Abstract
The authors report Myxobacteria virescens (M. virescens) mediated synthesis of silver nanoparticles (AgNPs) and its efficacy against Staphylococcus aureus (ATCC‐33591), Salmonella typhi (ATCC‐51812), Escherichia coli (E. coli) (ATCC‐14948), Klebsiella pneumoniae (MTCC‐4030) and Pseudomonas aeruginosa (MTCC‐4673). The organism exhibiting resistance to various antibiotics showed remarkable sensitivity, when used in combination of antibiotics and AgNPs. Antimicrobial property of AgNPs is playing a significant role in medicine and food storage. In this study, they have used M. virescens for the synthesis of AgNPs, which were characterised by using UV‐Vis spectrophotometer, nano‐particles tracking and analysis, zeta potential, Fourier transform infrared spectroscopy, X‐ray diffraction and transmission electron microscopy. Synthesised AgNPs were impregnated into paper by three different methods, i.e. glass rod method (without binder), glass rod method (with binder) and direct synthesis of AgNPs on paper. Nanoparticles synthesis on paper showed the significant antimicrobial activity against Staphylococcus aureus (ATCC‐33591), Salmonella typhi (ATCC‐51812), E. coli (ATCC‐14948), Klebsiella pneumoniae (MTCC‐4030) and Pseudomonas aeruginosa (MTCC‐4673). Paper impregnated with AgNPs was used for wrapping of fruits (apples) which increases their shelf life up to 15 days. This study demonstrates a new method for wrapping of fruits, which increases the shelf life of apples.
Inspec keywords: microorganisms, biotechnology, nanoparticles, wrapping, food packaging, antibacterial activity, ultraviolet spectra, electrokinetic effects, Fourier transform infrared spectra, X‐ray diffraction, transmission electron microscopy, food products
Other keywords: silver nanoparticles, wrapping paper impregnation, apple shelf life enhancement, Myxobacteria virescens mediated synthesis, glass rod method, transmission electron microscopy, X‐ray diffraction, Fourier transform infrared, zeta potential, nanoparticle analysis, nanoparticle tracking, UV‐Vis spectrophotometer, antimicrobial property, antibiotic resistance, MTCC‐4673, Pseudomonas aeruginosa, MTCC‐4030, Klebsiella pneumoniae, ATCC‐14948, E. coli, Escherichia coli, ATCC‐51812, Salmonella typhi, ATCC‐33591, Staphylococcus aureus, M. virescens mediated synthesis
1 Introduction
Nanotechnology is the study of matter at molecular and atomic level ranges from 1 to 100 nm. Chemical composition, size and shape of particles determine the action of nanoparticles. Metal nanoparticles, such as Au, Ag, Cu, Pt and Pd, are explored because of their broad applications in various fields, but amongst these silver nanoparticles (AgNPs) are gaining much of significance due to their remarkable antimicrobial, catalytic, fast acting, conductivity and tolerance free nature. Various methods of AgNPs synthesis are in practice such as chemical, physical and biological methods. In chemical method, a number of chemicals such as reducing and stabilising agents are used, while sputter deposition, laser ablation or cluster beam deposition are used in physical method. These methods are in practice, but contamination due to chemicals and the use of toxic solvent needs the development of eco‐friendly and safe method for synthesis of nanoparticles. Therefore, biological method is used as green route for the synthesis of AgNPs because they include biomolecules as the reducing and stabilising agent and the synthesis is aqueous based. Many plants are used for the synthesis of nanoparticles like Opuntia ficus‐indica [1], Murraya koenigii [2], Hydrilla verticillata [3], Ocimum sanctum [4] and seed extract of Brassica nigra [5]. A large number of fungi are also utilised for the synthesis of AgNPs such as, Fusarium oxysporum (F. oxysporum) [6, 7], Fusarium acuminatum [8], Fusarium graminearum, Fusarium scirpi [7], Phoma glomerata [9, 10], Fusarium culmorum [11], Penicillium citrinum [12], Humicola sp. [13, 14] and Alternaria alternata.
Bacterial system scores over other biological systems like fungi and plants for the synthesis of nanoparticles because they are able to synthesise reproducible particles with various shapes and sizes. Moreover, bacteria are simple in behaviour as related to their morphology and metabolic pathways and hence Klebsiella pneumoniae, Escherichia coli (E. coli), Enterobacter cloacae, Staphylococcus aureus, Bacillus subtilis, Lactobacillus acidophilus and Candida albicans were used for the synthesis of AgNPs by Minaeian et al. [15]. In addition, Pseudomonas aeruginosa has been exploited for synthesis of AgNPs [16].
The efficacy of AgNPs was evaluated by many researchers against pathogenic microorganisms such as bacteria, fungi and viruses. Feng et al. [17] evaluated the antibacterial activity of AgNPs against Staphylococcus aureus and E. coli. Sondi and Salopek‐Sondi [18] also demonstrated bactericidal activity of AgNPs against E. coli as a model for Gram negative bacteria. AgNPs are termed as new‐generation of antimicrobials [19]. The antifungal activity of nanoparticles was also evaluated [14] against Phoma glomerata, Phoma herbarum, Fusarium semitectum, Trichoderma sp. and Candida albicans. Joshi et al. [20] tested antifungal activity of AgNPs against Malassezia furfur. Kanhed et al. [21] demonstrated antifungal activity of copper nanoparticles against plant pathogenic fungi: Phoma destructiva, Curvularia lunata, Alternaria alternata and F. oxysporum. Gupta et al. [22] demonstrated the efficacy of nanoparticles against Candida albicans, Microsporum canis, Propionabacterium acne and Trichophyton mentagrophytes. Like bacteria and fungi, the efficiency of AgNPs was also demonstrated against viruses. Elechiguerra et al. [23] reported the interaction between AgNPs with the HIV‐I virus, which inhibit the virus from binding to host cell. Gaikwad et al. [7] also demonstrated the activity of AgNPs against Herpes simplex virus types 1 and 2 and Human para influenza virus type 3.
The Myxobacteria are heterotrophic aerobic, group of Gram negative eubacteria, belonging to the delta group of Proteobacteria. Most of them live in the soil rather than an aquatic environment. They do not possess flagella, but are able to glide through strange fibrils inside of the cell. They reproduce simply by division. Myxobacteria first aggregate into fruiting bodies [24], a process long‐through to be mediated by chemotaxis. These fruiting bodies can take different shapes and colours depending on the species. Within the fruiting bodies, cells begin as rod‐shaped vegetative cells and develops into rounded myxospore with thick cell walls. These myxospores, analogous to spores in other organisms. Moreover, wherever the myxospore get environmental friendly location, the cells divide and grow [25].
The aim of this study was impregnation of AgNPs on butter paper, which were synthesised by Myxobacteria and evaluation of the antibacterial activity of paper against pathogenic bacteria. The AgNP‐loaded antibacterial paper was used for the packing of apples. The main function of fruit and food packaging system is to protect it against microorganisms, chemical contaminants, oxygen and water vapour, extending shelf stability and improving food safety. Conventional food packaging systems are designed to protect the food in a passive way, that is to say, they are supposed not to interact with the food, but rather to act exclusively as a barrier between the food and the surrounding environment. On the other hand, active food packaging systems have been invented to perform some role other than providing an inert barrier to external conditions [26]. The most common active packaging systems are the antimicrobial ones, which release antimicrobial agents into the food surface, where microbial growth predominates, inhibiting or retarding microbial growth and spoilage.
2 Materials and methods
2.1 Procurement and maintenance of Myxobacteria
Myxobacteria (Myxococcus virescens) was procured from Department of Microbiology, Nicolaus Copernicus University, Torun, Poland. The Vy/2 agar medium [Baker's yeast (0.5%), CaCl2 ·2H2 O (0.1%), becosules/cynocobalamin (0.5%), agar (1.5%), pH 7.2%] was prepared as per given composition, bacterial culture was inoculated on Vy/2 agar plates and incubated at 37°C; simultaneously the slants were prepared and maintained at 4°C.
2.2 Biosynthesis of AgNP using M. virescens
The above myxobacterial culture was inoculated in 250 ml Erlenmeyer flask each containing Vy/2 broth and incubated at 37°C for three weeks until the proper growth was observed. The inoculated broth was centrifuged at 4000 rpm for 20 min. The bacterial pellet was re‐suspended in 100 ml distilled water for 24 h in 250 ml Erlenmeyer flasks followed by the centrifugation at 4000 rpm for 20 min. The supernatant was separated and challenged with 1 mM AgNO3 solution until its colour changes from colourless to brown or brick‐red, which indicates formation of AgNPs.
2.3 Detection of AgNPs
2.3.1 Visual detection
Metal nanoparticles exhibit phenomenon of surface plasmon resonance (SPR). Due to SPR phenomenon, after synthesis, the change in colour of the solution was observed. This is helpful to monitor the synthesis of nanoparticles.
2.3.2 Detection by UV‐Vis spectrophotometer
The synthesised AgNPs were detected by UV‐Vis spectrophotometer by scanning the absorbance spectra in the wavelength range of 200–800 nm. The samples of reaction mixtures were subjected to UV‐Vis analysis by using UV‐Vis spectrophotometer (Shimadzu UV‐1700, Japan).
2.4 Characterisation of AgNPs
AgNPs were characterised by using Fourier transform infrared (FTIR) spectroscopy, nanoparticle tracking and analysis system (LM 20), zeta potential measurements by Malvern's Zeta Seizer 90, transmission electron microscopy (TEM) analysis and X‐ray diffraction (XRD) method.
2.4.1 FTIR spectroscopy
Infrared spectroscopy is useful to identify unknown materials, determine the quality or consistency of a sample in solid, liquid and gaseous states, the amount of components in a mixture, and also it can be applied to the analysis of capping agent and role of molecules in AgNPs synthesis. KBr powder and AgNPs were mixed in 9:1 ratio by volume using mortar and pestle. The mixture was filled in the cavity and then this cavity was put in the cavity holder of FTIR instrument properly and the spectra were measured.
2.4.2 Nanoparticle tracking and analysis (NTA) system (LM 20)
The preliminary study about particle size and distribution was performed by LM‐20 (NanoSight Ltd., Amesbury, UK) using NTA. The sample was diluted with nuclease‐free water, and 0.5 ml of diluted sample was injected into the sample chamber and observed using NTA software. The size distribution of nanoparticles, which can be obtained on a particle‐by‐particle basis by NTA was studied. NTA enables separation of particles population by size and intensity, microscopic visualisation of individual nanoparticles in suspension and simultaneously determining their Brownian motion.
2.4.3 Zeta potential analysis
The zeta potential was measured by Malvern Zeta Sizer 90 (ZS 90, USA) to determine the stability of nanoparticle. Nanoparticles sample (30 µl) was diluted and sonicated for 15 min at 20 Hz to break down the larger particles. Then this mixture was filtered with the help of 0.2 µm filter and used for zeta potential analysis.
2.4.4 TEM analysis
The size and shape of AgNPs were studied by TEM (Philips, CM12 Eindhoven, The Netherlands), on conventional carbon coated copper grids (400 meshes, Plano Gmbh, Germany); it was cleaned using plasma treatment under oxygen for 45 s. A 5 μl of the sample was then placed on the grid and then dried at room temperature for 1 h. The samples were inspected by operating at 120 kV. In addition, crystalline nature of AgNPs was confirmed by TEM instrument having selected area electron diffraction (SAED) attachment.
2.4.5 X‐ray diffraction
XRD spectra were recorded on a PAN analytical (X PRT PRO, D‐8, Advanced Brucker instruments, Netherlands) and represented the number of Bragg reflections indexed on the basis of the face centred cubic (FCC) structure of metallic silver. XRD is a non‐destructive and analytical method for identification and quantitative analysis of various crystalline forms, also known as phases of the compound present in the samples. The X‐rays have wavelengths in the order of a few angstroms.
2.5 Coating of AgNPs on paper
There were mainly three methods applied for impregnation of AgNPs into butter paper.
2.5.1 Glass rod method (without binder)
AgNPs colloid was spread on to the surface of butter paper with glass rod and paper was dried at room temperature.
2.5.2 Glass rod method (with binder)
In this method, butter paper was coated with the mixture of binder (Fevicol™) and AgNPs. The Fevicol™ (one‐third volume) was mixed with AgNP colloids (two‐third) and this mixture was spread onto surface of butter paper using a glass rod and it was air dried.
2.5.3 Direct synthesis of AgNPs on paper
Butter paper sheet was flooded with bacterial filtrate then the paper was removed from filtrate and flooded with 1 mM silver nitrate solution for 15 min. Paper was allowed to air dry and then used for further study.
2.6 Evaluation of antimicrobial activity of AgNP coated papers
Antimicrobial efficacy of AgNPs coated papers was tested against pathogenic Staphylococcus aureus (ATCC‐33591), Salmonella typhi (ATCC‐51812), E. coli (ATCC‐14948), Klebsiella pneumoniae (MTCC‐4030) and Pseudomonas aeruginosa (MTCC‐4673). The antifungal activity of AgNPs was evaluated against the fungi Aspergillus niger (A. niger) and F. oxysporum by disc diffusion method. The plates containing Mueller Hinton agar medium and Potato Dextrose Agar were maintained. Overnight grown bacterial and fungal cultures were inoculated separately on the surface of respective agar media. Clinical and Laboratory Standards Institute (CLSI) guideline was used to assess the antifungal activity of AgNPs by disc diffusion method as well as antimicrobial activity of coated paper.
Three types of coated papers [glass rod method (without binder), glass rod method (with binder) and direct synthesis of AgNPs on paper] were used to check comparatively for bactericidal activity along with standard antibiotics.
2.7 Storage of apples by wrapping in AgNPs coated paper
The paper coated with AgNPs using the direct synthesis method as well as non‐coated paper was used to wrap apples to check the efficacy of coated paper for storage of fruits (apples). The experiment was performed in triplicate, for 45 days at room temperature.
3 Results and discussions
AgNPs were synthesised by using myxobacteria M. virescens. The colour of bacterial filtrate changed from colourless to brown after treatment of 1 mM aqueous silver nitrate solution revealed the synthesis of AgNPs (Fig. 1), which was further confirmed by UV‐Vis spectrophotometer. This showed a characteristic absorbance peak of AgNPs at 427 nm (Fig. 2). The brown colour in solution containing the bacterial cell filtrate and 1 mM AgNO3 clearly indicates the formation of AgNPs. The colour was obtained due to excitation of surface plasmon vibration in nanoparticles. This result corroborates the previous reports in [17, 27]. The authors reported the synthesis of AgNPs by using Pseudomonas aeruginosa and endophyte Pestalotia species.
Fig. 1.

Synthesis of AgNPs. A – Myxococcus virescens filtrate and B – filtrate treated with 1 mM AgNO3
Fig. 2.
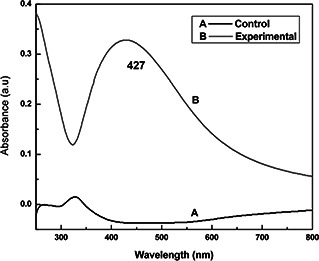
UV‐Vis spectra of AgNP synthesised from Myxococcus virescens. A – Myxococcus virescens filtrate and B – Myxococcus virescens filtrate+AgNO3
The FTIR spectra of synthesised AgNPs confirm the presence of different functional groups. In FTIR analysis, bacterial filtrate peaks were observed at 1201, 1622, 2931 and 3424 cm−1 (Fig. 3 spectra A) whereas, after the synthesis of AgNPs, these peaks were shifted to 1380, 1631, 2930 and 3429 cm−1, respectively (Fig. 3 spectra B). Peaks observed at 1380 and 1631 cm−1 associated with –C–H‐bond and N–H bending, respectively, whereas peaks at 2930 and 3429 cm−1 correspond to C–H and O–H stretching's, respectively. The peak exhibits the binding of the amide linkage with AgNPs, which was clearly indicated in the infrared region of the electromagnetic spectrum indicating the presence of protein as a capping agent for AgNPs [28, 29].
Fig. 3.
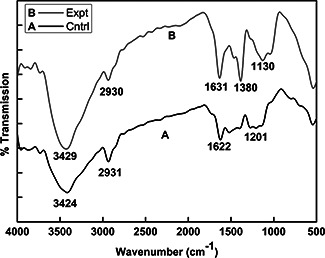
FTIR spectra of Myxococcus virescens cell filtrate and synthesised AgNPs. A – Myxococcus virescens filtrate (control) and B – AgNPs (experimental)
NTA was used to determine the average and particle size distribution of AgNP. The average size of particles was found to be 85 nm and having a particle size distribution from 12 to 200 nm with maximum population below 100 nm (Fig. 4). NTA is a technique, which allows analysis of nanoparticles on a particle‐by‐particle basis through real time sizing, counting and visualisation at higher resolution. It also allows a better understanding of aggregation than other methods such as dynamic light scattering and differential centrifugation sedimentation [30, 31].
Fig. 4.
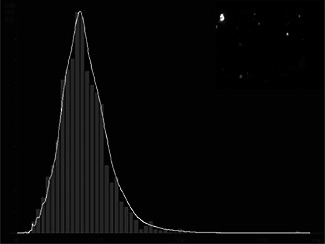
NTA analysis of AgNPs showing particle size distribution
To determine the potential stability of colloidal systems, it was characterised by surface zeta potential. Zeta potential measured for synthesised AgNPs was −25.2 mV at neutral pH 7 (Fig. 5). Particles in the colloidal either have large positive or negative charge due to which they will repel each other and no tendency to form aggregates [7]. Nanoparticles synthesised by M. virescens revealed zeta potential value −25.2 mV which proved the stable nanoparticle synthesis.
Fig. 5.
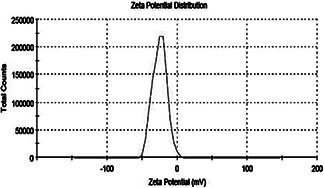
Zeta potential measurement of the AgNPs showing the zeta potential value −25.2 mV
The direct electron microscopic visualisation allows measuring the size and shape of the AgNP formed. TEM micrograph showed that synthesised nanoparticles were hexagonal in shape, in the size range of 4–21 nm (Fig. 6 a). Size determined by TEM analysis was found smaller as compared to measured by NTA [32, 33]. The difference in the results was mainly due to the bias from each specific method [34]. The SAED patterns from the samples revealed well‐defined diffraction spots in the form of rings, which indicate the polycrystalline nature of silver (Fig. 6 b). XRD patterns for the AgNPs produced by Myxobacteria virescens (M. virescens) observed at positions 38°, 44°, 66° and 78° of 2θ, depicting the presence of (111), (200), (220) and (311) facets of an FCC structure of AgNPs (Fig. 7). These values are in agreement with JCPD (Joint Committee on powder diffraction, standard file no. 04‐0783) which confirmed the presence of AgNPs crystal in the sample.
Fig. 6.
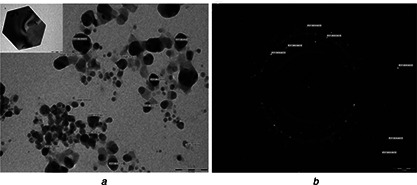
TEM micrograph showing synthesised nanoparticles were hexagonal in shape
a TEM Micrograph of AgNP (scale 100 nm)
b Diffraction pattern of AgNPs (scale 21 nm)
Fig. 7.
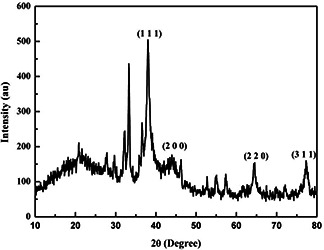
XRD pattern of AgNPs synthesised using M. virescens showing FCC structure
Three methods, i.e. glass rod method (without binder), glass rod method (with binder) and direct synthesis of AgNPs on paper were used for coating of AgNP on butter paper and their antibacterial activity was evaluated (Fig. 8). Also, antifungal activity of AgNPs was evaluated by disc diffusion method (Fig. 9). Among these methods for coating of AgNP on butter paper, paper with the direct synthesis of AgNP on it demonstrated the significant antibacterial potential against Staphylococcus aureus (ATCC‐33591), Salmonella typhi (ATCC‐51812), E. coli (ATCC‐14948), Klebsiella pneumoniae (MTCC‐4030) and Pseudomonas aeruginosa (MTCC‐4673) (Fig. 8). In further study, AgNPs revealed antifungal activity against A. niger and F. oxysporum at a great extent (Fig. 9). Simultaneously, for the assessment of antifungal activity, we also used antifungal agent ketoconazole, and from the results, it was evident that ketoconazol (antifungal agent) and AgNPs showed remarkable antifungal activity against tested fungus. The findings corroborate with the results of Dankovich and Gray [35]. The authors impregnated AgNPs in blotting paper, also known as bactericidal paper and concluded that AgNPs impregnated sheet exhibited antibacterial efficiency against E. coli and Enterococcus faecalis.
Fig. 8.
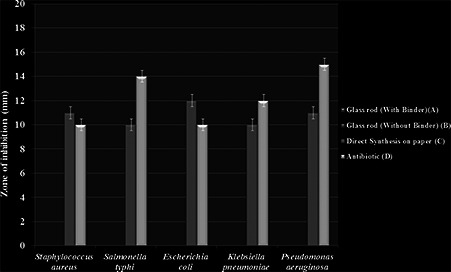
Comparative antibacterial activity of AgNPs coated papers. A – glass rod with binder, B – glass rod without binder, C – direct synthesis on paper and D – antibiotic
Fig. 9.
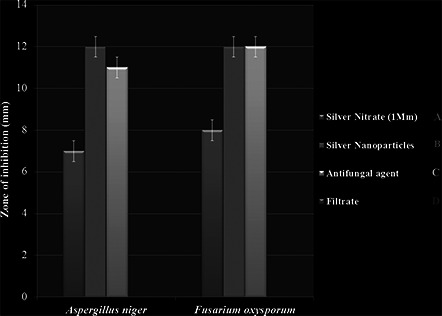
Antifungal activity of AgNPs against F. oxysporum and A. niger. A – silver salt (1 mM), B – AgNPs, C – antifungal agent and D – Myxococcus virescens filtrate
The paper with the direct synthesis of AgNP on it, demonstrated significant antibacterial potential; consequently, it was used for wrapping of apples to enhance its shelf life. Generally shelf life of apple is 3–4 weeks, after that it starts rotting by microorganisms. Considering this fact, we evaluated the effect of AgNPs on shelf life of apple and after 45 days of study. Apples wrapped in nanoparticles coated paper remain fresh while apples in non‐coated paper were found to be infected with microorganisms (Fig. 10). The results confirmed that the AgNPs coated paper increases the shelf life of apples.
Fig. 10.
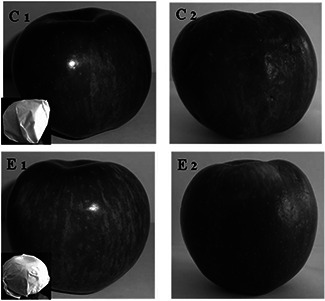
Effect of wrapped paper (direct synthesis of AgNPs on it) on shelf life of apple. C1 – apple wrapped in non‐coated paper on first day. C2 – apple wrapped in non‐coated paper after 45 days. E1 – apple wrapped in AgNPs coated paper (direct synthesis of AgNPs on it) on first day. E2 – apple wrapped in AgNPs coated paper (direct synthesis of AgNPs on it) after 45 days
4 Conclusions
We have evaluated the effect of AgNPs on shelf life of apples. In our 45 days study, we found that AgNPs coated paper increases shelf life of apples significantly. From experimental evidence, we concluded that the AgNPs coated paper demonstrated significant antimicrobial activity, which enhances the shelf life of apples up to a great extent, and hence can be used for storage of apples for several days.
5 References
- 1. Gade A.K. Gaikwad S.C. Tiwari V. et al.: ‘Biofabrication of silver nanoparticles by Opuntia ficus‐indica: In vitro antibacterial activity and study of the mechanism involved in the synthesis’, Curr Nanosci., 2010, 6, pp. 370 –375 (doi: 10.2174/157341310791659026) [DOI] [Google Scholar]
- 2. Bonde S.R. Rathod D.P. Ingle A.P. et al.: ‘ Murraya koenigii mediated synthesis of silver nanoparticles and its activity against three human pathogenic bacteria’, Nanosci. Methods, 2012, 1, pp. 25 –36 (doi: 10.1080/17458080.2010.529172) [DOI] [Google Scholar]
- 3. Sable N. Gaikwad S. Bonde S. et al.: ‘Phytofabrication of silver nanoparticles by using aquatic plant Hydrilla Verticilata ‘, Nusantara Biosci., 2012, 6, pp. 45 –49 [Google Scholar]
- 4. Mallikarjun K. Narsimha G. Dillip G.R. et al.: ‘Green synthesis of silver nanoparticles using Ocimum leaf extract and their characterization’, Digest J. Nanomater. Biostruct., 2010, 6, (1), pp. 181 –186 [Google Scholar]
- 5. Pandit R.: ‘Green synthesis of silver nanoparticles from seed extract of Brassica nigra and its antibacterial activity’, Nusantara Biosci., 2015, 7, (1), pp. 15 –19 [Google Scholar]
- 6. Duran N. Marcarto P.D. De souza G.I.H. et al.: ‘Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment’, J. Biomed. Nanotechnol., 2007, 3, pp. 203 –208 (doi: 10.1166/jbn.2007.022) [DOI] [Google Scholar]
- 7. Gaikwad S.C. Birla S.S. Ingle A.P. et al.: ‘Screening of different Fusarium species to select potential species for the synthesis of silver nanoparticles’, J. Braz. Chem. Soc., 2013, 24, (12), pp. 1974 –1982 [Google Scholar]
- 8. Ingle A. Gade A. Pierrat S. et al.: ‘Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria’, Curr. Nanosci., 2008, 4, pp. 141 –144 (doi: 10.2174/157341308784340804) [DOI] [Google Scholar]
- 9. Birla S.S. Tiwari V.V. Gade A.K. et al.: ‘Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus ’, Lett. Appl. Microbiol., 2008, 48, pp. 173 –179 (doi: 10.1111/j.1472-765X.2008.02510.x) [DOI] [PubMed] [Google Scholar]
- 10. Gade A. Gaikwad S. Duran N. et al.: ‘Green synthesis of silver nanoparticles by Phoma glomerata , Micron, 2014, 59, pp. 52 –59 (doi: 10.1016/j.micron.2013.12.005) [DOI] [PubMed] [Google Scholar]
- 11. Bawaskar M. Gaikwad S. Ingle A. et al.: ‘A new report on mycosynthesis of silver nanoparticles by Fusarium culmorum ’, Curr Nanosci., 2010, 6, pp. 376 –380 (doi: 10.2174/157341310791658919) [DOI] [Google Scholar]
- 12. Honary S. Barabadi H. Gharaei‐fathabad E. et al.: ‘Green synthesis of silver nanoparticles induced by the fungus Penicillium citrinum ’, Trop J. Pharma. Res., 2013, 12, (1), pp. 7 –11 [Google Scholar]
- 13. Syed A. Saraswati S. Kundu G.C. et al.: ‘Biological synthesis of silver nanoparticles using the fungus Humicola sp. and evaluation of their cytoxicity using normal and cancer cell lines’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2013, 114, pp. 144 –147 (doi: 10.1016/j.saa.2013.05.030) [DOI] [PubMed] [Google Scholar]
- 14. Gajbhiye M. Kesharwani J. Ingle A. et al.: ‘Fungus‐mediated synthesis of silver nanoparticle sands their activity against pathogenic fungi in combination with fluconazole’, Nanomed, 2009, 5, pp. 382 –386 [DOI] [PubMed] [Google Scholar]
- 15. Minaeian S. Shahverdi A.R. Nohi A.S. et al.: ‘Extracellular biosynthesis of silver nanoparticles by some bacteria’, J. Sci. IAU, 2008, 17, (66), pp. 1 –4 [Google Scholar]
- 16. Deshmukh S.D. Deshmukh S.D. Gade A.K. et al.: ‘ Pseudomonas aeruginosa mediated synthesis of silver nanoparticles having significant antimycotic potential against plant pathogenic fungi’, J. Bionanosci., 2012, 6, pp. 1 –5 (doi: 10.1166/jbns.2012.1066) [DOI] [Google Scholar]
- 17. Feng Q.L. Wu J. Chen G.Q. et al.: ‘A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus ‘, J. Biomed. Mater. Res., 2007, 52, (4), pp. 662 –668 (doi: ) [DOI] [PubMed] [Google Scholar]
- 18. Sondi I. Salopek‐Sondi B.: ‘Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram‐negative bacteria’, J. Colloid Interface Sci., 2007, 275, pp. 77 –82 [DOI] [PubMed] [Google Scholar]
- 19. Rai M. Yadav A. Gade A.: ‘Silver nanoparticles as a new generation of antimicrobials’, Biotechnol. Adv., 2009, 27, (1), pp. 76 –83 (doi: 10.1016/j.biotechadv.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 20. Joshi P.A. Bonde S.R. Gaikwad S.C. et al.: ‘Comparative studies on synthesis of silver nanoparticles by Fusarium oxysporum and Macrophomina phaseolina and its efficacy against bacteria and Malassezia furfur ’, J. Bionanosci., 2013, 7, pp. 1 –8 (doi: 10.1166/jbns.2013.1104) [DOI] [Google Scholar]
- 21. Kanhed P. Birla S. Gaikwad S. et al.: ‘In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi’, Mater. Lett., 2014, 115, pp. 13 –17 (doi: 10.1016/j.matlet.2013.10.011) [DOI] [Google Scholar]
- 22. Gupta A. Bonde S. Gaikwad S. et al.: ‘ Lawsonia inermis‐ mediated synthesis of silver nanoparticles: activity against human pathogenic Fungi and bacteria with special reference to formulation of a antimicrobial nanogel’, IET Nanobiotechnol., 2013, 8, (3), pp. 172 –178 (doi: 10.1049/iet-nbt.2013.0015) [DOI] [PubMed] [Google Scholar]
- 23. Elechiguerra J.L. Justine L.B. Yacaman M.J.: ‘Interaction of silver nanoparticles with HIV‐I’, J. Nanobiotechnol., 2005, 3, pp. 333 –336 (doi: 10.1186/1477-3155-3-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dawid W.: ‘Biology and global distribution of Myxobacteria in soils’, FEMS Microbiol. Rev., 2000, 24, pp. 403 –427 (doi: 10.1111/j.1574-6976.2000.tb00548.x) [DOI] [PubMed] [Google Scholar]
- 25. Rosenberg E. Vaks B. Zuckerberg A.: ‘Bactericidal action of an antibiotic produced by Myxococcus xanthus ’, Antimicrob. Agents Chemother, 1973, 4, pp. 507 –513 (doi: 10.1128/AAC.4.5.507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rooney M.L.: ‘Overview of active food packaging’, in Rooney M. (Eds.): ‘Active food packaging’ (Chapman & Hall, London, 1995), pp. 1 –37 [Google Scholar]
- 27. Raheman F. Deshmukh S. Ingle A. et al.: ‘Silver nanoparticles: novel antimicrobial agent synthesized from an endophytic fungus pestalotia sp. isolated from leaves of Syzygium cumini (L)’, Nano Biomed. Eng., 2011, 3, (3), pp. 174 –178 (doi: 10.5101/nbe.v3i3.p174-178) [DOI] [Google Scholar]
- 28. Sastry M. Ahmad A. Khan M. et al.: ‘Biosynthesis of metal nanoparticles using fungi and nanoparticles’, Curr. Sci., 2003, 85, (2), pp. 162 –170 [Google Scholar]
- 29. Huang J. Li Q. Sun D. et al.: ‘Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf’, Nanotechnology, 2007, 18, pp. 104 –107 [Google Scholar]
- 30. Montes‐burgos I. Walczyk D. Hole P. et al.: ‘Characterisation of nanoparticle size and state prior to nanotoxicological studies’, J. Nanopart. Res., 2010, 12, (1), pp. 47 –53 (doi: 10.1007/s11051-009-9774-z) [DOI] [Google Scholar]
- 31. Wright M.: ‘Nanoparticles in biology and medicine’ (Humana Press, Newyork, 2007), pp. 511 –520 [Google Scholar]
- 32. Farkas J. Christian P. Gallego‐urrea J.A. et al.: ‘Effects of silver and gold nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes’, Aquat. Toxicol., 2010, 96, (1), pp. 44 –52 (doi: 10.1016/j.aquatox.2009.09.016) [DOI] [PubMed] [Google Scholar]
- 33. Farkas J. Christian P. Gallego‐urrea J.A. et al.: ‘Uptake and effects of manufactured silver nanoparticles in rainbow trout (Oncorhynchus mykiss) gill cells’, Aquat. Toxicol., 2011, 101, pp. 117 –125 (doi: 10.1016/j.aquatox.2010.09.010) [DOI] [PubMed] [Google Scholar]
- 34. Hassellöv M. Readman J.W. Ranville J.F. et al.: ‘Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles’, Ecotoxicol., 2008, 17, pp. 344 –361 (doi: 10.1007/s10646-008-0225-x) [DOI] [PubMed] [Google Scholar]
- 35. Dankovich T.A. Gray D.G.: ‘Bactericidal paper impregnated with silver nanoparticles for point‐of‐use water treatment’, Environ. Sci. Technol., 2011, 45, pp. 1992 –1998 (doi: 10.1021/es103302t) [DOI] [PubMed] [Google Scholar]


