nucleic acid due to nanoparticles loads and also affects the development of conidiophores and conidia in fungi. The primarily mechanism for inhibiting the fungal growth by ZnNP might be due to the formation of free radicals by the electronic properties of the semiconductor material and/or the capacity of the ZnNP to disrupt electron transfer processes in biological systems [28, 29]. Previous studies had revealed antimicrobial activity of ZnNP due to formation of free radicals on the surface of the microbes and subsequent damage to the lipids cell membrane by these radicals, which consequently lead to the leakage and breakdown of cell membrane [30, 31]. Surface chemistry of ZnNP is also an important factor in nanoparticle–cell interactions which ultimately causes disruptions of cell membrane [29].
3.4 Malassezia yeasts biofilm eradication with ZnNP treatment
Malassezia biofilm formation was experimented with to establish the fact that Malassezia species can set up an excellent harmonious biofilm formation among its community. The biofilm formation capacity of Malassezia species was tested and the potential of ZnNP to eradicate it in as an antifungal agent was carried. A decrease in total population count was observed with an increasing amount of ZnNP (0–200 ppm). At 100 ppm, biofilm eradication was significantly high. Representative image of biofilm eradication of mix culture (M. furfur and M. globosa) in the presence of ZnNP was shown (Fig. 6). The morphological changes induced by fungal species by ZnNP treatment were studied by SEM. Fig. 6 shows inhibition of fungal growth and structural changes of the fungal cell membrane. Inhibition of conidial development was also seen in this experiment which demonstrates biofilm eradication potential of ZnNP. Structural changes in microbial cell membrane treated with nanoparticles have been reported by many authors [32, 33]. These authors also hypothesised that change in membrane integrity leads to cytoplasmic leakage and eventually the death of cells. Cytoplasmic leakage was studied by quantifying the amount of protein present in the supernatant of the ZnNP‐treated fungal cells in a static condition. Fig. 7 shows a gradual increase in protein contents in supernatant on account of ZnNP treatment at different time point.
Fig. 6.
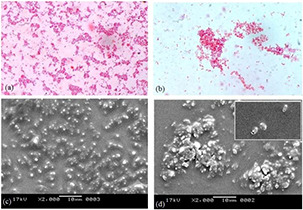
Biofilm formed by co‐culture of two Malessizia species (M. furfur and M. globosa)
(a, c) In the absence of nanoparticles, (b, d) In the presence of nanoparticles. (a, b) Light microscope image, (c, d) SEM image
Fig. 7.
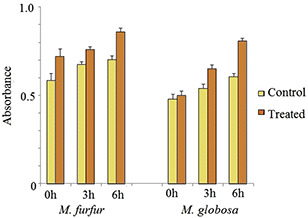
Cytoplasmic leakage (protein) from fungal cells due to membrane damaged by ZnNP treatment
3.5 Cell surface hydrophobicity (MATH test)
Microbial adhesion to surfaces is an essential step in the initial infection process. The MATH test helped elucidate the surface structure, which promotes or reduce the hydrophobic properties of the outermost cell surface of various microorganisms. The nanoparticles treatment to two Malassezia species has resulted in a reduction in absorbance at 600 nm in a dose‐dependent manner. The level of cell surface hydrocarbon inhibition of both M. furfur and M. globosa was found to be nearly same (Fig. 8). ZnNPs are reported to alter cell surface structure which helps in inhibition of biofilm formation in bacteria including yeasts [34, 35].
Fig. 8.
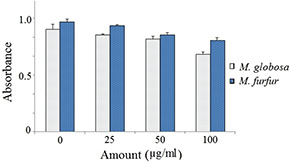
Effect of ZnNP on cell surface hydrophobicity of Malessizia species
4 Conclusion
Two most prevalent species of Malessizia (M. globosa and M. furfur) were successfully isolated from the skin of volunteers. ZnNP proved to be a robust antifungal agent to eradicate Malassezia growth significantly. Light microscopy and SEM analysis of biofilm growth in the absence and presence of nanomaterials demonstrate the inhibitory action of ZnNP. Synergistic effect of ZnNP and shampoo enhanced antifungal activity. Thus, it can be inferred that ZnNP in shampoo or medical oil formulation can efficiently cut down Malassezia proliferation on moist scalp, eliminating few of the many scalp problems one may face at adolescent age.
5 Acknowledgments
Authors thank departmental technical staffs for their technical help during the completion of this project. All volunteers are highly acknowledged for their consent to take dandruff samples.
References
- 1. Faergemann J.: ‘Lipophilic yeasts in skin disease’, Semin. Dermatol., 1985, 4, pp. 1173 –1184 [Google Scholar]
- 2. Faergemann J.: ‘Atopic dermatitis and fungi’, Clin. Microbiol. Rev., 2002, 15, (4), pp. 545 –563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta A.K. Batra R. Bluhm R. et al.: ‘Skin diseases associated with Malassezia species’, J. Am. Acad. Dermatol., 2004, 51, (5), pp. 785 –798 [DOI] [PubMed] [Google Scholar]
- 4. Joshi P. Bonde S. Gaikwad S. et al.: ‘Comparative studies on synthesis of silver nanoparticles by Fusarium oxysporum and Macrophomina phaseolina and it's efficacy against bacteria and Malassezia furfur ’, J. Bionanosci., 2013, 7, (4), pp. 378 –385 [Google Scholar]
- 5. Agarwal U.P. Prajakta S. Patki S. et al.: ‘Evaluation of clinical efficacy and safety of ‘anti dandruff hair cream’ for the treatment of dandruff’, Antiseptic, 2009, 106, pp. 37 –39 [Google Scholar]
- 6. Levin M.D. den Hollander J.G. van der Holt B. et al.: ‘Hepatotoxicity of oral and intravenous voriconazole in relation to cytochrome P450 polymorphisms’, J. Antimicrob. Chemother., 2007, 60, pp. 1104 –1107 [DOI] [PubMed] [Google Scholar]
- 7. Taxvig C. Hass U. Axelstad M. et al.: ‘Endocrine‐disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole’, Toxicol. Sci., 2007, 100, pp. 464 –473 [DOI] [PubMed] [Google Scholar]
- 8. Worth L. Blyth J.C.C. Booth D.L. et al.: ‘Optimizing antifungal drug dosing and monitoring to avoid toxicity and improve outcomes in patients with haematological disorders’, Intern. Med., 2008, 38, pp. 521 –537 [DOI] [PubMed] [Google Scholar]
- 9. Sanfilippo A. English J.C.: ‘An overview of medicated shampoos used in dandruff treatment’, P&T, 2006, 31, pp. 396 –400 [Google Scholar]
- 10. Nunes B. Braga M.R. Campos J.C. et al.: ‘Ecotoxicological effect of zinc pyrithione in the freshwater fish Gambusia holbrooki ’, Ecotoxicology, 2015, 24, (9), pp. 1896 –1905 [DOI] [PubMed] [Google Scholar]
- 11. Esteban‐Tejeda L. Prado C. Cabal B.: ‘Antibacterial and antifungal activity of ZnO containing glasses’, PLoS ONE, 2015, 10, (7), p. e0132709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rawat J. Ray S. Rao P.V.C. et al.: ‘Recent developments of nanomaterial doped paints for the minimization of biofouling in submerged structures’, Mater. Sci. Forum, 2010, 657, pp. 75 –82 [Google Scholar]
- 13. Yebra D. Kiil S. Weinell C. et al.: ‘Dissolution rate measurements of sea water soluble pigments for antifouling paints’, Prog. Org. Coat., 2006, 56, pp. 327 –337 [Google Scholar]
- 14. Mekewi M. Shebl A. Imam A.I.: ‘Screening the insecticidal efficacy of nano ZnO synthesized via in‐situ polymerization of crosslinked polyacrylic acid as a template’, J. Mater. Sci. Technol., 2012, 28, (11), pp. 961 –968 [Google Scholar]
- 15. Kavyashree D. Shilpa C. Nagabhushana J. et al.: ‘Zno superstructures as an antifungal for effective control of Malassezia furfur, dermatologically prevalent yeast: prepared by Aloe vera assisted combustion method’. ACS Sustain. Chem. Eng., 2015, 3, (6), pp. 1066 –1080 [Google Scholar]
- 16. Joshi P. Bonde S. Gaikwad S. et al.: ‘Biosynthesized silver nanoparticles performing as biogenic SERS‐nanotags for investigation of C26 colon carcinoma cells’, J. Bionanosci., 2013, 7, (4), pp. 378 –385 [DOI] [PubMed] [Google Scholar]
- 17. Singh B.N. Rawat A.K.S. Khan W. et al.: ‘Biosynthesis of stable antioxidant ZnO nanoparticles by Pseudomonas aeruginosa Rhamnolipids’, PLoSONE, 2014, 9, (9), p. e106937, doi:10.1371/journal.pone.0106937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He L. Liu Y. Mustapha A. et al.: ‘Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum ’, Microbiol. Res., 2011, 166, (3), pp. 207 –215 [DOI] [PubMed] [Google Scholar]
- 19. Guillot J.E. Guého M. Lesourd G.M. et al.: ‘Expression of rat neuronal nitric oxide synthase in Saccharomyces cerevisiae ’, J. Mycol. Med., 1996, 6, pp. 103 –110 [Google Scholar]
- 20. Anitha S. Ramachandran T. Koushik C.V. et al.: ‘Preparation and characterization of zinc oxide nanoparticles and a study of the anti‐microbial property of cotton fabric treated with the particles’, J. Text. Apparel, Tech. Manag., 2010, 6, (4), pp. 1 –6 [Google Scholar]
- 21. Goswamia S.R. Sahareena T. Singha M. et al.: ‘Role of biogenic silver nanoparticles in disruption of cell–cell adhesion in Staphylococcus aureus and Escherichia coli biofilm’, J. Ind. Eng. Chem., 2015, 26, pp. 73 –80 [Google Scholar]
- 22. Singh M.: ‘Elucidation of biogenic silver nanoparticles susceptibility towards Escherichia coli: an investigation on the antimicrobial mechanism’, IET Nanobiotech., 2016, 10, (5), pp. 276 –280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lowry O.H. Rosebrough N.S. Farr A. et al.: ‘Protein measurement with the folin phenol reagent’, J. Biol. Chem., 1951, 193, p. 265 [PubMed] [Google Scholar]
- 24. Rosenberg M. Gutnick D. Rosenberg E.: ‘Adherence of bacteria to hydrocarbons: a simple method for measuring cell‐surface hydrophobicity’, FEMS Microbiol Lett., 1980, 9, pp. 29 –33 [Google Scholar]
- 25. Zhang D.H. Xue Z.Y. Wang Q.P.: ‘Formation of ZnO nanoparticles by the reaction of zinc metal with aliphatic alcohols’, J. Phys. D, 2002, 35, (21), pp. 2837 –2840 [Google Scholar]
- 26. Geethaa M.S. Nagabhushana H. Shivananjaiah H.N.: ‘Green mediated synthesis and characterization of ZnO nanoparticles using Euphorbia jatropa latex as reducing agent’, J. Sci.: Adv. Mater. Devices, 2016, 1, (3), pp. 301 –310 [Google Scholar]
- 27. Cabañes F.J.: ‘Malassezia yeasts: how many species infect humans and animals?’, PLoS Pathol., 2014, 10, (2), p. e1003892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arciniegas‐Grijalba P.A. Patiño‐Portela M.C. Mosquera‐Sánchez L.P. et al.: ‘Zno nanoparticles (ZnO‐NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor ’, Appl. Nanosci., 2017, 7, pp. 225 –241 [Google Scholar]
- 29. Altunbek M. Baysal A. Çulha M.: ‘Influence of surface properties of zinc oxide nanoparticles on their cytotoxicity’, Colloids Surf. B Biointerfaces, 2014, 121, (1), pp. 106 –113 [DOI] [PubMed] [Google Scholar]
- 30. Reddy K.M. Feris K. Bell J. et al.: ‘Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems’, Appl. Phys. Lett., 2007, 90, (213902), pp. 2139021 –2139023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. López C. Rodríguez‐Páez J.E.: ‘Synthesis and characterization of ZnO nanoparticles: effect of solvent and antifungal capacity of NPs obtained in ethylene glycol’, Appl. Phys. A., 2017, 123, p. 748, DOI: 10.1007/s00339‐017‐1339‐x [Google Scholar]
- 32. Rao K.J. Paria S.: ‘Anti‐Malassezia furfur activity of natural surfactant mediated in situ silver nanoparticles for a better antidandruff shampoo formulation’, RSC Adv., 2016, 6, pp. 11064 –11069 [Google Scholar]
- 33. Ashajyothi C. Harish K.H. Dubey N.: ‘Antibacterial activity of biogenic zinc oxide nanoparticles synthesized from Enterococcus faecalis ’, J. Nanostruct. Chem., 2016, 6, pp. 329 –341 [Google Scholar]
- 34. Pati R. Mehta R.K. Mohanty S.A. et al.: ‘Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages’, Nanomedicine, 2014, 10, (6), pp. 1195 –1208 [DOI] [PubMed] [Google Scholar]
- 35. Borghi E. Sciota R. Biassoni C. et al.: ‘Cell surface hydrophobicity: a predictor of biofilm production in Candida isolates?’, J. Med. Microbiol., 2011, 60, pp. 689 –690 [DOI] [PubMed] [Google Scholar]


