Abstract
Cassia absus is used for medicinal purposes for a long time all over the world. In this study, the authors report the antimicrobial potential of C. absus extracts obtained with different solvents. The extract(s) obtained with ethyl acetate yielded the best antibacterial effects because of a rich supply of oxalates and alkaloids in it. The same extract was also exploited for reducing Ag+ ions (to metallic Ag0) for the synthesis of nanoparticles. Electron microscopy revealed that the silver nanoparticles were ∼18–25 nm in diameter. The Fourier‐transform infrared evaluation pointed towards the fact that flavonoids present in the plant extract were acting as reductants while amino groups were the bound stabilisation agents to the synthesised nanoparticles limiting the diameter to a certain threshold and avoiding aggregation naturally. A comparative antibacterial assay of C. absus versus Ag nanoparticles showed that the nanoparticles as well as organic (ethyl acetate) extract of the plant checked the growth of selected (MDR) superbugs. However, the biosynthesised Ag nanoparticles returned better antibacterial efficacies than ethyl acetate extract.
Inspec keywords: biomimetics, nanomedicine, nanoparticles, nanofabrication, reduction (chemical), microorganisms, silver, antibacterial activity, Fourier transform infrared spectra, biomedical materials, electron microscopy
Other keywords: antibacterial capacity, biomimetic synthesis, silver nanoparticles, antimicrobial potential, ethyl acetate, oxalates, alkaloids, electron microscopy, Fourier‐transform infrared evaluation, antibacterial efficacies, antibacterial assay, organic extract, Cassia absus, flavonoids, Ag
1 Introduction
Plants are self‐possessed with many compounds and they serve as a resource for several effective and influential (bio)active agents and/or drugs in many countries of the world [1, 2]. In many developing countries, conventional medicine is one of the most important healthcare systems [3, 4]. According to the World Health Organization, the statistics suggest that a substantial population in developed countries have resorted to the usage of traditional practices of health, particularly the use of the medicinal plants [5]. Among various remedial uses of plants, several species have antimicrobial, antifungal, antiparasitic, antihelminthic and antiviral properties [6, 7, 8].
Antibiotics are one of our most important armaments in fighting bacterial infections and have significantly benefited the health‐related features of human life since their discovery [6, 7, 8, 9, 10]. However, for the last few years these antibiotics had become less effective against infections because of the toxicities they cause inside our bodies and (mainly) due to the emergence of resistance in the microbial pathogens towards these administered drugs [11]. It had become indispensable for us to find new and/or novel alternative antibiotics/drugs with less or (ideally) no resistance from the microorganisms [12]. Current trends, though, point to the fact that the rate at which new drugs are discovered is (really slow and) declining [13, 14]. Natural products of higher plants may give an answer to the prevailing scenario as they harbour rich sources of antimicrobial agents which (would) have novel mechanisms of action [15, 16]. They are also burdened with a wide range of secondary metabolites such as saponins, tannins, terpenoids, alkaloids, flavonoids, glycosides and so on, which have been reported to act as antimicrobials through in‐vitro experiments [17, 18].
Cassia absus belongs to the family Fabaceae and commonly is known as ‘Chaksu’ in local language(s). It is disseminated throughout Pakistan, India and Sri Lanka and is also seen across the continents of Australia and Africa. It is an annual, erect and sparingly branched flowering plant [19]. The seed pulp contains 1.5% isochaksine and chaksine [20]. C. absus seeds extracts are used to treat blood glucose levels and mucous diseases. The efficacy of these seeds to treat hepatic and renal diseases is also already verified [21]. There are reports of the plant seeds to act as anti‐inflammatory and anti‐histaminic in eye drop formulations [19]. The paste of seed is also used for scrapping of dead tissues on the skin and the reduction of inflammation on the wounds. Powder made from seeds is used for relieving diarrhoea. It is also effectual in anuria renal stones and in aching urination [22].
In this paper, we report the antibacterial potency of extracts of C. absus followed by the synthesis and characterisation of Ag nanoparticles using the (ethyl acetate) extract of the plant as the reducing agent.
2 Materials and methods
2.1 Collection of plant materials
C. absus seeds were collected from the local markets of Abbottbad (Pakistan). The seeds were identified by a qualified Botanist at COMSATS Institute of Information Technology, Abbottabad, Pakistan. The seeds were washed three times by sterile distilled water, shade dried, crushed into powdered form and stored in air‐tight plastic containers for further investigations.
2.2 Preparation of plant extract
A number of organic solvent extracts were obtained for this study. C. absus seed powder (2 g) was added to 10 ml (each) of different solvents, i.e. carbon tetrachloride, methanol, ethyl acetate, ethanol, acetone and left for 24 h. The extracts were spun (Eppendorf 5810‐R, Germany) at 2500g for 15 min and filtered using Whatman filter paper no. 1 (Aldrich).
2.3 Test organisms
The test organisms (Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Pseudomonas aeruginosa, Bacillus cereus, Neisseria gonorrhea, Klebsiella pneumoniae) were provided by the Ayub Medical College and Hospital, Abbottabad (Pakistan). The organisms were cultured on Müller Hinton (MH) Agar (Oxoid, UK) and incubated at 37°C overnight.
2.4 Antibacterial assay
The antibacterial activity was performed by agar well diffusion method using 4 mm (diameter) wells. The test bacterial isolates were inoculated on MH agar plates. A volume of 30 µl plant extract of all solvents was poured into the respective wells. The plates were incubated at 37°C overnight and the antibacterial activity was evaluated by measuring the diameter of the zone of inhibition in mm.
2.5 Phytochemical analysis
Phytochemical screening of the extracts of the C. absus seeds was carried for alkaloids, carbohydrates, flavonoids, phenols, glycosides, saponins and sterols as reported by Odebiyi and Sofowora [23].
2.6 Preparation of silver nanoparticles
Since the ethyl acetate extract showed the best antimicrobial activity, therefore the Ag nanoparticles were prepared with this extract (this was our criterion for this particular study). A volume of 10 ml plant extract was mixed with 90 ml of (8 mM) AgNO3 solution (Sigma‐Aldrich) in a sterile conical flask and kept in dark conditions for incubation at room temperature for 2–3 days.
2.7 Fourier‐transform infrared (FTIR) spectroscopy
FTIR analysis was performed to determine the functional groups involved in the reduction of Ag+ ions to Ag nanoparticles. The spectra were collected using FTIR RX1 (Perkin Elmer) in the range of 500–4000 cm−1.
2.8 Electron microscopy
Electron microscopy of the biogenic Ag nanoparticles was performed using scanning electron microscopy (SEM) [JOEL (JSM5910, Japan)] and transmission electron microscopy (TEM) (JEM 2100 TEM) techniques. The SEM was performed by adding a drop of 5 µl of the sample on a pre‐cleaned Si wafer and was air dried. TEM was done by taking a sample volume of 7 µl and dispersing it onto a (carbon‐coated) Cu grid (Ted Pella, USA) and air dried for 5 min. The air‐dried grids were finally dried in a vacuum chamber for 45 min before installing it in the microscope for imaging.
2.9 Antimicrobial activity of silver nanoparticles
The antimicrobial testing of Ag nanoparticles was carried out by agar well diffusion method for comparison with natural seed extract on both susceptible as well as MDR bacteria.
3 Results
3.1 Antibacterial activity
Extracts of C. absus were tested for their antimicrobial properties relative to silver nanoparticles. The findings showed that all the extracts demonstrated good inhibitory activity against both, gram‐positive and gram‐negative bacteria. However, it was observed that the ethyl acetate extract had the best antimicrobial potential relative to its other counterparts used in this study. The results showed that it inhibited S. aureus, P. aeruginosa, N. meningitis, B. cereus and S. pyogenes, respectively, while at the same time showed bacteriostatic activity against A. baumonai and E. coli (see Fig. 1 a). To confirm the antibacterial potential of extract of C. absus seeds, a control experiment was performed through well diffusion assay where all the (eight) test bacterial strains were cultured under the effect of ethyl acetate. The results showed that ethyl acetate alone was not able to inhibit the growth of any of these strains (see Fig. 1 b). The other (methanol, ethanol, carbon tetrachloride) extracts of our selected plant did not result in the significant killing of bacterial cells. They eradicated either one or two of the strains. The overall results pointed out that the tested extracts of C. absus seeds had the potential to check bacterial growth of both, gram‐positive and gram‐negative strains.
Fig. 1.
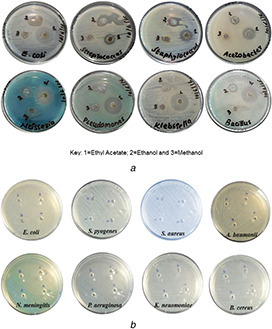
Well Diffusion susceptibility assay showing
(a) Antibacterial activity shown by clear zones of inhibition by the EAE of C. absus extract against selected bacterial strains, (b) Ethyly acetate alone (as negative control) did not show any antibacterial activity
A test for the phytochemical analysis of the C. absus seeds revealed the presence of a variety of bio‐active components in it. We found oxalate, quinines, terpenoids, tannins, saponins, flavonoids, glycosides, carbohydrates and alkaloids eluted with different organic solvents (see Table 1). This analysis led us to hypothesise why the ethyl acetate extract had best antibacterial capacities.
Table 1.
Phytochemical screening of seed extract of C. absus of different solvents
| Phytochemical constituents | Ethyl acetatae | Methanol | Ethanol | Acetone | Carbon tetrachloride |
|---|---|---|---|---|---|
| tannins | − | ++ | ++ | ++ | − |
| saponin | − | − | − | − | ++ |
| flavonoids | ++ | − | ++ | − | − |
| glycosides | − | ++ | ++ | − | − |
| carbohydrates | ++ | ++ | − | − | − |
| alkaloids | ++ | − | − | ++ | − |
| phenols | − | ++ | − | − | − |
| oxalates | ++ | − | − | − | − |
| quinones | − | ++ | ++ | ++ | − |
| terpenoids | − | − | − | − | − |
‘ + +’ sign represents abundance while ‘−’ sign represents absence of a specific biochemical.
3.2 Ethyl acetate extract of C. absus as a reductant
Since the C. absus extract obtained with ethyl acetate returned the best potential in terms of the antibacterial activity, we made this as a criterion for using this extract as a reductant for the synthesis of Ag nanoparticles. The ethyl acetate extract was added to a solution of AgNO3 and the resulting brown‐black precipitate was purified by filtration and centrifugation. Microscopy analysis showed that the Ag+ was reduced to Ag nanoparticles by the plant extract. SEM (Fig. 2 : inset) followed by scanning transmission electron microscopy (Fig. 2) showed that the particles were (roughly) spherical in shape. The diameter of the synthesised Ag nanoparticles (determined via electron microscopy imaging) was in the range of 18–25 nm and showed good monodispersity.
Fig. 2.
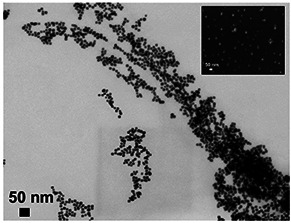
Electron microscopy (TEM) image of Ag nanoparticles synthesised with EAE of C. absus. Inset shows an SEM image of the same biomimetic nanoparticles
3.3 FTIR spectroscopy analysis
The FTIR investigations were carried for both the ethyl acetate extract and the silver nanoparticles obtained from C. absus seeds. From Fig. 3 a, it was observed that the ethyl acetate extract showed broadband stretching vibration at 2983, 1742 and 1369 cm−1 for C–H, C–C and C–N bonds, respectively. These results confirmed the presence of substituted flavonoids, amine and hydroxyl groups that are responsible for biological activity of C. absus. Similar observations were also detected in ethyl acetate extracts of Taraxacum officinale, Menthe longifolia [24] and Albizia adianthifolia [25].
Fig. 3.
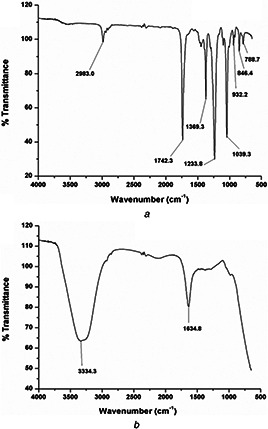
FTIR spectrum collected from
(a) EAE of C. absus seeds, (b) Ag nanoparticles synthesised with the EAE of C. absus seeds
The FTIR spectrum of the synthesised silver nanoparticles is shown in Fig. 3 b that exhibited spectral values at the stretching vibration band of 3334 cm−1 corresponded to an N–H stretching vibrations that is the presence of an amine group. The peak recorded at 1634 cm−1 indicates that the amino groups are partially utilised for the encapsulation and stabilisation of silver nanoparticles [26]. Further, it is observed that the flavonoids act as reducing agent and transformed Ag+ to Ag0 while the amino groups as a stabilising agent in the green synthesis of silver nanoparticles [27]. Thus, the FTIR study revealed the multifunctionality of the silver nanoparticles of C. absus extract where reduction and stabilisation occurred simultaneously. The silver nanoparticles showed antibacterial activity against many pathogenic strains and health industries [28, 29]. The FTIR analysis and the electron microscopy imaging results were in complete agreement to each other since microscopy showed them to be (sufficiently) monodispersed due to amino stabilisation of silver nanoparticles.
3.4 Plant extract versus Ag nanoparticles on susceptible and MDR bacterial isolates
After achieving good antimicrobial capacities with the ethyl acetate extract of C. absus and using it as a reductant for the synthesis of Ag nanoparticles, we performed an assay to compare the impact of ethyl acetate extract with Ag nanoparticles in terms of their capacities to kill bacterial cells. The quantity of (concentrated) ethyl acetate extract poured in each well was 30 µl, while for Ag nanoparticles a number of different volumes were used. The concentration of Ag nanoparticles, however, was kept the same, i.e. 25 mM. The comparison of C. absus extract of ethyl acetate and Ag nanoparticles did not show surprising outcome and as it would be expected; Ag nanoparticles showed a better antibacterial activity against the MDR bacterial cells than ethyl acetate extract (Figs. 4 a–d). However, an encouraging observation was the fact that despite proving less potent than Ag nanoparticles, the extract did check (prohibit) the growth of these superbugs. The diameters and the clear zones of inhibition produced by Ag nanoparticles and the ethyl acetate extract are summarised in Fig. 4.
Fig. 4.
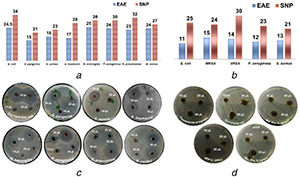
Bar chart diagrams and well diffusion assay images (with clear zones of inhibition) show relative antibacterial capacities of EAE versus Silver Nanoparticles (SNP) against
(a) & (c) susceptible and, (b) & (d) MDR bacteria
4 Discussion
Plants are known to contain various dynamic principles of remedial worth and hold natural activity against a number of diseases [30, 31]. In the last few years they (as extracts) had been extensively used as ‘green’ reductants for the synthesis of nanoparticles [32]. Using plants for the synthesis of nanoparticles has many advantages, e.g. they are cheap and less toxic than their conventional chemical counterparts [32, 33, 34]. It was observed (through FTIR analysis) that in our study the flavonoid(s) acted as reducing agent(s) and transformed Ag+ to Ag0 while the amino groups as a stabilising (capping) agents in the green synthesis of Ag nanoparticles. Flavonoids are a large group of polyphenolic compounds including anthocyanins, isoflavonoids, flavonols, chalcones, flavanones and so on. They are well known to chelate and reduce metallic ions to nanoparticles [32, 35].
The peaks recorded by FTIR spectroscopy at 1634 cm−1 of the extract of C. absus expressed in Ag nanoparticles indicated that the amino groups are utilised for the encapsulation and stabilisation of silver nanoparticles. Role of amino groups as capping end groups in nanoparticle synthesis via plant extracts is already well established [36, 37]. The FTIR analysis revealed the multifunctionality of the C. absus extract where reduction and stabilisation occurred simultaneously. Our FTIR and electron microscopy imaging observations were in complete agreement to each other since microscopy showed them to be (sufficiently) monodispersed, i.e. not making huge aggregated clumps. The reason for a good monodispersity came from functional groups mediated electrostatics that acted as capping agents.
The phytochemistry and medicial potential of Cassia plants had been already well documented [38]. The ethyl acetate extract of C. absus seeds revealed the presence of oxalates, flavonoids and alkaloids in abundance (see Table 1) and this was indeed the reason for its potent antimicrobial properties. Many reports show that plant extracts obtained with ethyl acetate can harvest oxalates, alkaloids, flavoids, phenols, saponins and tannins. The reason why these bioactive agents were present in abundance in this extract was because of a better elution of these biochemicals with ethyl acetate [39, 40]. It had been already established that plant alkaloids are excellent antimicrobial agents against susceptible as well as multiple drug‐resistant bacteria [41]. Plant alkaloids have been reported for inhibiting bacterial virulence in addition to antibacterial and antibiotic enhancing activity. Alkaloids work by disruption of virulence gene regulation, fimbriae and other adhesins, they inhibit bacterial secretion system and bacterial defence against host immune system [42]. Oxalates are produced in the plants naturally and they serve in multiple important roles. The presence of oxalates in seeds of C. absus is already well reported in the genus of Cassia such as C. fistula and other species [43]. One of the major roles of oxalates in plants is to minimise the attack of plant pathogens such as fungi and bacteria [44]. It is also evident from literature that oxalates have antimicrobial potential and in our study we counter verified it since the ethyl acetate extracts (which had abundance of oxalates) inhibited the microbial growth much efficiently that its other counterparts [44, 45]. Nanoparticles are also known as nano‐antibiotics, i.e. potent anti‐microbial agents [46]. The exact mechanism of how they tackle the eradication of microbial cells is still unknown. The hypothesis suggests that these nanoparticles penetrate bacterial cell wall, bringing structural changes and leads to cell death. They can also generate free radicals which make the cell membrane porous and resulting in loss of essential cytoplasmic salts and/or nutrients. Silver ions leaching from the nanoparticles can interact with thiol groups of many important bacterial enzymes, inhibiting cell functions, leading to cell damage and eventually death. DNA replication can also get affected by these nanoparticles as they have tendency to bind to sulphur and phosphorous of DNA [47]. The facts lying behind the antibacterial mode of action of Ag nanoparticles is controversial, yet we can speculate from our results that Ag0 nanoparticle rather than cationic silver ions have better anticellular capacities. The uptake of cationic silver is mediated by the cationic pumps while Ag0 nanoparticles are taken inside through non‐specific interactions which consequently result in higher concentrations of Ag content in the cells or across the bacterial cell wall. Moreover, the favourable redox potential of Ag0 atom at the surface of nanoparticles (which) triggers the generation of a higher concentration of free radicals leading to cytotoxic reactive oxygen species [48]. It is proved from this study that nanoparticles are still more potent than other natural solutions to tackle bacterial infections. Ag nanoparticles have four possible mechanisms of antibacterial activity: (i) interference during cell wall synthesis; the exact mechanism of how Ag nanoparticles interact with the cell wall of bacteria is not yet fully understood, yet it had been reported that they do play a role by interfering in the cell wall synthesis, (ii) suppression during protein bio‐synthesis (translation); Ag nanoparticles can suppress protein biosynthesis by interacting with ribosomes and denature them rendering them incapable of playing their role in the translation of mRNA molecules, (iii) interference or disruption of transcription process; Ag nanoparticles can intercalate into the DNA base pairs resulting in the breakage of hydrogen bonds disrupting the double helical structure. This eventually results in the blockage of gene expressions (transcription) of these microbes and (iv) disruption of primary metabolic pathways; Ag nanoparticles damage the cell membrane proteins by modifying their amino acids and the Ag‐protein conjugates result in the dysfunction of key enzymes which eventually obstruct primary metabolic processes [46, 49]. Nanoparticles have proved to be excellent antimicrobials so far but more efforts are needed to standardise the synthesis, characterisation and modification of nanoparticles to design the magic nano‐bullets against the prevailing pathogenic microbial infective agents including the superbugs.
5 Conclusion
This study could pave the way towards the usage of plants, herbs and their extracts to treat many more infections and diseases. Moreover, green synthesis is an answer to obtain nanoparticles and add stabilisation agents simultaneously. The ethyl acetate extract and biomimetic Ag nanoparticles can be used in the production of antibacterial drugs especially against the test organisms used in this study.
6 References
- 1. Khan A.A. Omer K.A. Talib A. et al.: ‘Green tropical phytoextracts‐promising anticancer alternative’, Braz. Arch. Biol. Technol., 2016, 59, p. e16160062 [Google Scholar]
- 2. Rasool Hassan B.A.: ‘Medicinal plants (importance and uses)’, Pharm. Anal. Acta, 2012, 3, p. e139 [Google Scholar]
- 3. Lam K.S.: ‘New aspects of natural products in drug discovery’, Trends Microbiol., 2007, 15, pp. 279 –289 [DOI] [PubMed] [Google Scholar]
- 4. Houghton P.J.: ‘The role of plants in traditional medicine and current therapy’, J. Altern. Complem. Med., 1995, 1, pp. 131 –143 [DOI] [PubMed] [Google Scholar]
- 5. Akerele O. Heywood V. Synge H.: ‘Conservation of medicinal plants’ (Cambridge University Press, Cambridge, UK, 1991) [Google Scholar]
- 6. Wink M.: ‘Medicinal plants: a source of anti‐parasitic secondary metabolites’, Molecules, 2012, 17, (11), pp. 12771 –12791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Q. Luyten W. Pellens K. et al.: ‘Antifungal activity in plants from Chinese traditional and folk medicine’, J. Ethnopharmacol., 2012, 143, (3), pp. 772 –778 [DOI] [PubMed] [Google Scholar]
- 8. Cecílio A.B. de Faria D.B. de Carvalho P. et al.: ‘Screening of Brazilian medicinal plants for antiviral activity against rotavirus’, J. Ethnopharmacol., 2012, 141, (3), pp. 975 –981 [DOI] [PubMed] [Google Scholar]
- 9. Demain A.L.: ‘Antibiotics: natural products essential to human health’, Med. Res. Rev., 2009, 29, (6), pp. 821 –842 [DOI] [PubMed] [Google Scholar]
- 10. Cragg G.M. Newman D.J.: ‘Biodiversity: a continuing source of novel drug leads’, Pure Appl. Chem., 2005, 77, pp. 7 –24 [Google Scholar]
- 11. Davies J. Davies D.: ‘Origins and evolution of antibiotic resistance’, Microbiol. Mol. Biol. Rev., 2010, 74, (3), pp. 417 –433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischbach M.A. Walsh C.T.: ‘Antibiotics for emerging pathogens’, Science, 2009, 325, (5944), pp. 1089 –1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conly J.M. Johnston B.L.: ‘Where are all the new antibiotics? The new antibiotic paradox’, Can. J. Infect. Dis. Med. Microbiol., 2005, 16, (3), pp. 159 –160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clardy J. Fischbach M.A. Walsh C.T.: ‘New antibiotics from bacterial natural products’, Nat. Biotechnol., 2006, 24, (12), p. 1541 [DOI] [PubMed] [Google Scholar]
- 15. Runyoro D.K.B. Matee M.I.N. Ngassapa O.D. et al.: ‘Screening of Tanzanian medicinal plants for anti‐Candida activity’, BMC Complement. Altern. Med., 2006, 6, p. 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonjar G.H.S.: ‘Evaluation of antibacterial properties of Iranian medicinal‐plants against Micrococcus luteus, Serratia marcescens, Klebsiella pneumoniae and Bordetella bronchoseptica ’, Asian J. Plant Sci., 2004, 3, pp. 82 –86 [Google Scholar]
- 17. Dubey N.K. Kumar R. Tripathi P.: ‘Global promotion of herbal medicine: India's opportunity’, Curr. Sci., 2004, 86, pp. 37 –41 [Google Scholar]
- 18. Kamboj V.P.: ‘Herbal medicine’, Curr. Sci., 2000, 78, (1), pp. 35 –39 [Google Scholar]
- 19. Pandya H. Kachwala Y. Sawant L. et al.: ‘Pharmacognostical screening of seeds of Cassia absus ’, Pharmacogn. J., 2010, 2, pp. 419 –426 [Google Scholar]
- 20. Aftab K. Ahmed S.I. Usmanghani K.: ‘Traditional medicine Cassia absus L. (chaksu)‐pharmacological evaluation’, Phytomedicine, 1996, 2, pp. 213 –219 [DOI] [PubMed] [Google Scholar]
- 21. Hussain F. Shahid M. Javed K.: ‘Antioxidant, antiglycation and alpha amylase inhibitory activities of Cassia absus seeds’, Int. Sci. Org. Curr. Sci. Perspect., 2015, 2, pp. 5 –9 [Google Scholar]
- 22. Hussain F. Shahid M. Javed K.: ‘Antioxidant, antiglycation and alpha amylase inhibitory activities of Cassia absus seeds’, Int. Sci. Org. Curr. Sci. Perspect., 2015, 2, (1), pp. 5 –9 [Google Scholar]
- 23. Odebiyi O.O. Sofowora E.A.: ‘Phytochemical screening of Nigerian medicinal plants II’, Lloydia, 1978, 41, (3), pp. 234 –246 [PubMed] [Google Scholar]
- 24. Saeidi S. Hassanpour K. Ghamgosha M. et al.: ‘Antibacterial activity of ethyl acetate and aqueous extracts of Mentha longifolia L. and hydroalcoholic extract of Zataria multiflora Boiss. Plants against important human pathogens’, Asian Pac. J. Trop. Med., 2014, 7, pp. S186 –S189 [DOI] [PubMed] [Google Scholar]
- 25. de Dieu Tamokou J. Mpetga D.J.S. Lunga P.K. et al.: ‘Antioxidant and antimicrobial activities of ethyl acetate extract, fractions and compounds from stem bark of Albizia adianthifolia (mimosoideae)’, BMC Complement. Altern. Med., 2012, 12, (1), p. 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paralikar P.: ‘Biogenic synthesis of silver nanoparticles using leaves extract of Epiphyllum oxypetalum and its antibacterial activity’, Austin J. Biotechnol. Bioeng., 2014, 1, (7), pp. 5 –9 [Google Scholar]
- 27. Kumar D.A. Palanichamy V. Roopan S.M.: ‘Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity’, Spectrochim. Acta, Part A, 2014, 127, pp. 168 –171 [DOI] [PubMed] [Google Scholar]
- 28. Kumar P.P.N.V. Pammi S.N.V. Kollu P. et al.: ‘Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity’, Ind. Crops Prod., 2014, 52, pp. 562 –566 [Google Scholar]
- 29. Veeraputhiran V.: ‘Bio‐catalytic synthesis of silver nanoparticles’, Int. J. Chem. Technol. Res., 2013, 5, (5), pp. 255 –2562 [Google Scholar]
- 30. Fridlender M. Kapulnik Y. Koltai H.: ‘Plant derived substances with anti‐cancer activity: from folklore to practice’, Front. Plant Sci., 2015, 6, (October), pp. 1 –9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karimi E. Jaafar H.Z.E. Ahmad S.: ‘Phytochemical analysis and antimicrobial activities of methanolic extracts of leaf, stem and root from different varieties of labisa pumila benth’, Molecules, 2011, 16, (6), pp. 4438 –4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmed S. Ahmad M. Swami B.L. et al.: ‘A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise’, J. Adv. Res., 2016, 7, (1), pp. 17 –28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar B. Smita K. Cumbal L. et al.: ‘Green synthesis of silver nanoparticles using Andean blackberry fruit extract’, Saudi J. Biol. Sci., 2017, 24, (1), pp. 45 –50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ankamwar B. Salgaonkar M. Sur U.K.: ‘Room temperature green synthesis of anisotropic gold nanoparticles using novel biological fruit extract’, Inorg. Nano‐Met. Chem., 2017, 14, (1), pp. 1 –10, (just‐accepted) [Google Scholar]
- 35. Iravani S.: ‘Green synthesis of metal nanoparticles using plants’, Green Chem., 2011, 13, (10), pp. 2638 –2650 [Google Scholar]
- 36. Nabikhan A. Kandasamy K. Raj A. et al.: ‘Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L . ’, Colloids Surf. B, Biointerfaces, 2010, 79, (2), pp. 488 –493 [DOI] [PubMed] [Google Scholar]
- 37. Bar H. Bhui D.K. Sahoo G.P. et al.: ‘Green synthesis of silver nanoparticles using seed extract of Jatropha curcas ’, Colloids Surf. A, Physicochem. Eng. Aspects, 2009, 348, (1), pp. 212 –216 [Google Scholar]
- 38. Ahmad S. Hassan A. Abbasi W.M. et al.: ‘Phytochemistry and pharmacological potential of Cassia absus –a review’, J. Pharm. Pharmacol., 2017, 70, (1), pp. 27 –41 [DOI] [PubMed] [Google Scholar]
- 39. Temitope O.O. Fasusi O.A. Odeluyi T.C. et al.: ‘The study of antimicrobial activity of partially purified ethyl acetate extracts of Bridelia ferruginea on clinical isolates’, Saudi J. Pathol. Microbiol., 2016, 1, (1), pp. 19 –28 [Google Scholar]
- 40. Krasteva I. Nikolova I. Danchev N. et al.: ‘Phytochemical analysis of ethyl acetate extract from Astragalus corniculatus Bieb. and brain antihypoxic activity’, Acta Pharm., 2004, 54, (2), pp. 151 –156 [PubMed] [Google Scholar]
- 41. Cowan M.M.: ‘Plant products as antimicrobial agents’, Clin. Microbiol. Rev., 1999, 12, (4), pp. 564 –582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cushnie T.P.T. Cushnie B. Lamb A.J.: ‘Alkaloids: an overview of their antibacterial, antibiotic‐enhancing and antivirulence activities’, Int. J. Antimicrob. Agents, 2014, 44, (5), pp. 377 –386 [DOI] [PubMed] [Google Scholar]
- 43. Mohideen S. Sasikala E. Aruhaj P.: ‘Pharmacognosy of Cassia Alata Linn –leaves’, Anc. Sci. Life, 2005, 24, (4), p. 192 [PMC free article] [PubMed] [Google Scholar]
- 44. Huang J. Huang C. Liebman M.: ‘Oxalate contents of commonly used Chinese medicinal herbs’, J. Tradit. Chin. Med., 2015, 35, (5), pp. 594 –599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anang D.M. Rusul G. Radu S. et al.: ‘Inhibitory effect of oxalic acid on bacterial spoilage of raw chilled chicken’, J. Food Prot., 2006, 69, (8), pp. 1913 –1919 [DOI] [PubMed] [Google Scholar]
- 46. Rafique M. Sadaf I. Rafique M.S. et al.: ‘A review on green synthesis of silver nanoparticles and their applications’, Artif. Cells Nanomed. Biotechnol., 2017, 45, (7), pp. 1272 –1291 [DOI] [PubMed] [Google Scholar]
- 47. Kim J.S. Poulose E.K.: ‘Antimicrobial effects of silver nanoparticles’, Nanomed. Nanotechnol. Biol. Med., 2007, 3, (1), pp. 95 –101 [DOI] [PubMed] [Google Scholar]
- 48. Pandey J.K. Swarnkar R.K. Soumya K.K. et al.: ‘Silver nanoparticles synthesized by pulsed laser ablation: as a potent antibacterial agent for human enteropathogenic gram‐positive and gram‐negative bacterial strains’, Appl. Biochem. Biotechnol., 2014, 174, (3), pp. 1021 –1031 [DOI] [PubMed] [Google Scholar]
- 49. Dakal T.C. Kumar A. Majumdar R.S. et al.: ‘Mechanistic basis of antimicrobial actions of silver nanoparticles’, Front. Microbiol., 2016, 7, p. 1831 [DOI] [PMC free article] [PubMed] [Google Scholar]


