Abstract
The study was focused on the phytochemicals‐mediated biosynthesis of silver nanoparticles using leaf extracts and infusions from Cynara scolymus. To identify the antioxidant activity and total phenolic content, the 1,1‐diphenyl‐1‐picrylhydrazyl and Folin–Ciocalteau methods were applied, respectively. The formation and stability of the reduced silver ions were monitored by UV–vis spectrophotometer. The particle sizes of the silver nanoparticles were characterised using the dynamic light scattering technique and scanning electron microscope. The phase composition of the obtained silver nanoparticles was characterised by X‐ray diffraction. The silver nanoparticles suspension, artichoke infusion, and silver ions were separately tested towards potential cytotoxicity and pro‐inflammatory effect using mouse fibroblasts and human monocytes cell line, respectively. The total phenolic content and antioxidant activity of ethanol extract and infusion were found significantly higher as compared to aqueous extract and infusion. The UV–visible spectrophotometric analysis revealed the presence of the characteristic absorption band of the Ag nanoparticles. Moreover, it was found that with the increasing volume of plant extract, the average size of particles was increased. Biocompatibility results evidently showed that silver nanoparticles do not induce monocyte activation, however in order to avoid their cytotoxicity suspension at a concentration <2 ppm should be applied.
Inspec keywords: pharmaceuticals, health and safety, renewable materials, toxicology, organic compounds, antibacterial activity, X‐ray diffraction, nanomedicine, nanoparticles, nanofabrication, suspensions, ultraviolet spectra, visible spectra, scanning electron microscopy, silver, particle size
Other keywords: phytochemicals‐mediated biosynthesis; antioxidant activity; total phenolic content; dynamic light scattering technique; silver nanoparticles suspension; scanning electron microscopy; Cynara scolymus; 1,1 diphenyl‐1‐picrylhydrazyl method; cytotoxicity; immune compatibility; leaf extracts; UV‐vis spectrophotometry; particle size; Folin‐Ciocalteau methods; phase composition; X‐ray diffraction; artichoke infusion; pro‐inflammatory effect; mouse fibroblasts; human monocytes cell line; Ag
1 Introduction
Nanoparticles are currently an area of ardent scientific research, due to their wide range of prospective applications in biomedical [1, 2, 3], environmental [4, 5, 6] and electronics [7, 8, 9] fields. Their utilisation is caused by exceptional features of nanoparticles arising from differences in properties between nano‐ and macroscale (bulk) materials, which is the main reason for the growing interest in nanosized structures. In particular, nanoparticles have begun to play a major role in various fields of biomedicine, owing to their bactericidal [10, 11], antifungal [12, 13] and anticancer [14, 15] activity. Silver nanoparticles for the sake of their antimicrobial properties have found numerous application in molecular diagnostics and dentistry, as well as a component of materials applied in burn wounds healing [16, 17]. Due to the growing microbial resistance against metal ions, antibiotics and the development of resistant strains, the metallic nanoparticles are considered as one of the most promising antimicrobial agents. Antimicrobial properties of silver nanoparticles (AgNPs) are caused by their anchoring and penetrating to the cell wall of bacteria resulting in modulation of cellular signalling and disturbing cell viability and division leading to cell death [18]. It is well reported that the bactericidal properties of AgNPs are dependent on many factors including their size, shape, concentration and crystal structure, thus extensive research devoted to synthesising and characterising NPs has been performed. Studies on silver nanoparticles synthesis and bactericidal effect were mainly focused on spherical‐shape NPs interaction with gram (+) and gram (−) microorganisms [19]. Shape‐dependent effect of AgNPs of different morphology on their bactericidal activity against Escherichia coli was for the first time described by Pal et al. [20]. The authors compared antibacterial properties of spherical, rod‐shaped and truncated triangular AgNPs showing that the last one has the strongest toxic effect against E. coli. Moreover, the authors conclude that the differences in the observed trends in E. coli inhibition can be favoured by high‐atom‐density facets such as {111} that are the most abundant in truncated triangular AgNPs as compared to nanoparticles of spherical and rod‐shaped morphology. Although, studies performed by Raza et al. [21] showed that the smallest‐sized spherical AgNPs revealed a stronger antimicrobial effect against Pseudomonas aeruginosa and E. coli strains in comparison with the triangular and larger spherical shaped AgNPs.
Many methods have been devised to produce metallic nanoparticles. The silver nanocrystals are usually fabricated from Ag+ containing solutions, derived from salt like silver nitrate (AgNO3). Initially, metal ions are reduced to atoms by reaction with reducing agent. Subsequently, atoms form small clusters that nucleate to particles. The size and shape of the nanoparticles can be easily controlled by the silver ions to reducing agent concentration ratio [22]. Methods of synthesis of AgNPs can be broadly categorised as chemical methods, physical methods, and biological methods. However, considering the intensive use of synthetic reactants and energy consumption in chemical and physical methods, there is a fundamental need to amplify environmentally harmless and inexpensive procedures for the metallic nanoparticles ‘green’ synthesis that use eco‐friendly reactants. The development of biologically inspired methods for the syntheses of AgNPs is evolving into an important field of nanotechnology. Silver nanoparticles produced by green chemistry methods are extremely promising agents in application to the biological system. For this purpose, extracts of various plants have been examined as a reducing and stabilising agent because of their antioxidant or reducing properties typically responsible for the reduction of metal ions [23, 24, 25].
The use of plants, especially as the source of reducing and stabilising agents in AgNPs production has accrued utmost interest, owing to its facile, eco‐friendly, non‐pathogenic and economical character that make plant extracts an excellent choice for AgNPs synthesis. It is proved that the source of the plant extract can influence the characteristics of the NPs, because of variable concentrations and combinations of organic reducing agents, involving various plant metabolites such as alkaloids, phenolic compounds, terpenoids, and coenzymes as well [26]. The main mechanism of NPs formation in biological methods considered for the process is phytochemicals‐assisted reduction caused by the presence of water‐soluble phytochemicals in plants extracts resulting in the immediate reduction of the Ag ions upon contact of the extract with the ions solution. Additionally, the great advantages of plant extracts employment in AgNPs preparation are the availability of abroad variety of plant species and safety of their metabolites in most cases. Reagents used for AgNPs production applied as an active substance in disease treatment cannot induce any toxic side effect on tissues, therefore plants extracts and infusions are especially suited for the production of nanoparticles intended to use as therapeutic agents in medical application [22].
In fact, an interesting approach to give an added value to AgNPs therapeutic activity is the use of health‐promoting plants extracts as a reducing and stabilising agents in NPs preparation. An attractive plant material for potential use in preparation of metal nanoparticles is artichoke (Cynara scolymus). Various reports related to artichoke composition have revealed its health‐protective potential especially in cancer treatment, hepatoprotective and hypocholesterolemic activity [27, 28, 29]. Antioxidant activity of artichoke extracts is associated with the high content of caffeic acid and its derivatives with chlorogenic acid as the most important of these derivatives. Also, other phenolic compounds such as the flavonoids (apigenin and luteolin) as well as different cyanidin caffeoylglucoside derivatives have been identified [30, 31, 32]. Moreover, it is well proved that bioactive substances, e.g. caffeic acid, chlorogenic acid, cynarin and luteolin that are found in artichoke extract can decrease the production of reactive oxygen species (ROS) and reduce cytotoxicity induced by AgNPs in normal cells [33].
Considering the vast potential of plants as a source of health‐promoting substances with antioxidative potential, this work aims to apply a phytochemical‐mediated green technique for the synthesis of silver nanoparticles as an alternative to chemical and physical methods. In this regard, water and ethanol extracts and infusions of leaves of C. scolymus a species of family Compositeae (Asteraceae) were used for the bioconversion of Ag ions to AgNPs. Silver nanoparticles suspensions were produced at various concentrations of leaf extracts and infusions in order to optimise route to AgNPs obtaining. The proposed method is facile, cost‐effective and sustainable. The silver nanoparticles preparation with the use of C. scolymus was previously investigated by Sampaio and Viana [34]. This paper was focused on AgNPs obtaining with the use of an aqueous extract of artichoke flower petals under various pH conditions. The purpose of this paper was to give a characterisation of electrical conductivity of such‐prepared AgNPs. Green synthesis of AgNPs with artichoke was also performed by Erci et al. [35]. The authors presented research on AgNPs preparation by using the aqueous brew of C. scolymus leaves. This paper includes characteristic of the morphology of the obtained AgNPs and their cytotoxicity assessment, as well as size characteristic performed with DLS technique. In comparison with these reports, our studies introduce some additional information regarding antioxidant properties of artichoke leaves extracts and infusions, and cytotoxicity and inflammatory assessment of infusion and AgNPs as well. Moreover, we showed the effect of an additional polymeric stabilising agent on the formation of nanosized silver structure.
2 Experimental
2.1 Preparation of plant extracts and infusions
Cynara scolymus leaf extracts and infusions were utilised to prepare silver nanoparticles suspensions on the basis of cost‐effectiveness, antioxidant properties, and availability. Artichoke heads were purchased from local greengrocer shop. Plant leaves were separated, cleaned with running tap water, followed by distilled water and air dried at room temperature. Dry leaves were subsequently cut into small pieces with knife mill. Around 10 g of finely cut leaves were used to prepare water and ethanol extracts and infusions. Extraction was carried out in Soxhlet apparatus for 10 h with 150 mL of water or ethanol (96%, POCh SA). In order to obtain infusions, 10 g of dry artichoke leaves were extracted in 150 mL of water or ethanol at the solvents boiling point. The beakers with mixtures were covered with a watch glass and allowed to infuse for 30 min. Such‐obtained extracts were subsequently filtered through filter paper. The filtrates were cooled down, stored at 4°C and used after 1 day.
2.2 Preparation of silver nanoparticles suspensions
As a source of silver ions in AgNPs synthesis silver nitrate solution was used. Then, 5, 10 and 15 mL of extract were added separately to 45, 40 and 35 mL of silver nitrate water or ethanol solutions. The final mixtures contained 500 ppm of Ag+. Simultaneously, in order to access the effect of an additional stabilising agent on AgNPs formation, the identical silver nitrate mixtures in the solution of poly(N ‐vinylpyrrolidone) (PVP, MW 8000, Acros Organics) were prepared. The final mixtures contained 500 ppm of Ag+ in 3% PVP solution. Synthesis of nanoparticles was carried out under constant stirring using a magnetic stirrer. The colour change of solutions from colourless to brown suggested a successful reduction of Ag+ to Ag0. Such‐prepared nanoparticles suspensions were stored at 4°C.
2.3 Characterisation of extracts
In order to examine antioxidation activity of prepared plant extracts and infusion measurement of total antioxidant capacity using 1,1‐diphenyl‐1‐picrylhydrazyl (DPPH) radical and total content of phenolic compounds using the Folin–Ciocalteau (F–C) method was utilised. The antioxidant activity of artichoke leaves extracts and infusions was assessed using DPPH assay. In this method, DPPH reagent which is a stable free radical is reduced by phytochemicals extracted from plants possessing antioxidative properties. The DPPH ethanol solution has a deep purple colour and has a maximum absorption value at 517 nm. The presence of antioxidant substances results in DPPH reduction which in turn causes colour changing from deep purple to yellow or colourless. These changes can be recorded by spectrophotometry technique. The decrease in absorption is proportional to the amount of the reduce indicator in the mixture [36]. Around 0.5 mM ethanol solution of DPPH was prepared by dissolving 19.71 mg of DPPH (Sigma‐Aldrich) in 100 mL of ethanol. Subsequently, the such‐prepared solution was diluted with ethanol to the absorbance signal of ∼ 0.9. At first, the absorbance of the free radical solution was recorded. The control sample was prepared by adding to cuvette 2 mL of diluted DPPH ethanol solution, and 60 μL of ethanol (96%) and subsequent absorbance measurement (A 0) was carried out. Tested samples were prepared by adding 2 mL of diluted DPPH solution and 60 μL of infusion or extract. Absorbance measurement was recorded after 10 min at room temperature at 517 nm. For each specimen, the measurement was carried out three times and the average value (A) was calculated. Such obtained results were expressed as percent inhibition and were calculated in accordance with the equation
| (1) |
To determine total phenolic content (TPC) the colourimetric F–C assay was employed. This method is based on electron transfer between phenolic compounds and F–C reagent (POCh SA) (in an alkaline solution as a result of which blue colour formation is observed). The colour change is proportional to the concentration of phenols and can be analysed by measuring the absorbance of the solution with a spectrophotometer at 765 nm. As a standard for absorbance measurement gallic acid (Acros Organics) was utilised and the TPC was expressed as gallic acid equivalents (GAE, mg g−1). As a working solution the gallic acid aqueous solution of the concentration of 5 mg mL−1 was used and exploited to prepare calibration standard at concentrations of 0.05; 0.15; 0.25; 0.35; 0.5 mg mL−1. At first, the absorbance of calibration solutions was recorded by adding to cuvette 20 µL of calibration standards, 1.58 mL of double distilled water and 100 µL of F–C reagent. Subsequently, after 3 min, 300 µL of saturated sodium carbonate (POCh SA) solution was added. Standards were kept in the thermostat set at 40°C for 30 min. The tested samples were prepared in the manner identical to the calibration curve solutions. The measurements were carried out at 765 nm against blank sample without gallic acid in triplicates.
2.4 Characterisation of silver nanoparticles
UV–vis spectral analysis was done by using Evolution 220 spectrophotometer (Thermo Scientific) with a resolution of 1 nm between 300 and 700 nm in cuvettes with an optical path length of 10 mm. An analysis was carried out at room temperature. UV–vis spectral analysis was used to examine long‐term stability of the obtained silver suspensions. In order to determine the average particle size and size distribution of AgNPs dynamic light scattering method (DLS) was employed. The measurement was carried out at room temperature with Zetasizer Nano ZS apparatus (Malvern Instruments Ltd) and average diameter was determined by taking an arithmetic average of five runs. Qualitative phase analysis of AgNPs was performed using X‐ray diffractometry employing XRD‐Philips X'Pert diffractometer with Cu Kα radiation (λ = 0.15418 nm), equipped with graphite monochromator PW 1752/00 operating at 40 kV and 30 mA in 2θ range of 10°–60°. The SEM studies were performed with SEM Zeiss Ultra Plus microscope equipped with EDS microanalysis system Quantax 400 V (Bruker) with ultra‐fast detector with 127 eV energy resolution.
2.4.1 Direct contact cytotoxicity assay
For risk assessment purposes, it is essential to evaluate the biosafety of AgNPs on the in vitro level. This step enables to exclude potentially cytotoxic doses and help to determine the concentrations of AgNPs that remain safe and most relevant for the further investigation. Cytotoxicity evaluation of the selected infusion, AgNPs suspension, and AgNO3 solution used for AgNPs preparation were carried out in accordance with the international standard protocol for cytotoxicity evaluation of medical devices (ISO 10993‐2009‐5).
As target cells in MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide) reduction assay mice L929 fibroblasts (NCTC clone 929: CCL 1) were utilised. Prior the experiments the viability and morphology was confirmed by trypan blue exclusion assay and light microscopy, respectively. Cellular suspension of a density of 2 × 105 cells/mL was distributed in a volume of 100 µL/well into 96 well‐culture microtiter plate. In order to avoid the influence of the medium evaporation on the cell viability, 100 µL of medium was distributed into the peripheral wells and incubated for 24 h (37°C, 5% CO2). Subsequently, all wells with semi‐confluent monolayer were checked with a phase contrast microscope to affirm that cell growth is relatively even across the microtitre plate. Serial twofold dilutions were made from aqueous artichoke extract and its nanoparticles suspension to give working concentrations of 100–0.003% for artichoke infusion and 500–0.015 ppm Ag for AgNPs suspension. Next, cells were incubated with 100 μL of various concentrations of test solutions in cell culture medium, in sextuplicate under standard conditions for 24 h. The positive control (PC) of cells viability was performed using cell suspension in growth medium alone instead of plant extract or AgNPs. Following the 24 h incubation, the cell viability was evaluated microscopically for the presence or otherwise of potential cellular alterations, the medium was replaced with fresh one and 20 µL of MTT solution (Sigma‐Aldrich) was added to each well. After the 4 h incubation period (37°C, 5% CO2) the plates were centrifuged (1400 rpm/min, 10 min), supernatants were removed, and the intracellularly stored MTT formazan was solubilised in 100 µL of isopropanol. Absorbance was measured at a reference wavelength of 570 nm using a plate reader spectrophotometer Victor2 (Wallac, Turku, Finland).
2.4.2 Pro‐inflammatory assay
In order to determine whereas the tested samples induce the inflammatory effect on the in vitro level, THP1‐XBlue™ human monocytic cell line was used. RPMI 1640 growth medium composed of 10% heat‐inactivated fetal bovine serum (Cytogen, Łódź, Polska), 2 mM l ‐glutamine, 25 mM HEPES supplemented with penicillin–streptomycin (100 U/mL–100 µg/mL), and selective agents: normocine (100 µg/mL), and blastocidine (10 µg/mL), were used for cell cultures (37°C, 5% CO2).
The viability of THP1‐XBlue™ cells using trypan blue exclusion assay was carried out to generate a suspension of a density 5 × 105 cells/mL and viability exceeding 95%. Subsequently, 180 μL/well of the cell suspension was introduced into 96‐well culture plates. Then, to selected wells 20 μL of tested samples in serial dilutions (in sextuplicate) were introduced and incubated at standard conditions for 24 h. The tested solutions were selected based on previous cytotoxicity studies. As a negative control (NC) of monocyte activation, the wells filled with cells in cell culture medium were used, while the monocytes cultures treated with E. coli lipopolysaccharide (LPS) O55:B5 (Sigma‐Aldrich) in a final concentration of 1 µg/mL, served as positive controls (PC). After incubation, the plates were centrifuged for 10 min at 1400 rpm/min. Afterward, individual supernatants (20 μL) were transferred into corresponding wells of a plate containing Quanti‐Blue detection reagent (180 μL/well) and incubated for 3 h. Absorbance measurement was performed at 620 nm wavelength using a Spectramax® multi‐detection reader (ThermoFisher Scientific).
3 Results
3.1 Antioxidant activity of C. scolymus extracts and infusions
The total polyphenols content and antioxidant activity were evaluated for two solvents and methods for artichoke extraction. Results are listed in Table 1. Maximum content of TPC was obtained using ethanol extract and corresponded to 102.4 ± 7.4 mg GAE g−1 dry weight (d.w.) The TPC of the ethanol infusion (70.1 ± 1.9 mg GAE g−1 d.w.) is not significantly higher than that of the aqueous extract (57.3 ± 9.4 mg GAE·g−1 d.w.), whereas the TPC of the water infusion (59.3 ± 6.9 mg GAE·g−1 d.w.) is significantly less than ethanol extract. DDPH method evaluated the capacity of compounds present in artichoke solutions to reduce DDPH radical. DPPH radical scavenging activity of each sample range from 26.2 ± 2.3% to 40.5 ± 6.7%. The clear differences between water and ethanol solutions were observed; ethanol infusion and extract have the highest antioxidant activity, 39.4 ± 2.7% and 40.5 ± 6.7%, respectively. Due to the chosen extraction methods and solvents, the water infusion has the lowest value of antioxidant activity.
Table 1.
Total phenolic content and antioxidant activity of artichoke extracts and infusions
| Artichoke solution | Phenolic content, mg GAE g−1 d.w. | Antioxidant activity (Inhibition %) |
|---|---|---|
| aqueous extract | 57.3 ± 9.4 | 28.0 ± 4.2 |
| ethanol extract | 102.4 ± 7.4 | 40.5 ± 6.7 |
| aqueous infusion | 59.3 ± 6.9 | 26.2 ± 2.3 |
| ethanol infusion | 70.1 ± 1.9 | 39.4 ± 2.7 |
3.2 UV–visible spectral analysis
The formation of silver nanoparticles at different time intervals, plant extract and infusion volume and solvents was monitored by UV–visible spectrophotometer at 300–750 nm. An intense absorption band recorded in the wavelength range of 380–450 nm was recognised as AgNPs surface plasmon resonance (SPR) induced by the excitation of free electrons in the nanoparticles. Since the intensity of absorbance of the SPR band was much higher, the solutions in all the cases were diluted with distilled water or ethanol in order to perform absorbance measurements. The detailed description of the prepared silver nanoparticles suspensions is shown in Table 2.
Table 2.
Silver nanoparticles suspensions detailed description
| Artichoke solution content, ml | Artichoke solution type | PVP presence | |||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | |||||
| sample symbol | A | sample number | 1 | 3 | 5 | aqueous extract | − |
| B | aqueous extract | + | |||||
| C | ethanol extract | − | |||||
| D | ethanol extract | + | |||||
| A | 2 | 4 | 6 | aqueous infusion | − | ||
| B | aqueous infusion | + | |||||
| C | ethanol infusion | − | |||||
| D | ethanol infusion | + | |||||
UV–vis absorption spectra of silver nanoparticles synthesised by the reduction of AgNO3 with 5 ml of plant extracts and infusions are shown in Figs. 1 and 2, respectively. It was observed that the formation of AgNPs with the use of aqueous extract and infusion proceed faster without the use of PVP as an additional stabilising agent. However, in the case of ethanol extract and infusion, the reverse trend is observed. Moreover, it is shown that as the nanoparticles formation proceeds, the maxima of surface plasmon resonance bands of AgNPs prepared with aqueous extract and infusion slightly shifted toward the longer wavelength region ∼450 nm as compared to AgNPs synthesised with ethanol solutions. Additionally, recorded bands were wide and non‐symmetric that suggest the obtained nanoparticles were polydisperse. UV–vis absorption spectra of silver nanoparticles prepared by reduction of AgNO3 with ethanol extract and infusion as a reducing agent were sharp and symmetric. Furthermore, the maxima of their surface plasmon resonance bands were shifted towards shorter wavelengths. To study the effect of the extract of C. scolymus, the volume of the extract was varied from 5 to 15 ml at a constant concentration of AgNO3. Figs. 3 and 4 present UV–vis absorption spectra of AgNPs suspension prepared with 10 and 15 ml of extracts, while nanoparticles obtained with 10 and 15 ml of infusion are shown in Figs. 5 and 6, respectively. Experiments show that higher content of plant extracts and infusions in reaction mixtures results in slower AgNPs formation. Moreover, broadening of recorded bands was observed.
Fig. 1.
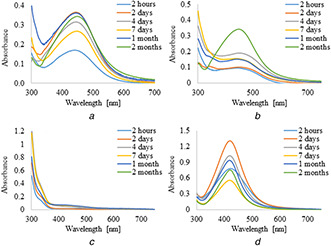
UV–visible spectra of AgNPs obtained using leaves extracts (5 ml) of C. scolymus at different time intervals
Fig. 2.
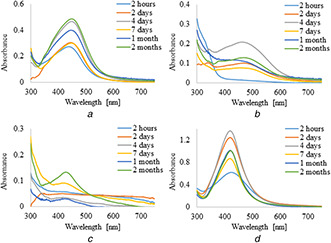
UV–visible spectra of AgNPs obtained using leaves infusions (5 ml) of C. scolymus at different time intervals
Fig. 3.
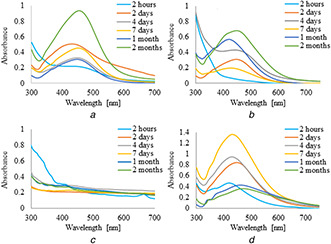
UV–visible spectra of AgNPs obtained using leaves extracts (10 ml) of C. scolymus at different time intervals
Fig. 4.
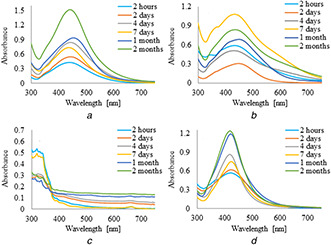
UV–visible spectra of AgNPs obtained using leaves infuisons (10 ml) of C. scolymus at different time intervals
Fig. 5.
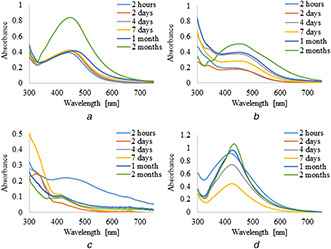
UV–visible spectra of AgNPs obtained using leaves extracts (15 ml) of C. scolymus at different time intervals
Fig. 6.
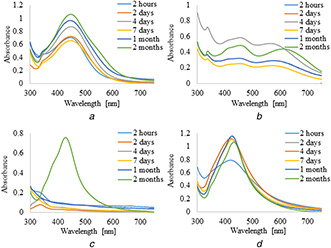
UV–visible spectra of AgNPs obtained using leaves infusions (15 ml) of C. scolymus at different time intervals
3.3 Dynamic light scattering analysis
In order to determine silver nanoparticles average size distribution in selected mixtures dynamic light scattering (DLS) was employed. All measurements in this report were taken at a temperature of 25°C. Based on the results, the mean average diameters of AgNPs were at the range from 42 ± 0.8 nm to 181 ± 6.7 nm. The particles intensity‐averaged diameters and polydispersity indexes are summarised in Table 3. The intensity particle size distributions obtained from the analysis are shown in Fig. 7. AgNPs aqueous suspension prepared with the use of the lowest concentration of infusion (2A) revealed a bimodal distribution with the main peak mode <100 nm diameter and a second peak mode at 10 nm, whereas specimen 2D shows broader size distribution in range of 2 to 400 nm. Samples 2A and 2D were characterised with polydispersity index (PDI) equal to 0.335 ± 0.029 and 0.340 ± 0.053, respectively. For AgNPs suspensions 4A and 3A prepared with 10 ml of extracts the main peak modes were shifted to 60 and 200 nm, respectively. However, sample 4A revealed second peak mode at 5 µm and higher value of PDI (0.428 ± 0.004) in comparison with sample 3A (0.225 ± 0.012). Samples 4D and 3D prepared with addition of PVP exhibited bimodal distribution with the main peak mode located at 150 nm, and second peak mode at 6 and 15 nm, respectively. The particle size distributions of samples 5D and 6D were both monomodal. However, PDI of sample 6D (0.262 ± 0.007), as compared with 5D sample PDI (0.244 ± 0.047) was relatively low. Moreover, maximum intensity of the size distribution was centered at ∼ 200 nm in the case of the specimen 6D, while located at ∼ 220 nm for sample 5D.
Table 3.
Intensity‐averaged diameter in nanometers and the polydispersity index values obtained by DLS
| AgNPs sample | D average ± SD, nm | PDI ± SD |
|---|---|---|
| 2A | 42 ± 0.8 | 0.335 ± 0.029 |
| 2D | 69 ± 2.8 | 0.340 ± 0.053 |
| 4A | 54 ± 1.1 | 0.428 ± 0.004 |
| 3A | 148 ± 0.7 | 0.225 ± 0.012 |
| 4D | 97 ± 1.8 | 0.354 ± 0.058 |
| 3D | 109 ± 8.7 | 0.479 ± 0.031 |
| 6D | 151 ± 1.4 | 0.262 ± 0.007 |
| 5D | 181 ± 6.7 | 0.244 ± 0.047 |
Fig. 7.
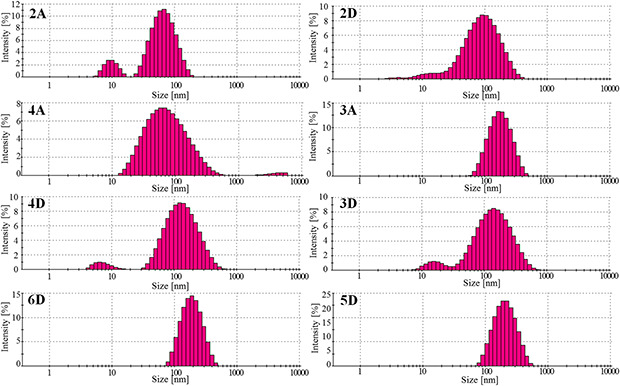
DLS analysis of AgNPs showing percentage distribution of different sized AgNPs suspensions
3.4 XRD study
Fig. 8 shows the X‐ray diffraction pattern (XRD) of silver nanoparticles prepared with C. scolymus aqueous infusion (5 ml). Bragg reflections corresponding to the (111) and (200) sets of lattice planes were recorded. In accordance with International Center for Diffraction Data (No. 04‐0783) these reflections were assigned to the face‐centered cubic (fcc) structure of silver, and the calculated lattice parameter was a = 4.0862 Å. Intense peaks located at angles lower than 30° were related with the applied holder.
Fig. 8.
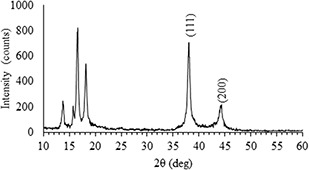
XRD diffraction pattern of AgNPs
3.5 SEM analysis
In order to characterise size and morphology of nanoparticles scanning electron microscope was utilised. SEM image in Fig. 9 shows the morphology of the fabricated silver nanoparticles obtained with artichoke aqueous infusion (5 ml).
Fig. 9.
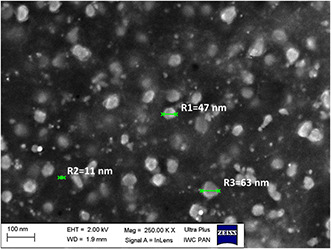
SEM image of AgNPs
As shown in the SEM image, the prepared AgNPs had sphere‐like morphology. Moreover, differences in their size were observed. The presented SEM image revealed non‐uniformity in AgNPs diameters, which stays in agreement with DLS measurement. It was showed using SEM that prepared AgNPs were in the size range from few nm to about 65 nm.
3.6 Direct contact cytotoxicity assay
In accordance with ISO standards, the material with the medical application is considered cytotoxic, when causing the reduction of cell viability by >30%. With a view to excluding the prospective influence of plant extracts on cytotoxicity effect of AgNPs suspension, artichoke infusion, and Ag+ as well were tested with direct contact cytotoxicity assay as well (Fig. 10). For biocompatibility assessment, nanoparticles suspension prepared with 5 ml of infusion due to the most favourable characteristic was used. For this purpose, a dilution series of infusion, AgNPs suspension and Ag ions solution in the range from 500 to 0.015 ppm were prepared. The conducted cytotoxicity tests revealed that the applied artichoke infusion at concentrations of 100 and 50% has strong cytotoxic activity resulting in a significant reduction in the viability of mouse L929 fibroblast cells to 13.1 ± 1.3% and 41.6 ± 1.5%, respectively. However, the obtained results indicate no significant cytotoxic effect when using a fourfold dilution of the initial infusion, a cell viability treated with these compounds remain on the biosafety in vitro level: 82.3 ± 6.7%. Additionally, subsequent tested dilutions do not affect significantly cells viability, which ranges from 80.1 ± 2.1% to 91.2 ± 6.0%. The obtained results clearly demonstrate that the possible cytotoxicity of AgNPs will not be caused by the presence of a plant extract due to the volume of artichoke infusion used for the preparation of AgNPs suspensions.
Fig. 10.
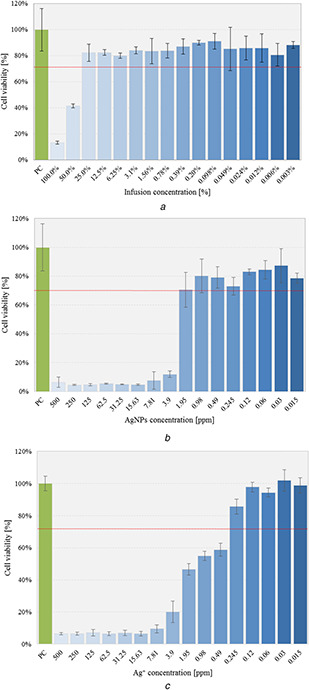
Viability of cells cultured in the presence of tested solutions evaluated in the MTT reduction assay on L929 fibroblasts; diagrams present mean ± standard deviation (n = 6)
(a) Artichoke infusion, (b) AgNPs suspension, (c) Ag ions
Tests carried out on a selected suspension of silver nanoparticles proved the existence of a strong relationship between cytotoxicity and the concentration of the initial suspension of nanoparticles. The applied AgNPs concentrations in the range of 500–3.9 ppm, corresponding to Ag contents, lead to extensive cell lysis, as evidenced by the high cytotoxicity level of ∼88–95%. This may suggest that high AgNPs concentration inhibit proper metabolic activity and division ratio of cells. As can be seen, the acceptable ISO standard dose of silver nanoparticles was a dilution corresponding to 1.95 ppm concentration of AgNPs, for which the cellular survival reached 70.6 ± 6.1%. The research allowed us to determine the dose of silver nanoparticles, which will not cause a cytotoxic effect whereupon applied in the tissue environment. As is shown in Fig. 10 C, mitochondrial activity decreased in an Ag+ concentration‐dependent manner, and induce cell lysis at an Ag+ concentration range of 500–0.49 ppm.
3.7 Pro‐inflammatory assay
Considering the interaction of the immunological cells with material dedicated to the biomedical application that would result in monocyte activation, and thus to local inflammation, tissue damage and even systemic consequences, it is crucial to detect prospective pro‐inflammatory effect caused by possible interference with the tissue environment. Immunocompatibility assay using THP1‐XBlue™ human monocytic cell line (Invivogen) was used to evaluate the pro‐inflammatory activity of the prepared dilutions of nanoparticle suspensions, artichoke infusion and silver nitrate (V) solution. The activation of THP1‐XBlue™ cells via one of the toll‐like receptors (TLR) induces the nuclear factor NF‐κB transcription and subsequently, the release of (SEAP) detected in the cell culture using Quanti‐Blue™ reagent (Invivogen). The physiological level of monocyte's NF‐κB induction remained within the range of: 0.086 ± 0.01, and was significantly upregulated in THP1‐XBlue™ monocytes stimulated with E. coli LPS (1 µg/mL) to the level of 1.47 ± 0.022 (p = 0.001). Nor artichoke infusion extract, either AgNPs or silver ions did induce significant activation of THP1‐XBlue™ monocytes, in comparison to NC (Fig. 11). Although, slight THP1‐XBlue™ activation was observed in cell cultures treated with artichoke infusion in the concentration range from 25% to 0.39%, this activation was not statistically significant; this phenomenon might be explained by the antioxidant and immunostimulatory activity of polyphenols present in the artichoke infusion extract towards innate immunity cells. Additionally, there was no pro‐inflammatory effect in case of THP1‐XBlue™ cells treated with artichoke infusion in concentration below 0.39%. The studies revealed that artichoke infusion diluted below 0.39% does not causing NF‐κB activation in monocytes which might result with a consequent inflammation. Similarly, the levels of monocyte activity induced by the presence of individual AgNPs and silver nitrate(V) dilutions did not exceed the limit values determined by the cellular response of untreated cells.
Fig. 11.
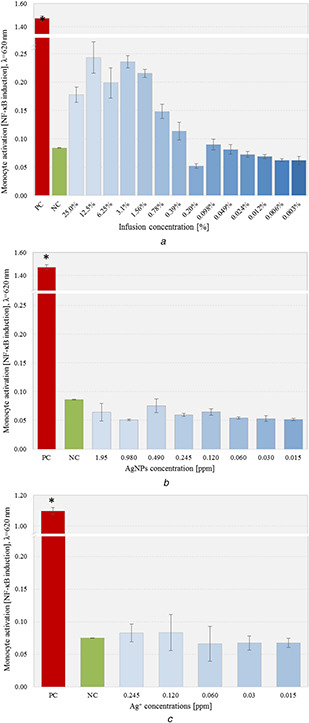
THP1‐XBlue™ screening assay evaluating pro‐inflammatory potential in comparison with positive control (PC) and untreated cells (NC);data are shown as a mean ± standard deviation (n = 6)
(a) Artichoke infusion, (b) AgNPs suspension, (c) Ag ions
4 Discussion
The antioxidant activity of C. scolymus was evaluated using the F–C method and DPPH radical scavenging assay. In general, the concentration of phenolic compounds and antioxidant activity showed a higher value for ethanol systems which is in agreement with the reports of Salem et al. and Emanue et al. [37, 38]. The water extract may contain more non‐phenolic compounds or possess phenolic compounds that contain a smaller number of active groups than ethanol extracts. Moreover, aqueous extract and infusion did not reveal any significant differences in values of TPC and antioxidant activity. Lower TPC and antioxidant activity of aqueous plant solution may also be a result of thermolabile substances decomposition during the processing of plant material extraction by water due to the higher boiling point of water than ethanol. C. scolymus leaf extracts and infusions were used as a reducing and capping agents to prepare AgNPs on the basis of cost‐effectiveness, ease of availability and its health‐promoting property. In our study, we confirmed the synthesis of Ag nanoparticles with the proposed plant extracts visually by the change of colour of a reaction mixture, with UV–visible spectral analysis which showed absorption band in the characteristic visible region 380–450 nm due to the surface plasmon excitation. Interestingly, UV–visible spectral analysis showed that only aqueous infusions and extracts possess the ability to reduce Ag+ to Ag0 and stabilising the formed nanostructures as well. However, AgNPs formation was observed in a reaction mixture containing ethanol extracts and infusions with additional polymer capping agent (PVP). Therefore, it can be concluded that extraction of C. scolymus with ethanol leads to isolation phytochemicals with strong antioxidant activity and no stabilising ability. Dosi et al. [39] performed an analysis of the nutritional value of artichoke globe and revealed that 100 g of the fresh plant consists of ∼3 g of proteins, depending on sampling area. Proteins, in addition to their nutritional properties, were also identified as an excellent capping agent in silver nanoparticles preparation caused by strong affinity to metals [40]. However, proteins solubility studies performed by Pace et al. [41] suggest that their solubility is markedly lower in polar solvents such as ethanol, therefore no stabilising ability of applied extract and infusion can be explained. The results of this work showed that C. scolymus, when a proper extraction solvent is established, could serve as a reducing and stabilising agent in silver nanoparticles preparation.
In order to perform an analysis of the size distribution of nanoparticles in suspensions DLS method was employed. The proposed technique allows the measurement of particle sizes down to 1–2 nm. However, it is worth emphasising that for the sake of lack of fractionation of suspension the measurement can be affected by agglomerates and polydispersity. Differences in particles sizes measured with various methods were well reported by Dieckmann et al. [42] and showed that DLS measured AgNPs size was slightly larger than the TEM and XRD sizes. This reflects the fact that DLS measures the hydrodynamic diameter that includes the particle and molecules and ions attached to its surface. Nevertheless, the DLS technique due to its facility and accessibility plays an important role in optimising the size of nanoparticles. DLS analysis showed that the particles size distributions for most samples were bimodal. The lowest values of intensity‐averaged diameters were observed for samples prepared with the lowest concentration of extracts. It was also observed that the increase in extract volume used in AgNPs synthesis contribute to the increase in average diameter of NPs and can be the result of plant organic matrix constituents on particles surface attaching as well as their presence in solution in general; that effect was especially clear for the samples 2D, 4D, and 6D. Moreover, it is shown that maximum intensities of the size distribution for AgNPs suspensions prepared with the use of plant infusions were placed at higher values of nanoparticles diameters as compared to NPs suspensions obtained with extracts that can be associated with lower TPC concentrations in extracts, resulting in less effective nanostructures stabilisation effect. XRD analysis revealed that the nanoparticles with the fcc structure were formed. Analysis of the prepared AgNPs morphology performed with SEM confirmed nanosized structure formation in the range from few nanometres to ∼65 nm.
For biocompatibility assessment, nanoparticles suspension prepared with 5 ml of infusion was taken. The results show dose‐dependent cytotoxic effects of AgNPs, which reach up to 95% as measured by mitochondrial activity. However, AgNPs used at concentrations below 2 ppm did not induce cytotoxic effect towards L929 fibroblasts. The viability of cells treated with various dilutions of artichoke leaves infusion reached acceptable value defined in ISO standard for infusion diluted at least four times for which cell viability was 82.3 ± 6.7%. The cytotoxic effect of the infusion used in the highest concentrations can be associated with a high concentration of organic compounds that can cause cell lysis or inhibit its metabolic activity. However, this effect was dose dependent and it was not observed at lower infusion concentration. Therefore, it can be concluded that the observed cytotoxicity of the studied AgNPs suspension at higher concentration was not associated with the applied infusion, but the cytotoxicity mediated by the AgNPs itself. A number of studies suggested that AgNPs toxicity is closely related with ROS generation and oxidative stress which induced cell death. Generally, it is believed that the interaction of silver nanoparticles with mammalian cells can cause membrane damage, including altering of membrane permeability [43, 44]. Studies performed by Cheng et al. [45] showed that AgNPs can cause cell membranes damage resulting in cellular apoptosis through oxidative stress. Moreover, Arora et al. proved that the presence of AgNPs in the mitochondria of primary fibroblast and liver cells can trigger the antioxidant mechanisms [46]. The studies on the influence of nanoparticles size on the viability of L929 cell line was performed by Park et al. [27]. The authors showed that AgNPs with 20 nm diameter were most effective in decreasing metabolic activity of L929 cells than larger particles (80 and 113 nm). Silver nitrate(V) solution at variable concentrations was tested towards potential cytotoxic effect as well. According to the obtained results, the cytotoxic effect of Ag+ was proved to be dose dependent and was observed at a concentration range of 500–0.49 ppm. In accordance with the obtained results, AgNPs do not induce cell lysis at eight times higher concentration in comparison with Ag+. Cytotoxicity of Ag+ with the use of AgNO3 as a source of silver ions was thoroughly investigated by many researchers, and similarly to AgNPs, cytotoxicity of silver ions was dose dependent. Studies performed by Liu et al. indicate that Ag+ at a concentration of 1 ppm killing 80% of investigated fibroblasts (L929 cell line) while treating cells with 10 ppm of Ag+ results in the whole colony death [47]. Cytotoxic effect of Ag ions was also examined by Heidenau et al. [48]. In accordance with this research, lethal dose 50 (LD50) of ionic silver for L929 fibroblasts is 3.5 × 10−3 mM (0.375 ppm). Furthermore, this cell line was more sensitive to the effects of silver nanoparticles compared with murine peritoneal macrophage cell line (RAW 264). Here we report that the obtained nanoparticles do not cause the induction of NF‐κB transcription factor responsible for monocyte activation. Several studies reported on the anti‐inflammatory properties of the artichoke‐derived compounds. Majority of the reports on downregulatory properties of artichoke extracts were based on the samples obtained by the ethanol extraction, which contain higher polyphenolics content, enriched with tannins and flavonoids [37, 49, 50]. However, the aqueous phase of the artichoke extracts contain polysaccharides such as inuline‐insoluble sugar composed of β (2‐1) polyfructofuranosyl α‐d ‐glucose polymer chains. Plants of the Compositae family, including artichoke produce inulin as a storage carbohydrate, which according to recent studies regulate the gut microbiota as well as downregulates the obesity‐ and diabetes‐mediated inflammation, by the interaction with monocytes [51]. Inulin was shown to interact with monocytes and enhance LPS‐mediated IL‐10 secretion via TLR dependent manner and that the NF‐κB inhibitor fully prevented these effects [51]. Whereas Gill et al. shown that inulin demonstrated pro‐inflammatory properties and induce intracellular TNF‐α expression in the monocytes [52]. Therefore, the slight activation of THP1‐XBlue™ monocytes observed and mediated by aqueous artichoke extract might be the effect of the inulin‐mediated activation. It can be concluded that the induction of the NF‐κB transcription factor in monocytes was not caused by nanoparticles, but the activation of monocytes could be associated with the presence of reducing or carbohydrate compounds present in the infusion, which after reduction of silver ions to Ag0 lost their pro‐inflammatory properties. Our results showed that this effect can be removed by applying the appropriate dilution of the infusion. Furthermore, Golinska and Dahm [53] observed the activation of NF‐κB factor of biogenic silver nanoparticles at the concentration range of 10–100 μg/mL, the mean size of obtained nanoparticles was 19.9 nm (±13 nm), which suggests that the nature of this effect is AgNPs size dependent. Here we showed that AgNPs prepared via phytochemical‐mediate synthesis do not exhibit cytotoxicity at low concentration and possess no ability to activation of monocytes. Pro‐inflammatory assessment of Ag+ originating from AgNO3 was examined by Herzog et al. [54]. The authors present a comparison of dose‐dependent releasing of TNF‐α marker examined with enzyme‐linked ELISA assay and proved that Ag+ did not induce TNF‐α releasing, which stays in agreement with results obtained in our investigation.
Based on the obtained data, we assume that synthesised AgNPs can be a promising and safe medical agent and should be further investigated towards their antimicrobial properties.
5 Conclusion
This study presents the method of obtaining silver nanoparticles using water and ethanol extracts and infusions of C. scolymus. Thanks to the conducted research, the appropriate conditions for the synthesis of AgNPs with the use of plant raw material have been selected. In the case of aqueous extracts and infusions, the synthesis of nanoparticles did not require the presence of an additional polymeric stabiliser. The experiment indicates that the plant extracts and infusions were not only efficient in reducing Ag+ to nanoparticles but also played a crucial role in stabilising the obtained nanostructures. Moreover, it was proved that ethanol extracts and infusions do not reveal the stabilising effect on Ag nanoparticles, however, the formation of nanoparticles was recorded when PVP addition was applied. Additionally, a significant influence of the applied volume of plant extracts resulting in differences in average particles size was recorded. Furthermore, the biocompatibility tests of the obtained AgNPs showed no effect of activation of NF‐κB. Considering the potential application of nanoparticles in the biomedical field, attention should be paid to their impact on living cells. Therefore, direct contact cytotoxicity assay was also carried out, determining the maximum dose of AgNPs not causing cells lysis.
6 Acknowledgments
We gratefully acknowledge financial support from National Science Centre in the frame of UMO‐2016/21/D/ST8/01697.
7 References
- 1. Ang L.Y. Lim M.E. Ong L.C. et al.: ‘Applications of upconversion nanoparticles in imaging, detection and therapy review’, Nanomedicine, 2011, 6, (7), pp. 1273 –1288 [DOI] [PubMed] [Google Scholar]
- 2. Aberasturi D.J. De Serrano‐montes A.B. Liz‐marzán L.M.: ‘Modern applications of plasmonic nanoparticles: from energy to health’, Adv Opt Mater., 2015, 3, (5), pp. 1 –16 [Google Scholar]
- 3. Haider A. Kang I.‐K.: ‘Preparation of silver nanoparticles and their industrial and biomedical applications: a comprehensive review’, Adv. Mater. Sci. Eng., 2015, 2015, pp. 1 –16 [Google Scholar]
- 4. Liu W.: ‘Nanoparticles and their biological and environmental applications’, J. Biosci. Bioeng., 2006, 102, (1), pp. 1 –7 [DOI] [PubMed] [Google Scholar]
- 5. Kaur A. Gupta U.: ‘A review on applications of nanoparticles for the preconcentration of environmental pollutants’, J. Mater. Chem., 2009, 19, (44), pp. 8279 –8289 [Google Scholar]
- 6. Tang S.C.N. Lo I.M.C.: ‘Magnetic nanoparticles: essential factors for sustainable environmental applications’, Water Res.., 2013, 47, (8), pp. 2613 –2632 [DOI] [PubMed] [Google Scholar]
- 7. Kataja M. Hakala T.K. Julku A. et al.: ‘Surface lattice resonances and magneto‐optical response in magnetic nanoparticle arrays’, 2015. [DOI] [PMC free article] [PubMed]
- 8. Permyakov D. Sinev I. Markovich D. et al.: ‘Probing magnetic and electric optical responses of silicon nanoparticles’, Appl. Phys. Lett., 2015, 106, p. 1171110 [Google Scholar]
- 9. Liao J. Blok S. Van Der Molen J. et al.: ‘Ordered nanoparticle arrays interconnected by molecular linkers: electronic and optoelectronic’, Chem. Soc. Rev., 2015, 44, (4), 999 –1014 [DOI] [PubMed] [Google Scholar]
- 10. Holla G. Yeluri R. Munshi A.K.: Evaluation of minimum inhibitory and minimum bactericidal concentration of nano‐silver base inorganic anti‐microbial agent (Novaron ®) against streptococcus mutans, Contemp. Clin. Dentistry., 2012, 3, (3), 288 –293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lara H.H. Garza‐treviño E.N. Ixtepan‐turrent L. et al.: ‘Silver nanoparticles are broad‐spectrum bactericidal and virucidal compounds’, J. Nanobiotechnol., 2011, 9, (30), pp. 2 –9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jebali A.L.I. Haji F. Hajjar E. et al.: ‘Silver and gold nanostructures: antifungal property of different shapes of these nanostructures on Candida species’, Int. Soc. Hum. Anim. Mycol., 2013, 52, (1), pp. 1 –7 [DOI] [PubMed] [Google Scholar]
- 13. Medda S. Hajra A. Dey U.: ‘Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp.’, Appl. Nanosci., 2015, 5, (7), pp. 875 –880 [Google Scholar]
- 14. Balasubramani G. Ramkumar R. Krishnaveni N. et al.: ‘Structural characterization, antioxidant and anticancer properties of gold nanoparticles synthesized from leaf extract(decoction)of Antigonon leptopus Hook. & Arn.’, J. Trace Elem. Med. Biol., 2015, 30, pp. 83 –89 [DOI] [PubMed] [Google Scholar]
- 15. Transactions C.S. Lokina S. Narayanan V.: ‘Antimicrobial and anticancer activity of gold nanoparticles synthesized from grapes fruit extract †’, Chem. Sci. Trans., 2013, 2, (S1), pp. S105 –S110 [Google Scholar]
- 16. Li Q. Li X.: ‘Nanosilver particles in medical applications: synthesis, performance, and toxicity’, Int. J. Nanomed., 2014, 9, (1), pp. 2399 –2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boonkaew B. Suwanpreuksa P. Cuttle L. et al.: ‘Hydrogels containing silver nanoparticles for burn wounds show antimicrobial activity without cytotoxicity’, J. Appl. Polym. Sci., 2014, 131, (9), 10 pp. [Google Scholar]
- 18. Beyth N. Houri‐haddad Y. Domb A. et al.: ‘Alternative antimicrobial approach: nano‐antimicrobial materials’, Evidense‐Based Complement. Altern. Med., 2015, 2015, 16pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Press D.: ‘Nanomaterials for alternative antibacterial therapy’, Int. J. Nanomed., 2017, 2017, pp. 8211 –8225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pal S. Tak Y.K. Song J.M.: ‘Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticlea study of the gram‐negative bacterium Escherichia coli ’, Appl. Environ. Biol., 2007, 73, (6), pp. 1712 –1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raza M.A. Kanwal Z. Rauf A. et al.: ‘Size‐ and shape‐dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes’, no date [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prabhu S. Poulose E.K.: ‘Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects’, Int. Nano Lett., 2012, 2, (32), pp. 1 –10 [Google Scholar]
- 23. Sharma V.K. Yngard R.A. Lin Y.: ‘Silver nanoparticles: green synthesis and their antimicrobial activities’, Adv. Colloid Interface Sci., 2009, 145, (1–2), pp. 83 –96 [DOI] [PubMed] [Google Scholar]
- 24. Elia P. Zach R. Hazan S.: ‘Green synthesis of gold nanoparticles using plant extracts as reducing agents’, Int. J. Nanomed., 2014, 9, (1), pp. 4007 –4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malik P. Shankar R. Malik V. et al.: ‘Green chemistry based benign routes for nanoparticle synthesis nanoparticles: a glance’, J. Nanoparticles, 2014, 2014, 14pp. [Google Scholar]
- 26. Kumar V. Yadav S.K.: ‘Plant‐mediated synthesis of silver and gold nanoparticles and their applications’, J. Chem. Technol. Biotechnol., 2009, 176061, pp. 151 –157 [Google Scholar]
- 27. Rangboo V. Noroozi M. Zavoshy R. et al.: ‘The effect of artichoke leaf extract on alanine aminotransferase and aspartate aminotransferase in the patients with nonalcoholic steatohepatitis’, Int. J. Hepatol. 2016, 2016, 6pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mileo A.M. Venere D.D.I. Linsalata V. et al.: ‘Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer cell line MDA‐MB231’, J. Cell Physiol., 2011, 227, (9), pp. 3301 –3309 [DOI] [PubMed] [Google Scholar]
- 29. Rondanelli M. Giacosa A. Opizzi A. et al.: ‘Beneficial effects of artichoke leaf extract supplementation on increasing HDL‐cholesterol in subjects with primary mild hypercholesterolaemia: a double‐blind, randomized, placebo‐controlled trial’, Int. J. Food Sci. Nutrition, 2013, 64, pp. 7 –15 [DOI] [PubMed] [Google Scholar]
- 30. Oma F.R.A.T. Arbera Ä.S. Erreres F.E.F.: ‘Artichoke (Cynara scolymus L.) byproducts as a potential source of health‐promoting antioxidant phenolics’, J. Agri. Food Chem., 2002, 50, (12), pp. 3458 –3464 [DOI] [PubMed] [Google Scholar]
- 31. Aymond R.L.O.: ‘Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities’, J. Agri. Food Chem., 2004, 52, (24), pp. 7272 –7278 [DOI] [PubMed] [Google Scholar]
- 32. Li H. Xia N. Brausch I. et al.: ‘Flavonoids from artichoke (Cynara scolymus L.) up‐regulate endothelial‐type nitric‐oxide synthase gene expression in human endothelial cells’, J. Pharmacol. Exp. Therapeutics, 2004, 310, (3), pp. 926 –932 [DOI] [PubMed] [Google Scholar]
- 33. Martirosyan A. Grintzalis K. Polet M. et al.: ‘Tuning the inflammatory response to silver nanoparticles via quercetin in Caco‐2 (co‐) cultures as model of the human intestinal mucosa’, Toxicol. Lett., 2016, 253, pp. 36 –45 [DOI] [PubMed] [Google Scholar]
- 34. Sampaio S. Viana J.C.: ‘Production of silver nanoparticles by green synthesis using artichoke (Cynara scolymus L.) aqueous extract and measurement of their electrical conductivity’, Adv. Nat. Sci., Nanosci. Nanotechnol., 2018, 9, (4), p. 045002 [Google Scholar]
- 35. Erci F. Cakir‐Koc R. Isildak I.: ‘Green synthesis of silver nanoparticles using Thymbra spicata L. var. spicata (zahter) aqueous leaf extract and evaluation of their morphology‐dependent antibacterial and cytotoxic activity’, Artif. Cells Nanomed. Biotechnol., 2017, 46, (Suppl. 1), pp. 1 –9 [DOI] [PubMed] [Google Scholar]
- 36. Cuvelier M.E. Berset C.: ‘Use of a free radical method to evaluate antioxidant activity’, Food Sci. Technol., 1995, 28, (1), pp. 25 –30 [Google Scholar]
- 37. Salem M. Ben Affes H. Athmouni K. et al.: ‘Chemicals compositions, antioxidant and anti‐inflammatory activity of cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC’, Evidense‐Based Complement. Altern. Med., 2017, 2017, 14pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alghazeer R. El‐saltani H. Saleh N.A. et al.: ‘Antioxidant and antimicrobial activities of Cynara scolymus l. Rhizomes’, Mod. Appl. Sci., 2012, 6, (7), pp. 54 –63 [Google Scholar]
- 39. Dosi R. Daniele A. Guida V. et al.: ‘Nutritional and metabolic profiling of the Globe artichoke (Cynara scolymus L. ‘Capuanella’ heads) in province of Caserta, Italy’, 2013. [Google Scholar]
- 40. Using N. Yadav A. Rai M.: ‘Bioreduction and mechanistic aspects involved in the synthesis of silver nanoparticles using holarrhena antidysenterica’, J. Bionanosci., 2011, 5, (1), pp. 70 –73 [Google Scholar]
- 41. Pace C.N. Treviño S. Prabhakaran E. et al.: ‘Protein structure, stability and solubility in water and other solvents’, 2004, 359, (1448), pp. 1234 –1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dieckmann Y. Co H. Hofmann H. et al.: ‘Particle size distribution measurements of manganese‐doped ZnS nanoparticles’, Anal. Chem., 2009, 81, (10), pp. 3889 –3895 [DOI] [PubMed] [Google Scholar]
- 43. Mcshan D. Ray P.C. Yu H.: ‘Molecular toxicity mechanism of nanosilver’, J. Food Drug Anal., 2014, 22, (1), pp. 116 –127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baruwati B. Simmons S.O. Varma R.S. et al.: ‘‘Green’ synthesized and coated nanosilver alters the membrane permeability of barrier (intestinal, brain endothelial) cells and stimulates oxidative stress pathways in neurons’, ACS Sustainable Chem. Eng., 2013, 1, (7), pp. 753 –759 [Google Scholar]
- 45. Cheng X. Zhang W. Ji Y. et al.: ‘Revealing silver cytotoxicity using Au nanorods/Ag shell nanostructures: disrupting cell membrane and causing apoptosis through oxidative damage’, RSC Adv., 2013, 3, (7), pp. 2296 –2305 [Google Scholar]
- 46. Arora S. Jain J. Rajwade J.M. et al.: ‘Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells’, Toxicol. Appl. Pharmacol., 2009, 236, (3), pp. 310 –318 [DOI] [PubMed] [Google Scholar]
- 47. Liu H.L. Dai S.A. Fu K.Y. et al.: ‘Antibacterial properties of silver nanoparticles in three different sizes and their nanocomposites with a new waterborne polyurethane’, Int. J. Nanomedicine, 2010, 5, (1), pp. 1017 –1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heidenau F. Mittelmeier W. Detsch R. et al.: ‘A novel antibacterial titania coating: metal ion toxicity and in vitro surface colonization’, J. Mater. Sci. Mater. Med., 2005, 16, (10), pp. 883 –888 [DOI] [PubMed] [Google Scholar]
- 49. Jung Y.J. Kim B.O. Kwak J.H. et al.: ‘Inhibitory effect of methyl 2‐(4 # ‐methoxy‐4 # ‐oxobutanamido) benzoate from Jerusalem artichoke (Helianthus tuberosus) on inflammatory paracrine loop between macrophages and adipocytes’, J. Agri. Food Chem., 2016, 64, (49), pp. 9317 –9325 [DOI] [PubMed] [Google Scholar]
- 50. Samal L. Chaturvedi B. Saikumar G. et al.: ‘Prebiotic potential of Jerusalem artichoke (Helianthus tuberosus L.) in Wistar rats: effects of levels of supplementation on hindgut fermentation, intestinal morphology, blood metabolites and immune response’, J. Sci. Food Agri., 2015, 95, (8), pp. 1689 –1696 [DOI] [PubMed] [Google Scholar]
- 51. Ahmed W. Rashid S.: ‘Functional and therapeutic potential of inulin: a comprehensive review’, Crit. Rev. Food Sci. Nutr., 2019, 59, (1), pp. 1 –13 [DOI] [PubMed] [Google Scholar]
- 52. Gill S.K. Islam N. Shaw I. et al.: ‘Immunomodulatory effects of natural polysaccharides assessed in human whole blood culture and THP‐1 cells show greater sensitivity of whole blood culture’, Int. Immunopharmacol., 2016, 36, pp. 315 –323 [DOI] [PubMed] [Google Scholar]
- 53. Golinska M.S.P. Dahm K.R.H.: ‘Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles’, Med. Microbiol. Immunol., 2016, 205, (6), pp. 603 –613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Herzog F. Clift M.J.D. Piccapietra F. et al.: ‘Exposure of silver‐nanoparticles and silver‐ions to lung cells in vitro at the air‐liquid interface’, Part. Fibre Toxicol., 2013, 10, (1), pp. 11 –14 [DOI] [PMC free article] [PubMed] [Google Scholar]


