Abstract
This paper investigated the green synthesis of silver nanoparticles (AgNPs) using aqueous extract of silky hairs of corn (Zea mays L.) which is a waste material of the crop, as both a reducing and stabilising/capping agent. The AgNPs were characterised by UV‐visible spectroscopy, scanning electron microscopy (SEM), energy dispersive X‐ray analysis (EDX), thermogravimetric analysis (TGA), X‐ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT‐IR). The average size of AgNPs was found to be 249.12 nm. The AgNPs displayed strong antibacterial activity against five different foodborne pathogenic bacteria with diameter of inhibition zones ranged between (9.23 − 12.81 mm). It also exhibited potent synergistic antibacterial activity together with standard antibiotics, kanamycin (10.6 − 13.65 mm inhibition zones) and rifampicin (10.02 − 12.86 mm inhibition zones) and anticandidal activity with amphotericin b (10.57 − 13.63 mm inhibition zones). The AgNPs exhibited strong antioxidant activity in terms of nitric oxide scavenging (IC50 91.56 µg/mL), ABTS (2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid) radical scavenging (IC50 115.75 µg/mL), DPPH (1,1‐diphenyl‐2‐picrylhydrazyl) radical scavenging (IC50 385.87 µg/mL), and reducing power (IC0.5 23.14 µg/mL). This study demonstrated the synthesis of spherical AgNPs with strong antibacterial, anticandidal and antioxidant properties that could potentially be utilised in the biomedical, cosmetic, food and pharmaceutical industries.
Inspec keywords: silver, nanoparticles, nanomedicine, antibacterial activity, biomedical materials, nanofabrication, botany, ultraviolet spectra, visible spectra, scanning electron microscopy, X‐ray chemical analysis, Fourier transform infrared spectra, crystallites
Other keywords: biomedical industry; cosmetic industry; food industry; pharmaceutical industry; Ag; crystallite size; 1,1‐diphenyl‐2‐picrylhydrazyl radical scavenging; 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid) radical scavenging; nitric oxide scavenging; amphotericin b; anticandidal activity; rifampicin; kanamycin; standard antibiotics; inhibition zones; foodborne pathogenic bacteria; Fourier transform infrared spectroscopy; X‐ray diffraction; thermogravimetric analysis; energy‐dispersive X‐ray analysis; scanning electron microscopy; ultraviolet‐visible spectroscopy; Zea mays L; antioxidant potential; anticandidal synergistic activity; antibacterial synergistic activity; corn; silky hair aqueous extract; silver nanoparticles biosynthesis
1 Introduction
The syntheses of metal nanoparticles and their various scientific and technological applications have garnered a great deal of attention in the 21st century. Metal nanoparticles have been widely applied as catalysts, biomedical materials and in micro‐electronics [1, 2, 3, 4, 5]. Among various methods for the synthesis of nanoparticles, green technology is most widely adopted in the present scientific world and most useful for the application as biomedical materials [6]. This is because biological molecules present in natural sources can provide strong reducing and stabilising/capping agents for nanoparticle synthesis [7]. Moreover, use of biological sources in nanoparticle synthesis is non‐toxic, environmentally friendly and cost effective [6, 8].
Recently, various agricultural wastes, medicinal plants and their byproducts have been tested for the synthesis of metal nanoparticles [5, 7, 8]. In maize (Z. mays), only kernels are edible, while rest of the crop is discarded. However, different portions of maize have been effectively utilised in traditional medicines as strong therapeutic agents [9]. For example, the extract of corn silk hairs of Z. mays possesses medicinal potential with rich flavonoid glycosides content [10]. Additionally, the silky hairs of corn contain bioactive compounds such as carbohydrates, proteins, vitamins, flavonoids, minerals and salts, as well as volatile components [9]. These silky hairs are used in the treatment of renal diseases including benign prostate hyperplasia, chronic nephritis and cystitis [11, 12, 13, 14]. They also help to pass stones from the kidney and urinary tract and prevent the inflammatory effects. Z. mays hair also has anti‐prostatitis and anti‐spasmodic activities [15]. Thus, formulation of drugs or natural tea from these hairs could potentially be utilised in disease treatments or health drinks.
In the present paper, we attempted to synthesise silver nanoparticles (AgNPs) using corn silky hairs aqueous extract (CHE) and to test their antibacterial and antioxidant potential. Though silky hairs are used to produce beverages for diet and to induce urination, they are mainly treated as waste. AgNPs have been shown to be effective antibacterial agent against bacteria, fungi and viruses [7, 16]. Therefore, AgNPs were synthesised using CHE toward a novel approach to waste utilisation. Moreover, its application as antibacterial, anticandidal and antioxidant compounds was also evaluated.
2 Experimental
2.1 Preparation of aqueous extract and chemicals
Whole corn was purchased from a local market located in Gyeonsan, Republic of Korea and the silky hairs were collected (Fig. 1) and cut into small pieces. Next, 20 g aliquots were placed into 100 ml of deionised water in a 250 ml conical flask and boiled for 10 min with continuous stirring. The samples were then cooled, filtered and used for the synthesis of AgNPs. All chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA).
Fig. 1.
Photograph showing
a Corn kernels
b Silky hairs of corn used for synthesis of AgNPs
2.2 Green synthesis of AgNPs and characterisation
AgNPs were prepared by suspending 10 ml of CHE into 100 ml of 1 mM silver nitrate (AgNO3) in a 250 ml conical flask and stirring continuously at room temperature. The reduction process was carried out up to 24 h and the change in the colour of the solution from yellow to reddish brown was taken to confirm the completion of the reduction reaction with the formation of AgNPs. After 24 h, the synthesised AgNPs solution was centrifuged at 12, 000 rpm for 30 min using a high‐speed centrifuge (Supra 22K, Hanil Science Industrial, Republic of Korea). The supernatant was discarded and the pallet was washed three times with distilled water to remove any compounds from the CHE and dried under vacuum to get powdered AgNPs. The synthesised AgNPs were then characterised by ultraviolet–vis (UV–vis) spectroscopy, Fourier‐transform infrared spectroscopy (FT‐IR), scanning electron microscopy (SEM), energy‐dispersive X‐ray (EDX), thermogravimetric analysis (TGA) and X‐ray powder diffraction (XRD).
UV–vis spectroscopy: The UV–vis spectra of AgNO3 solution and reduction reaction for the formation of AgNPs were monitored by measuring the absorption spectra between 350 and 550 nm at a resolution of 1 nm using a micro‐plate reader (Infinite 200 PRO NanoQuant, TECAN, Mannedorf, Switzerland).
FT‐IR: FT‐IR analysis of powdered AgNPs was conducted using an FT‐IR spectrophotometer (Model no. Jasco 5300, JASCO, Mary's Court, Easton, MD, USA) in the wavelength range of 400–4000 cm−1.
SEM and EDX analyses: SEM analysis of AgNPs was conducted using an SEM‐S‐4200 (Hitachi, Japan). Prior to analysis, the AgNPs were spread and sputter coated with platinum in an ion coater for 120 s. Elemental analysis was then conducted using a liquid nitrogen cooled EDX detector attached to the SEM machine. The particle size distribution of the AgNPs was calculated from an enlarged SEM image.
TGA analysis: The TGA analysis of AgNPs was conducted using an SDT Q600 TGA machine (TA Instruments, New Castle, DE, USA) with an alumina pan. Samples were analysed at 20–700 °C at a ramping rate of 10 °C/min.
XRD analysis: The particle sizes and nature of AgNPs were determined by XRD (X'Pert MRD model, PANalytical, Almelo, The Netherlands) at 30 kV, 40 mA, with Cu Kα radians at 2θ.
2.3 Antimicrobial activity
2.3.1 Determination of antibacterial activity
The antibacterial activity of AgNPs was determined by the standard disc diffusion method [17] against five foodborne bacteria (Bacillus cereus ATCC 13061, Listeria monocytogenes ATCC 19115, Staphylococcus aureus ATCC 49444, Escherichia coli ATCC 43890 and Salmonella Typhimurium ATCC 43174) obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Six millimetre filter paper disc (Advantec, Toyo Roshi Kaisha Ltd., Japan) was impregnated with AgNPs at 50 µg/disc was taken as the test sample and 5% dimethyl sulphoxide (DMSO) was used as the negative control. The result was measured after 24 h of incubation at 37 °C in terms of the diameter of zones of inhibition around the filter paper disc in the nutrient agar plate.
2.3.2 Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration
The MIC and minimum bactericidal concentration (MBC) of AgNPs against the five different foodborne pathogenic bacteria were determined by the two‐fold dilution method [18]. The AgNPs were first dissolved in 5% DMSO and 200 µl of this solution was incorporated into a tube containing 1780 µl of nutrient broth medium to obtain a final concentration of 400 µg/ml, then it was serially diluted into different tubes to attain 200, 100, 50, 25 and 12.5 µg/ml concentrations. Then, 10 µl of each pathogenic bacterial suspension (∼107 cfu/ml) was transferred to each set of the tubes separately. The tubes containing only 5% DMSO and the bacterial suspensions were taken as the negative control. All tubes were incubated at 37 °C for 24 h. The lowest concentration of AgNPs that did not show any visible growth of bacteria after 24 h of incubation was taken as the MIC value which was expressed in µg/ml. Furthermore, the lowest concentration of AgNPs at which there is no growth of bacteria colony when seeded on nutrient agar plates, was considered as the MBC value for the AgNPs.
2.3.3 Determination of synergistic antibacterial activity
The synergistic effects of AgNPs were determined using two antibiotics (kanamycin and rifampicin at 5 µg separately) combined with AgNPs (25 µg) by the disc diffusion method [19]. Prior to use, both the mixture of AgNPs and each antibiotics were sonicated for 10 min, filtered and used for the antibacterial assay.
2.3.4 Determination of synergistic anticandidal activity
Five different pathogenic Candida species (Candida albicans KACC 30003 and KACC 30062, Candida glabrata KBNO6P00368, Candida geochares KACC 30061 and Candida saitoana KACC 41238) obtained from the Korean Agricultural Culture Collection (KACC, Suwon, Republic of Korea) were used in the present paper. The synergistic anticandidal potential of AgNPs with amphotericin b (standard antifungal agent) was determined by the disc diffusion method [20]. AgNPs at 2000 µg/ml and amphotericin b at 200 µg/ml were mixed together at 1:1 ratio and sonicated for 15 min at room temperature. Antifungal discs were prepared by adding 50 µl of the mixture solution containing AgNPs (50 µg) and amphotericin b (5 µg) to a 6 mm filter paper disc. Prior to the assay the Candida species were grown on potato‐dextrose broth media at 28 °C. The pathogens were loaded uniformly on potato‐dextrose agar plates and the antifungal discs were placed on it. After incubating the plates at 28 °C for 48 h, the data were obtained by measuring the diameters of the zones of inhibition around each disc.
2.4 Antioxidant activity
2.4.1 Nitric oxide (NO) radical scavenging assay
The NO radical scavenging potential of AgNPs was determined by the standard method, with slight modification [21]. Different concentrations (20–100 µg/ml) of AgNPs and the standard reference compound, ascorbic acid were taken for the assay. The results are presented in terms of half maximal inhibitory concentration (IC50) values (the concentration of AgNPs displaying 50% inhibition activity).
2.4.2 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid (ABTS) radical scavenging assay
The ABTS radical scavenging activity of AgNPs was measured according to the method described by Thaipong et al. [22], with minor modification. Different concentrations (20–100 µg/ml) of AgNPs and the standard reference compound, ascorbic acid were taken for the assay and the scavenging activity was measured by taking the absorbance value at 750 nm. The results are presented in terms of IC50 values (the concentration of AgNPs/ascorbic acid displaying 50% inhibition activity).
2.4.3 1,1‐diphenyl‐2‐picrylhydrazyl (DPPH) free radical scavenging assay
The DPPH free radical scavenging potential of AgNPs was determined by the standard procedure, with slight modification [23]. Different concentrations (20–100 µg/mL) of AgNPs and the standard reference compound, ascorbic acid were taken for the assay. The absorbance of both the test sample and the standard was recorded at 517 nm using a 96‐well micro‐plate reader (Infinite 200 PRO NanoQuant, TECAN, Mannedorf, Switzerland). The results are presented in terms of IC50 values (the concentration of AgNPs/ascorbic acid displaying 50% inhibition activity).
2.4.4 Reducing power assay
The reducing power of AgNPs was determined by the method described by Sun et al. [24]. The absorbance of the reaction mixtures was recorded using a micro‐plate reader (Infinite 200 PRO NanoQuant, TECAN, Mannedorf, Switzerland). The results are presented in terms of IC0.5 values defined as the concentration of AgNPs required to obtain an optical density (OD) of 0.5. Ascorbic acid was used as the reference compound.
2.5 Statistical analysis
All experiments were repeated three times and the results were expressed as the mean value of three independent replicates ± the standard deviation (SD). Significance differences between the mean values obtained among treatments were identified by one‐way analysis of variance followed by Duncan's test at the 5% level of significance (P < 0.05) using the statistical analysis software (SAS) (Version: SAS 9.4, SAS Institute Inc., Cary, NC).
3 Results and discussion
3.1 Characterisation of AgNPs
The addition of CHE to reaction medium containing AgNO3 resulted in a gradual change in colour from yellow to reddish brown (Fig. 2) due to the surface plasmon resonance (SPR), confirming the formation of AgNPs [25]. The absorbance spectra of only AgNO3 and the CHE did not show any peak in the range 350–550 nm (Fig. 2, blue and red line). However, the increase in SPR peaks started from 3 h of incubation till 24 h with the absorption maxima at 435 nm at 24 h incubation time that indicated complete synthesis of AgNPs after 24 h. The reduction of Ag+ to Ag0 was confirmed by UV–vis spectroscopy. The bioactive compounds present in CHE might have provided both reducing and stabilising/capping agent to the Ag+ to convert it to AgNPs [9, 10, 26]. Furthermore, FT‐IR analysis of both CHE and the synthesised AgNPs was conducted to determine the functional groups involved in the reduction and stabilising/capping of AgNPs and the interactions between them. The FT‐IR spectra showed absorption peaks at 3641.97, 3350.76, 2353.14, 2148.44, 1645.37, 687.10 and 649.24 cm−1 for the CHE and 3394.70, 2924.27, 2374.51, 1654.92, 1127.19, 671.22 and 486.06 cm−1 for AgNPs (Fig. 3). The very intense broadband located at 3394 and 3350 cm−1 in the spectra of AgNPs and CHE corresponded to N−H stretching of the amine group. The peaks located at 1654 and 1645 cm−1 for AgNPs and CHE, respectively, corresponded to –C=C−H stretching of alkenes, whereas those located at 671 and 649 cm−1 for AgNPs and CHE, respectively, corresponded to −C≡C–H:C–H stretching of alkyne groups [27, 28, 29, 30]. The slight shifting of different functional groups in the AgNPs from the CHE displayed the progress of the reduction processes with capping and stabilisation [25, 31, 32].
Fig. 2.
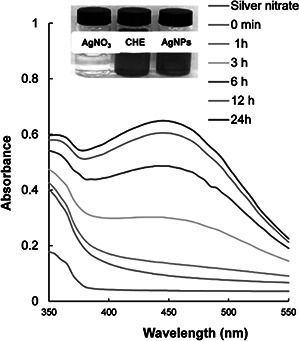
UV–Vis spectra of AgNPs synthesised by silky hairs aqueous extract (CHE); inset; colour change during synthesis of AgNPs
Only AgNO3 (colourless)
Only CHE (yellowish colour)
AgNPs (reddish brown colour)
Fig. 3.
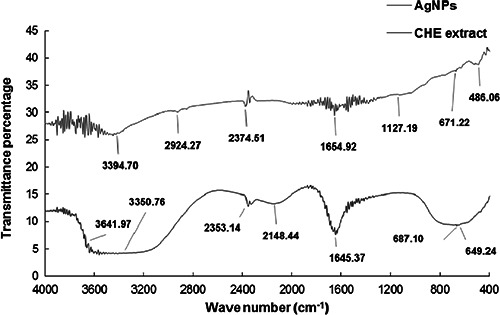
FT‐IR analysis of AgNPs synthesised by silky hairs aqueous extract (CHE)
SEM revealed a spherical shape of the synthesised AgNPs (Fig. 4 a). The particle size distribution of the synthesised AgNPs ranged from 100 to 450 nm (Fig. 4 b). The average particle size of the AgNPs was found out to be 249.12 nm. The EDX analysis of AgNPs is shown in Fig. 4 c. The elements contained in the AgNPs revealed by the EDX analysis included carbon, oxygen, aluminium, silicon (Si), chlorine and Ag. The Si peak was attributed to the Si substrate [33]. Since AgNPs were synthesised using CHE (biomaterials), the presence of carbon and oxygen elements was inevitable. Ag was present in the highest percentage, confirming that the synthesised particles were composed of Ag.
Fig. 4.
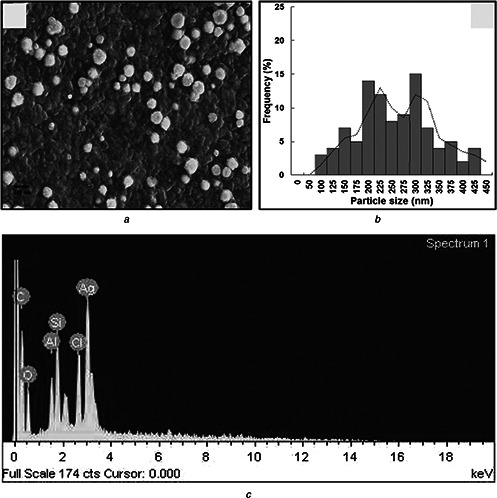
SEM revealed a spherical shape of the synthesised AgNPs
a SEM image
b Particle size distribution
c EDX analysis of AgNPs synthesised by silky hairs aqueous extract (CHE)
TGA analysis of AgNPs is presented in Fig. 5. The results indicated three stages of weight loss, 43.95% at 22.45–242.26 °C (weight loss due to absorbed moisture), 242.46–389.02 °C (weight loss due to organic moieties present as capping agents around AgNPs) and 394.65–690.00 °C (weight loss due to organic moieties and decomposition of calcium) [34]. The remaining 56% of the residue was AgNPs. The multistep gravimetric study indicated the good thermal stability of AgNPs.
Fig. 5.
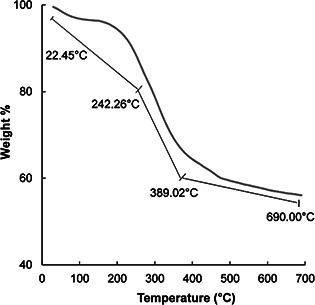
TGA analysis of AgNPs synthesised by silky hairs aqueous extract (CHE)
The XRD pattern of AgNPs is presented in Fig. 6. Four diffraction peaks corresponding to the (111), (200), (220) and (311) planes were present [7, 35, 36]. All resultant peaks were matched to the face centred cubic phase of Ag0 standard (Joint Committee on Powder Diffraction Standards, JCPDS Card no. 04‐0783) [7, 35]. Some unassigned peaks (*) were also observed, which might have been due to crystallisation of the bio‐organic phases on the surface of AgNPs [37].
Fig. 6.
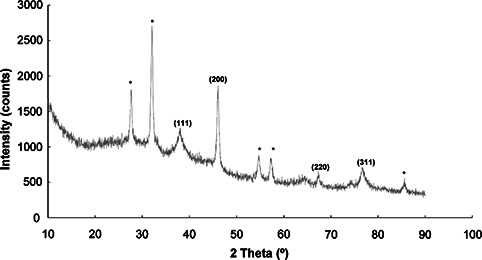
XRD pattern of AgNPs synthesised by silky hairs aqueous extract (CHE)
3.2 Bioactive potential of AgNPs
The bioactive potential of AgNPs was determined in terms of its antibacterial activity, synergistic antibacterial and anticandidal activity and antioxidant potential. Strong antibacterial activity of AgNPs against all five foodborne pathogens was observed, with zone of inhibitions ranging from 9.23 to 12.81 mm (Table 1, Fig. 7). The MIC and MBC values of AgNPs against the five different foodborne pathogenic bacteria ranged between 25 and 100 µg/ml (Table 1). The AgNPs with low MIC and MBC could be potentially useful in various applications including formulation of antibacterial drugs, antibacterial food packaging and drug delivery. When the synergistic potentials of AgNPs combined with kanamycin or rifampicin against five foodborne pathogens were measured, the inhibition zones ranged from 10.02 to 13.65 mm (Table 2 and Figs. 8 and 9). The antibacterial activity of AgNPs synthesised using the extracts of baby corn have previously been reported [26]. However, when compared with the results of previous studies, the present study is the first to report synthesis of AgNPs using waste material from corn hairs. The strong antibacterial effects of AgNPs indicate that they have the potential for use in the agricultural, cosmetic, food and pharmaceutical industries. The use of AgNPs together with the antibiotics could reduce the use of higher concentration of the antibiotics and also helps in easy penetration of the antibiotics AgNPs mixture into the cell membrane of the pathogens, thereby destructing them. The use of less concentration of antibiotics in the agriculture could also be able to reduce the adverse toxicity effect of the antibiotics to the food crops and also the consumers who feed on them.
Table 1.
Antibacterial activity of AgNPs against foodborne pathogenic bacteria
| Foodborne pathogenic bacteria | AgNPs (50 µg/disc) | MIC (µg/ml) | MBC (µg/ml) |
|---|---|---|---|
| B. cereus ATCC 13061 | 12.81 ± 2.36a | 25 | 50 |
| E. coli ATCC 43890 | 9.82 ± 0.23c | 50 | 100 |
| L. monocytogenes ATCC 19115 | 9.65 ± 0.45c | 50 | 100 |
| S. aureus ATCC 49444 | 10.49 ± 0.77b | 50 | 100 |
| S. Typhimurium ATCC 43174 | 9.23 ± 0.46c | 50 | 100 |
Data are expressed as the mean zone of inhibition in mm ± SD. Values with different superscript letters are statistically significant different at P < 0.05.
Fig. 7.
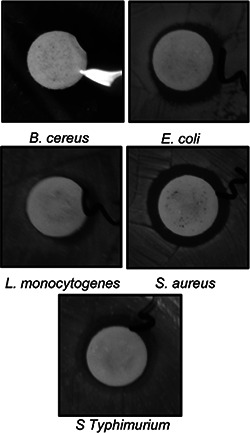
Antibacterial activity of AgNPs synthesised by silky hairs aqueous extract (CHE) against foodborne pathogens
Table 2.
Synergistic antibacterial activity of AgNPs and standard antibiotics (kanamycin and rifampicin) against foodborne pathogenic bacteria
| Foodborne pathogenic bacteria | AgNPs (25 µg) + kanamycin (5 µg) | AgNPs (25 µg) + rifampicin (5 µg) |
|---|---|---|
| B. cereus ATCC 13061 | 13.65 ± 0.28a | 12.86 ± 0.15a |
| E. coli ATCC 43890 | 11.05 ± 0.39b | 11.48 ± 0.17b |
| L. monocytogenes ATCC 19115 | 10.62 ± 0.23b | 10.02 ± 0.42c |
| S. aureus ATCC 49444 | 13.95 ± 0.24a | 11.57 ± 0.34b |
| S. Typhimurium ATCC 43174 | 10.6 ± 0.88b | 10.52 ± 0.25c |
Data are expressed as the mean zone of inhibition in mm ± SD. Values in each column with different superscript letters are statistically significant different at P < 0.05
Fig. 8.
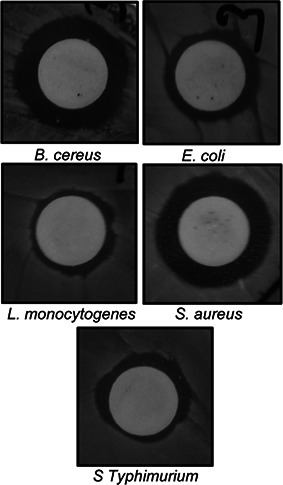
Synergistic antibacterial activity of AgNPs (25 µg) with kanamycin (5 µg) against foodborne pathogens
Fig. 9.
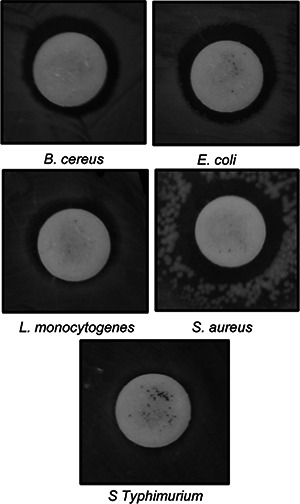
Synergistic antibacterial activity of AgNPs (25 µg) with rifampicin (5 µg) against foodborne pathogens
The anticandidal activity of AgNPs at a concentration of 50 µg/disc and amphotericin b at a concentration of 50 µg/disc was initially tested. However, both AgNPs and amphotericin b did not exhibit any inhibition activity against any of the tested Candida species. Thus, their synergistic anticandidal potential was tested and the results are presented in Table 3 and Fig. 10. The mixture of AgNPs and amphotericin b displayed high inhibition potential against all the five pathogenic Candida species with 10.57–13.63 mm inhibition zone (Table 3 and Fig. 10). Candida species are among the most representative pathogenic yeast in humans, and there are a limited number of effective antifungal drugs including amphotericin b available for its treatment [38, 39]. Thus, the synergistic anticandidal potential of AgNPs along with the lower concentration of amphotericin b could be beneficial in the treatment of infections caused by the pathogenic Candida species which also can minimise the toxicity effect of high‐dose drugs.
Table 3.
Synergistic anticandidal activity of AgNPs (50 µg) with standard antifungal agent, amphotericin b (5 µg), against pathogenic Candida species
| Candida species | WMP‐AgNPs + amphotericin b |
|---|---|
| C. albicans KACC 30003 | 11.07 ± 0.78b |
| C. albicans KACC 30062 | 13.24 ± 0.74a |
| C. glabrata KBNO6P00368 | 12.74 ± 0.15a |
| C. glochares KACC 30061 | 13.63 ± 0.28a |
| C. saitoana KACC 41238 | 10.57 ± 0.76b |
Data are expressed as the mean zone of inhibition in mm ± SD. Values with different superscript letters are statistically significant different at P < 0.05
Fig. 10.
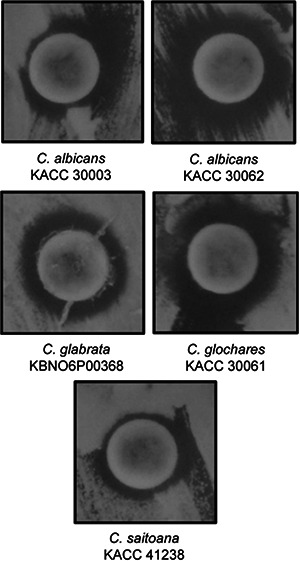
Synergistic anticandidal activity of AgNPs (50 µg) with amphotericin b (5 µg) against Candida pathogens
AgNPs displayed strong antioxidant activity in terms of NO radical scavenging (IC50 = 91.56 µg/ml), ABTS radical scavenging (IC50 = 115.75 µg/ml), DPPH radical scavenging (IC50 = 385.87 µg/ml) and reducing power (IC0.5 = 23.14 µg/ml) (Table 4). The bioactive compounds present in CHE could have bound to AgNPs during synthesis, which might have contributed to their strong antioxidant potentials [27]. Strong antioxidant effects of the AgNPs make it as a potential candidate for application in food processing and preservation. It can also be utilised in preparation of various packaging materials that are used by different food industries for packaging of various food products. Apart from these, the AgNPs synthesised through green technology route utilising an industrial waste could be a potential source of waste utilisation.
Table 4.
In vitro antioxidant activity of AgNPs synthesised by silky hairs aqueous extract (CHE)
| Antioxidant parameters | AgNPs | Ascorbic acid |
|---|---|---|
| NO scavenginga | 91.56 ± 1.48 | 153.28 ± 2.70 |
| ABTS radical scavenginga | 115.75 ± 2.06 | 44.17 ± 4.28 |
| DPPH free radical scavenginga | 385.87 ± 0.24 | 71.83 ± 0.26 |
| reducing powerb | 23.14 ± 0.01 | 49.46 ± 0.10 |
a Data are expressed in terms of IC50 value (µg/ml)
b Data are expressed in terms of IC0.5 value (µg/ml).
4 Conclusions
In this paper, a non‐toxic, environment friendly approach to synthesise AgNPs using waste products (silky hairs) from the Z. mays plant was developed. The method described herein represents a novel approach toward utilisation of waste material for synthesis of nanoparticles. The results of the UV–vis spectral, SEM, EDX and XRD analyses indicated the stable formation of AgNPs. The strong antibacterial and antioxidant potentials of AgNPs using this green biosynthesis have potential applications in the cosmetic, food and pharmaceutical industries.
5 Acknowledgment
This work was carried out with the support of ‘Cooperative Research Program for Agriculture Science & Technology Development (project no. PJ010934042015)’ Rural Development Administration, Republic of Korea.
6 References
- 1. Tsunoyama H. Sakurai H. Ichikuni N. et al.: ‘Colloidal gold nanoparticles as catalyst for carbon–carbon bond formation: application to aerobic homocoupling of phenylboronic acid in water’, Langmuir, 2004, 20, (26), pp. 11293 –11296 (doi: 10.1021/la0478189) [DOI] [PubMed] [Google Scholar]
- 2. Mirkin C.A. Letsinger R.L. Mucic R.C. et al.: ‘A DNA‐based method for rationally assembling nanoparticles into macroscopic materials’, Nature, 1996, 382, pp. 607 –610 (doi: 10.1038/382607a0) [DOI] [PubMed] [Google Scholar]
- 3. Daniel M.C. Astruc D.: ‘Gold nanoparticles: assembly, supramolecular chemistry, quantum‐size‐related properties, and applications toward biology, catalysis, and nanotechnology’, Chem. Rev., 2004, 104, (1), pp. 293 –346 (doi: 10.1021/cr030698+) [DOI] [PubMed] [Google Scholar]
- 4. Schultz D.A.: ‘Plasmon resonant particles for biological detection’, Curr. Opin. Biotechnol., 2003, 14, (1), pp. 13 –22 (doi: 10.1016/S0958-1669(02)00015-0) [DOI] [PubMed] [Google Scholar]
- 5. Ramamurthy C.H. Padma M. Daisy mariya samadanam I. et al.: ‘The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties’, Colloid Surf. B, 2013, 102, pp. 808 –815 (doi: 10.1016/j.colsurfb.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 6. Patra J. Baek K.H.: ‘Green nanobiotechnology: factors affecting synthesis and characterization techniques’, J. Nanomater., 2014, 2014, pp. 1 –12 (doi: 10.1155/2014/417305) [DOI] [Google Scholar]
- 7. Basavegowda N. Lee Y.R.: ‘Synthesis of silver nanoparticles using Satsuma mandarin (Citrus unshiu) peel extract: a novel approach towards waste utilization’, Mater. Lett., 2013, 109, pp. 31 –33 (doi: 10.1016/j.matlet.2013.07.039) [DOI] [Google Scholar]
- 8. Nezamdoost T. Bagherieh‐Najjarn M.B. Aghdasi M.: ‘Biogenic synthesis of stable bioactive silver chloride nanoparticles using Onosma dichroantha Boiss. root extract’, Mater. Lett., 2014, 137, pp. 225 –228 (doi: 10.1016/j.matlet.2014.08.134) [DOI] [Google Scholar]
- 9. Solihah M.A. Wan Rosli W.I. Nurhanan A.R.: ‘Phytochemicals screening and total phenolic content of Malaysian Zea mays hair extracts’, Int. Food Res. J., 2012, 19, (4), pp. 1533 –1538 [Google Scholar]
- 10. Nessa F. Ismailb Z. Mohamed N.: ‘Antimicrobial activities of extracts and flavonoid glycosides of corn silk (Zea mays L)’, Int. J. Biotechnol. Wellness. Ind., 2012, 1, (2), pp. 115 –121 [Google Scholar]
- 11. Habtemariam S.: ‘Extract of corn silk (stigma of Zea mays) inhibits tumour necrosis factor‐α ‐ and bacterial lipopolysaccharide‐induced cell adhesion and ICAM‐1 expression’, Planta Med., 1998, 64, (4), pp. 314 –318 (doi: 10.1055/s-2006-957441) [DOI] [PubMed] [Google Scholar]
- 12. Ribeiro R.A. de Barros F. de Melo M.M. et al.: ‘Acute diuretic effects in conscious rats produced by some medicinal plants used in the state of Sao Paulo, Brazil’, J. Ethnopharmacol., 1988, 24, (1), pp. 19 –29 (doi: 10.1016/0378-8741(88)90136-5) [DOI] [PubMed] [Google Scholar]
- 13. Maksimovic Z. Dobric S. Kovacevic N. et al.: ‘Diuretic activity of Maydis stigma extract in rats’, Pharmazie, 2004, 59, (12), pp. 193 –197 [PubMed] [Google Scholar]
- 14. Tahraoui A. El Hilaly J. Israili Z.H. et al.: ‘Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south‐eastern Morocco (Errachidia province)’, J. Ethnopharmacol., 2007, 110, (1), pp. 105 –117 (doi: 10.1016/j.jep.2006.09.011) [DOI] [PubMed] [Google Scholar]
- 15. Buhner S.H.: ‘The natural testosterone plan; for sexual health and energy’ (Healing Arts Press, Rochester, Vermont, 2007) [Google Scholar]
- 16. Sharma V.K. Yngard R.A. Lin Y.: ‘Silver nanoparticles: green synthesis and their antimicrobial activities’, Adv. Colloid Interface. Sci., 2009, 145, (1–2), pp. 83 –96 (doi: 10.1016/j.cis.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 17. Diao W.R. Hu Q.P. Feng S.S. et al.: ‘Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens’, J. Agric. Food Chem., 2013, 61, (25), pp. 6044 –6049 (doi: 10.1021/jf4007856) [DOI] [PubMed] [Google Scholar]
- 18. Kubo I. Fujita K. Kubo A. et al.: ‘Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis ’, J. Agric. Food Chem., 2004, 52, (11), pp. 3329 –3332 (doi: 10.1021/jf0354186) [DOI] [PubMed] [Google Scholar]
- 19. Naqvi S.Z.H. Kiran U. Ali M.I. et al.: ‘Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug‐resistant bacteria’, Int. J. Nanomed., 2013, 8, pp. 3187 –3195 (doi: 10.2147/IJN.S49284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray P.R. Baron E.J. Pfaller M.A. et al.: ‘Manual of clinical microbiology’ (ASM Press, Washington DC, 1995, 6th edn.) [Google Scholar]
- 21. Makhija I.K. Aswatha‐Ram H.N. Shreedhara C.S. et al.: ‘In vitro antioxidant studies of Sitopaladi Churna, a polyherbal ayurvedic formulation’, Free Radic. Antioxid., 2011, 1, (2), pp. 37 –41 (doi: 10.5530/ax.2011.2.8) [DOI] [Google Scholar]
- 22. Thaipong K. Boonprakoba U. Crosbyb K. et al.: ‘Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts’, J. Food Compos. Anal., 2006, 19, (6–7), pp. 669 –675 (doi: 10.1016/j.jfca.2006.01.003) [DOI] [Google Scholar]
- 23. Braca A. Tommasi N.D. Bari L.D. et al.: ‘Antioxidant principles from Bauhinia tarapotensis’, J. Nat. Prod., 2001, 64, pp. 892 –895 (doi: 10.1021/np0100845) [DOI] [PubMed] [Google Scholar]
- 24. Sun L. Zhang J. Lu X. et al.: ‘Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.)’, Food Chem. Toxicol., 2011, 49, (10), pp. 2689 –2696 (doi: 10.1016/j.fct.2011.07.042) [DOI] [PubMed] [Google Scholar]
- 25. Basavegowda N. Idhayadhulla A. Lee Y.R.: ‘Phyto‐synthesis of gold nanoparticles using fruit extract of Hovenia dulcis and their biological activities’, Ind. Crops Prod., 2014, 52, pp. 745 –751 (doi: 10.1016/j.indcrop.2013.12.006) [DOI] [Google Scholar]
- 26. Deb S.: ‘Synthesis of silver nano particles using Murraya Koenigii (Green Curry Leaves), Zea Mays (Baby Corn) and its antimicrobial activity against pathogens’, Int. J. PharmTech Res., 2014, 6, (1), pp. 91 –96 [Google Scholar]
- 27. Dauthal P. Mukhopadhyay M.: ‘In‐vitro free radical scavenging activity of biosynthesized gold and silver nanoparticles using Prunus armeniaca (apricot) fruit extract’, J. Nanoparticle Res., 2013, 15, p. 1366 (doi: 10.1007/s11051-012-1366-7) [DOI] [Google Scholar]
- 28. Reddy N.J. Vali D.N. Rani M. et al.: ‘Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit’, Mater. Sci. Eng. C, 2014, 34, pp. 115 –122 (doi: 10.1016/j.msec.2013.08.039) [DOI] [PubMed] [Google Scholar]
- 29. Anandalakshmi K. Venugobal J. Ramasamy V.: ‘Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity’, Appl. Nanosci., 2016, 6, pp. 399 –408 (doi: 10.1007/s13204-015-0449-z) [DOI] [Google Scholar]
- 30. Ibrahim H.M.M.: ‘Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms’, J. Radiat. Res. Appl. Sci., 2015, 8, pp. 265 –275 (doi: 10.1016/j.jrras.2015.01.007) [DOI] [Google Scholar]
- 31. Jeeva K. Thiyagarajan M. Elangovan V. et al.: ‘ Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens’, Ind. Crops Prod., 2014, 52, pp. 714 –720 (doi: 10.1016/j.indcrop.2013.11.037) [DOI] [Google Scholar]
- 32. Shankar S.S. Rai A. Absar‐Ahmad A. et al.: ‘Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using neem (Azadirachta indica) leaf broth’, J. Colloid Interface. Sci., 2004, 275, pp. 496 –502 (doi: 10.1016/j.jcis.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 33. Yousefzadi M. Rahimi Z. Ghafori V.: ‘The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen) J. Agardh’, Mater. Lett., 2014, 137, pp. 1 –4 (doi: 10.1016/j.matlet.2014.08.110) [DOI] [Google Scholar]
- 34. Parveen A. Ali T. Wahid M. et al.: ‘Facile biological approach for immobilization, physicochemical characterization and antibacterial activity of noble metals nanocomposites’, Mater. Lett., 2015, 148, pp. 86 –90 (doi: 10.1016/j.matlet.2015.02.059) [DOI] [Google Scholar]
- 35. Lee K.J. Park S.H. Govarthanan M. et al.: ‘Synthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogens’, Mater. Lett., 2013, 105, pp. 128 –131 (doi: 10.1016/j.matlet.2013.04.076) [DOI] [Google Scholar]
- 36. Zuas O. Hamim N. Sampora Y.: ‘Bio‐synthesis of silver nanoparticles using water extract of Myrmecodia pendan (Sarang Semut plant)’, Mater. Lett., 2014, 123, pp. 156 –159 (doi: 10.1016/j.matlet.2014.03.026) [DOI] [Google Scholar]
- 37. Philip D. Unni C. Aromal S.A. et al.: ‘ Murraya koenigii leaf‐assisted rapid green synthesis of silver and gold nanoparticles’, Spectrochim. Acta A, 2011, 78, (2), pp. 899 –904 (doi: 10.1016/j.saa.2010.12.060) [DOI] [PubMed] [Google Scholar]
- 38. Khan Z.U. Chandy R. Metwali K.E.: ‘ Candida albicans strain carriage in patients and nursing staff of an intensive care unit: a study of morphotypes and resistotypes’, Mycoses, 2003, 46, (11–12), pp. 476 –486 (doi: 10.1046/j.0933-7407.2003.00929.x) [DOI] [PubMed] [Google Scholar]
- 39. Klepser M.E.: ‘Antifungal resistance among Candida species’, Pharmacotherapy, 2001, 21, (8P2), pp. 24 –132 [DOI] [PubMed] [Google Scholar]


