Abstract
In this study, extracellular extract of plant growth promoting bacterium, Nitrobacter sp. is used for the bioconversion of AgNO3 (silver nitrate) into Ag2 O (silver oxide nanoparticles). It is an easy, ecofriendly and single step method for Ag2 O NPs synthesis. The bio‐synthesized nanoparticles were characterized using different techniques. UV‐Vis results showed the maximum absorbance around 450 nm. XRD result shows the particles to have faced centered cubic (fcc) crystalline nature. FTIR analysis reveals the functional groups that are involved in bioconversion such as C–N, N–H and C=O. Energy‐dispersive X‐ray spectroscopy (EDAX) spectrum confirms that the prepared nanoparticle is Ag2 O NPs. Particle size distribution result reveals that the average particle size is around 40 nm. The synthesized Ag2 O NPs found to be almost spherical in shape. Biosynthesized Ag2 O NPs possess good antibacterial activity against selected Gram positive and Gram negative bacterial strains namely Salmonella typhimurium, Staphylococcus aureus, Escherichia coli and Klebsiella pneumoniae when compared to standard antibiotic. In addition, Ag2 O NPs exhibits excellent free radical scavenging activity with respect to dosage. Thus, this study is a new approach to use soil bacterial extract for the production of Ag2 O NPs for biomedical application.
Inspec keywords: nanomedicine, nanoparticles, silver compounds, antibacterial activity, ultraviolet spectra, visible spectra, X‐ray diffraction, Fourier transform infrared spectra, X‐ray chemical analysis, particle size, free radicals
Other keywords: free radical scavenging activity, Ag2 O, AgNO3 , Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, Salmonella typhimurium, Gram negative bacterial strains, Gram positive bacterial strains, particle size distribution, energy‐dispersive X‐ray spectroscopy spectrum, functional groups, Fourier transform infrared analysis, faced centred cubic crystalline nature, XRD, UV‐Vis results, bio‐synthesised nanoparticles, silver oxide nanoparticles, silver nitrate bioconversion, plant growth promoting bacterium, extracellular extract, biomedical application, antibacterial potential, antioxidant potential, Ag2 O NPs, extract mediated biosynthesis, Nitrobacter sp
1 Introduction
Currently, nanotechnology is promising and upcoming area which deals with nanoparticles for various applications. In recent times, biosynthesis of nanoparticles has quickly augmented using different sources such as different microorganisms and plant extracts due to its cost effective, less time consuming and eco‐friendly route [1]. Compare with almost all nanoparticles, silver oxide nanoparticles (Ag2 O NPs) comprise efficient and guarantee different applications in the area of nanotechnology such as catalytic, antibacterial properties and electrical conductivity [2].
There are different approaches which are widely used in the production of Ag2 O NPs, such as chemical and physical methods. However, these methods are not much promising due to the use of toxic chemicals as stabilising and reducing agents. Hence, the biosynthesis approach is the best reliable and eco‐friendly approach for the synthesis of silver oxide nanoparticles [3]. The utilisation of bacterial extracellular extracts as stabilising and reducing agents was found to be more beneficial than the use of other bio sources because it is very easy to use and a more eco‐friendly approach [4].
Several modern researches mainly focused to utilise different bacteria as prospective agents for the biosynthesis of silver oxide nanoparticles such as Bacillus cereus [5], Chryseobacterium artocarpi [6], Lactobacillus casei [7], Escherichia coli [8] and Pseudomonas putida [9]. However, till date, there is no evidence about the use of Nitrobacter sp. for the biosynthesis of Ag2 O NPs.
Nitrobacter sp. is a plant growth promoting bacterium which is Gram negative, rod shaped, chemoautotrophic and motile [10]. This bacterium is well known to have the capacity to promote the growth of plants by nitrogen fixation [11]. The bacterium requires different nitrite oxidoreductase enzymes for nitrogen fixation [12]. Thus, this essential soil bacterium can be a useful candidate for Ag2 O NPs production.
Thus, keeping the above findings in mind, we have planned our experiment for the synthesis of Ag2 O NPs using Nitrobacter sp. extracellular extract. This plan of research will provide a new area of biosynthesis using a novel, non‐toxic and eco‐friendly approach for the production of Ag2 O NPs. The produced Ag2 O NPs will also be used to analyse its antibacterial and antioxidant potentials.
2 Materials and methods
2.1 Materials used
Silver nitrate (AgNO3) was procured from Sigma Aldrich (ACS reagent) with 99% purity and was used without any additional purification steps. Mueller Hinton Agar, Stanier's medium and 2, 2‐Diphenyl‐1‐picrylhydrazyl (DPPH) were procured from HiMedia Laboratories, Mumbai, India for the analysis of antibacterial and antioxidant activities.
2.2 Biosynthesis of Ag2 O NPs
Nitrobacter sp. (NCIM 5067), the bacterium used in the experiment was obtained from the National Collection of Industrial Microorganisms (NCIM), Pune, India. Stanier's modified medium (250 ml), a special medium for the bacterium was inoculated with the culture, followed by incubation at 37°C for 24 h in a shaker at 300 rpm. After incubation, the medium was centrifuged at 6000 rpm for 30 min to collect the supernatant (extracellular extract). The obtained supernatant was further used for the biosynthesis of Ag2 O NPs.
The extracellular extract and 0.1 M silver nitrate were mixed in the volume ratio of 1:1 and was incubated for 24 h under dark condition for the bioconversion. After 24 h of incubation, the change in colour from light brown to dark colloidal brown was observed (Fig. 1). The colloidal brown solution was precipitated by centrifugation at 6000 rpm for 30 min. The supernatant was removed and the obtained pellet was re‐suspended 3 times in deionised water and centrifuged again at 6000 rpm for 30 min to achieve a clear supernatant. Thus, the obtained final pellet was dried in a hot air oven for 24 h at 60°C. The dried powder was further crushed using mortar and pestle into a fine powder and kept in a dry container and used further for its complete characterisation.
Fig. 1.

Biosynthesis of Ag2 O NPs, A: AgNO3, B: Extracellular extract and C: Ag2 O NPs
2.3 Characterisation of Ag2 O NPs
To study the different properties of Ag2 O NPs, the powder was subjected to different characterisation techniques. The bioconversion of Ag2 O NPs was confirmed via evaluating the wave length of the powder suspension by UV‐Visible spectrum under spectrophotometer (LAMBDA 35, PerkinElmer, USA). The scanning range for the sample was between 200–800 nm at a speed of 480 nm per minute. The nature and the crystalline phase of the reduced Ag2 O NPs samples were identified by X‐ray powder diffraction patterns (X'Pert PRO; PANalytical, the Netherlands) using CuKα as a radiation (l51.54060 Å) source. The diffractometer was scanned in the 2θ range from 10° to 80° at scanning rate of 5° min−1. The observed peak positions and the relative intensities of the powder pattern were identified in comparison with the reference powder diffraction data.
The presence of functional groups and chemical bonds on the surface of Ag2 O NPs was investigated using Fourier transform infrared (FTIR) spectrophotometer (Spectrum 100; PerkinElmer, USA). The sample spectrum was recorded between the ranges of 4000–400 cm−1 at a resolution of 4 cm−1. The presence of silver was confirmed by energy dispersive spectrum (EDS) (EDX‐720; Shimadzu, Japan). The samples were focused directly at 10 mm/5 mm on a thin film of Mylar without further sample preparation. Particle size distribution (PSD) was used to determine the particle size using particle size analyser (Nanophox; Sympatec, Germany) using the well‐known dynamic light‐scattering technique (DLS). Transmission electron microscopy (TEM) was used to figure out the dimension and the form of Ag2 O NPs. The sample was analysed using high resolution TEM (TEMCM200; Philips, USA). For analysis, the well dispersed samples were loaded on the copper grids and scanned at 120 kV. Particle size was then manually calculated by taking into account, the maximum and minimum particle size as marked in the observed image.
2.4 Antibacterial activity
Antibacterial activity of the synthesised Ag2 O NPs was determined against selected Gram positive and Gram negative clinical bacterial strains (collected from NCIM, India) such as Salmonella typhimurium (NCIM 2501), Staphylococcus aureus (NCIM 2127), E. coli (NCIM 2065) and Klebsiella pneumoniae (NCIM 2883) using Kirby–Bauer disk diffusion method [13] in Muller–Hinton Agar medium. Sterile media (10 ml) was prepared and poured into petri dishes. Once the medium got solidified, 100 μl of freshly prepared overnight inoculums were spread on to it, using sterile swabs. Sterile filter disc was loaded over the solid medium, separated at equal distance. The prepared nanoparticles suspension was loaded over the disk under aseptic condition. The plates were subsequently incubated at 37°C for 24 to 48 h after which the zone of inhibition (in mm diameter) was measured and tabulated.
2.5 Antioxidant activity
Antioxidant activity of the Ag2 O NPs was analysed by following DPPH method available in literature with minor modifications [14]. Different masses of Ag2 O NPs such as 1, 5, 10 and 100 mg was taken and mixed with 1.7 ml of freshly prepared DPPH solution and vortexed for about 3 min. Control (C) tube contained only DPPH reagent whereas Ag2 O NPs added tubes served as test (T). The mixture was then incubated for 30 min at room temperature followed by centrifugation at 1000 rpm for 2 min. The absorbance of the supernatant was then measured using UV–Vis spectrophotometer (U‐2900/2910, Japan) at 517 nm using methanol as blank solution. The inhibition percentage of the Ag2 O NPs was calculated by applying the formula as given below
whereas, CA (control) and TA (test) absorbance.
3 Results and discussion
3.1 UV‐Visible analysis
After 24 h of incubation of the extracellular extract and 01 M silver nitrate mixture, the change in colour (Fig. 1) of the solution from yellow to dark brown indicated the bioconversion of silver nitrate into Ag2 O NPs. The change in colour might be due to the changes in the excitation energy of the particle's surface plasma resonance. Similar result was observed for Bacillus licheniformis and Fusarium oxysporum, used for the production of silver nanoparticles [15, 16]. In addition, the formation of nanoparticles may be attributed to the nitrate reductase enzyme or the proteins in the supernatant [17, 18]. The prepared powder was dispersed in deionised water for UV‐Visible analysis. UV‐Visible analysis (Fig. 2) showed that the wavelength for silver nanoparticles production ranged between 350 to 450 nm whereas for extracellular extract and AgNO3 no absorption peak was observed. The shift of colour is due to the variation in shape and size of the silver nanoparticles [19].
Fig. 2.
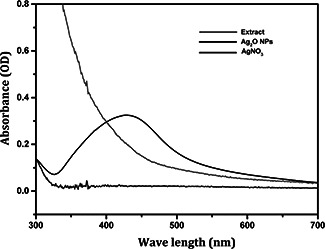
UV‐Visible absorbance of Ag2 O NPs
3.2 XRD analysis
The XRD analysis result shown in Fig. 3 revealed the crystalline nature of the prepared Ag2 O NPs. The observed 2θ values were well matched with ICDD standard data (JCPDS File No: 43‐0997), which clearly indicated that the synthesised nanoparticles (Ag2 O) had cubic crystal system. XRD pattern exhibited typical reflections from (110), (111), (211), (220), (310), and (321) Miller's planes at ∼28°, ∼32°, ∼48°, ∼54°, ∼58° and ∼68°, respectively, and correlated with the literature results [20].
Fig. 3.
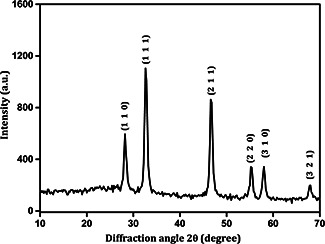
XRD pattern of Ag2 O NPs
3.3 FTIR analysis
FTIR result as represented in Fig. 4 revealed the presence of functional groups in the synthesised Ag2 O NPs. The presence of proteins was observed in the range of 1036–1150 cm−1. The signature of phenols was indicated in the band 1761 cm−1. Presence of esterified groups was revealed in the peak of 1330 cm−1 [21]. Peak at 3456 cm−1 indicated the existence of hydroxyl group from phenol. In addition, an asymmetrical band of methyl and methylene was observed at 2411 and 2733 cm−1 [21]. An intense sharp peak between 817–1761 cm−1 was due to the reduction of Ag+ to Ago. Thus, from the above observations, it was clear that the functional groups in the supernatant could be responsible for the bioconversion of Ag2 O NPs. These functional groups might have acted as reducing, capping and stabilising agents during Ag2 O NPs formation. This result coincided with a study in which Lactobacillus casei was being used in the biosynthesis of silver nanoparticles [7].
Fig. 4.

FTIR spectrum of Ag2 O NPs
3.4 Energy‐dispersive X‐ray spectroscopy (EDAX) analysis
EDAX analysis shown in Fig. 5 showed the strong signals due to surface plasma resonance at 2.98 keV. EDAX results clearly demonstrated the synthesis of Ag2 O NPs. The surface of the Ag2 O NPs showed the presence of oxygen and carbon which might be due to the influence of organic molecules in the extract used for synthesis. The two peaks observed below 1 keV indicated the presence of carbon as previously reported [22, 23]. The obtained result was in well agreement with the available reports wherein silver nanoparticles was synthesised using E. coli [8] and Pseudomonas putida [9].
Fig. 5.

Elemental analysis (EDS) of Ag2 O NPs
3.4 DLS analysis
To determine the dimension and distribution of the Ag2 O NPs, DLS method was employed. The sample graphs (Fig. 6) clearly depicted the average PSD of Ag2 O NPs was to be 40 nm, respectively.
Fig. 6.
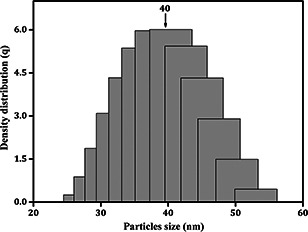
PSD of Ag2 O NPs
3.5 TEM imaging
TEM analysis (Fig. 7) of Ag2 O NPs samples reveals that the nanoparticles share ellipsoidal and spherical morphology. The diameter of the particles is found to be in the range of 20 to 40 nm. The respective patterns in SAD are obtained with diffraction rings with the spots and d‐spacing indexed as a crystalline structure [faced centred cubic (fcc)] according to JCPDS file 43‐0997. In the analysis, it is evident that the Ag2 O NPs appears to have good crystalline structure, as the patterns of SAD reveal a strong existence of bright spots along the crystal orientations appearing in the diffraction rings. The measurement of the diameters from the centre along the rings was consistent with the d‐spacing and corresponds with fcc phase of the ions of silver as determined in the XRD.
Fig. 7.

TEM image of Ag2 O NPs
3.6 Antibacterial activity
The antibacterial potential of the prepared Ag2 O NPs was analysed using well known disk diffusion method. The observed results are depicted in Fig. 8 and given in Table 1. It is clear that, as the concentration of the Ag2 O NPs was increased, there was an increase in the zone of inhibition when compared with the standard antibiotic streptomycin. The antibacterial property also depended on the type of the bacterium used. In our study, we have used two Gram positive bacteria (S. typhimurium and S. aureus), and two Gram negative bacteria (E. coli and K. pneumoniae). The result revealed difference in antibacterial activity of the Ag2 O NPs with respect to the bacterium used. The Gram positive bacteria S. typhimurium and S. aureus showed less zone of inhibition that is of 12 ± 0.54 mm and 12 ± 0.28 because of the presence of thick peptidoglycan on the surface of the cell, which inhibited the entry of Ag2 O NPs inside the bacterial cell [24]. In contrast, Ag2 O NPs found to exhibit higher order of inhibition against Gram negative bacteria E. coli and K. pneumoniae, which was about 14 ± 0.15 mm and 13 ± 0.45 mm size. This was because, Gram negative bacterium possess thin layer of peptidoglycan, which made it easier for the Ag2 O NPs to enter the cell and kill it [25]. Thus, this result clearly showed that, antibacterial activity not only depends on the type of particle, but also on the surface composition of the tested bacterium. In a recent study, it was shown that the antibacterial activity was due to the electrostatic interaction between the bacterial cell wall and Ag2 O NPs [26].
Fig. 8.
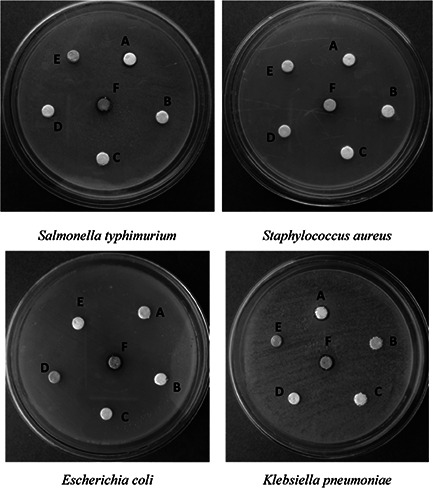
Antibacterial activity of Ag2 O NPs (A: 100 µg/ml antibiotic, B: Extracellular extract, C&D: only disk, E: distilled water, F: 100 µg/ml Ag2 O NPs)
Table 1.
Antibacterial activity of Ag2 O NPs synthesised from Nitrobacter sp. extracellular extract
| Microorganisms | Zone of inhibition [Mean ± SD (mm)] | |
|---|---|---|
| Streptomycin (100 µg/ml) | Ag2 O NPs (100 µg/ml) | |
| S. typhimurium | 12 ± 0.45 | 12 ± 0.54 |
| S. aureus | 11 ± 0.16 | 12 ± 0.28 |
| E. coli | 10 ± 0.47 | 14 ± 0.15 |
| K. pneumoniae | 12 ± 0.58 | 13 ± 0.45 |
3.7 Antioxidant activity
The free radical scavenging activity of synthesised Ag2 O NPs is shown in Fig. 9. It was observed that as the concentration of the Ag2 O NPs increased, its scavenging activity also increased correspondingly: 25% (1 mg), 43% (5 mg), 63% (10 mg) and 90% (100 mg). Similar antioxidant activity was being reported for iron and nickel oxide nanoparticles [27, 28]. In living systems, free radicals are being generated continuously due to various biochemical reactions. Biosynthesised Ag2 O NPs will be very helpful to scavenge these free radicals [29]. The small size with high surface area of these particles helps them to react easily with the free radicals and thereby scavenging them. Currently in the field of biomaterial and medicine, generation of free radicals is being considered a serious issue [30]. Hence, these Ag2 O NPs could be used as an additive in biomedical applications to scavenge the free radicals.
Fig. 9.
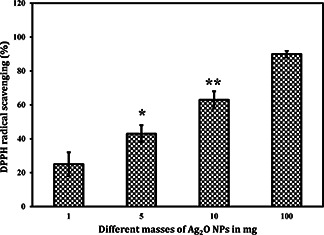
Antioxidant activity of Ag2 O NPs (*, ** Represents level of significant at p < 0.05)
4 Conclusions
In the present investigation, a search for new source for the production of Ag2 O NPs is successfully being carried out. This method is very rapid, eco‐friendly, economical and easy for the synthesis of Ag2 O NPs. In addition, this is a single step method without the use of toxic chemicals and less time consuming. By this method, we have successfully synthesised Ag2 O NPs with unique size and morphology. The bacterial extracts act as reducing, capping and stabilising agents. FTIR analysis evidenced about the organic groups involved in the bioconversion of Ag2 O NPs. XRD pattern reveals that the produced Ag2 O NPs are crystalline with face centred geometry. From the EDAX spectrum analysis, it is clear that the prepared nanoparticle is Ag2 O NPs. TEM analysis reveals that the synthesised Ag2 O NPs found to be almost spherical in shape. The antibacterial susceptibility assay reveals that the Ag2 O NPs exhibits good antibacterial property against both Gram negative and Gram positive bacteria such as S. typhimurium, S. aureus, E. coli and K. pneumoniae when compared with standard antibiotic streptomycin. In addition, Ag2 O NPs possess good antioxidant potential which is dose dependent. Thus, these findings will serve as a base for the development of Ag2 O NPs into nano‐medicine for the treatment of bacterial diseases, in future.
5 Acknowledgment
The authors gratefully acknowledge the financial support of the Ministry of Education and Science of the Russian Federation in the framework of Increase Competitiveness Program of NUST «MISiS» (№ К4‐2015‐017).
6 References
- 1. Dhand V. Soumya L. Bharadwaj S. et al.: ‘Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity’, Mater. Sci. Eng. C, 2016, 58, pp. 36 –43 (doi: 10.1016/j.msec.2015.08.018) [DOI] [PubMed] [Google Scholar]
- 2. Castro L. Blazquez M.L. Gonzalez F. et al.: ‘Biosynthesis of silver and platinum nanoparticles using orange peel extract: characterisation and applications’, IET Nanobiotechnol., 2015, 9, pp. 252 –258 (doi: 10.1049/iet-nbt.2014.0063) [DOI] [PubMed] [Google Scholar]
- 3. Mittal A.K. Tripathy D. Choudhary A. et al.: ‘Bio‐synthesis of silver nanoparticles using Potentilla fulgens Wall. ex Hook. and its therapeutic evaluation as anticancer and antimicrobial agent’, Mater. Sci. Eng. C, 2015, 53, pp. 120 –127 (doi: 10.1016/j.msec.2015.04.038) [DOI] [PubMed] [Google Scholar]
- 4. Paul B. Bhuyan B. Purkayastha D.D. et al.: ‘Photocatalytic and antibacterial activities of gold and silver nanoparticles synthesized using biomass of Parkia roxburghii leaf’, J. Photochem. Photobiol. B Biol., 2016, 154, pp. 1 –7 (doi: 10.1016/j.jphotobiol.2015.11.004) [DOI] [PubMed] [Google Scholar]
- 5. Sunkar S. Nachiyar C.V.: ‘Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus ’, Asian Pac. J. Tropical Biomed., 2012, 2, pp. 953 –959 (doi: 10.1016/S2221-1691(13)60006-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venil C.K. Sathishkumar P. Malathi M. et al.: ‘Synthesis of flexirubin‐mediated silver nanoparticles using Chryseobacterium artocarpi CECT 8497 and investigation of its anticancer activity’, Mater. Sci. Eng. C, 2016, 59, pp. 228 –234 (doi: 10.1016/j.msec.2015.10.019) [DOI] [PubMed] [Google Scholar]
- 7. Korbekandi H. Iravani S. Abbasi S.: ‘Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp. casei’, J. Chem. Technol. Biotechnol., 2012, 87, pp. 932 –937 (doi: 10.1002/jctb.3702) [DOI] [Google Scholar]
- 8. Ghorbani H.R.: ‘Biosynthesis of silver nanoparticles by Escherichia coli ’, Asian J. Chem., 2013, 25, pp. 1247 –1249 [Google Scholar]
- 9. Thamilselvi V. Radha K.V.: ‘Synthesis of silver nanoparticles from Pseudomonas Putida NCIM 2650 in silver nitrate supplemented growth medium and optimization using response surface methodology’, Dig. J. Nanomaterials Biostructures, 2013, 8, pp. 1101 –1111 [Google Scholar]
- 10. Cakmakc R.I. Aydin D.F. Sahin A.F.: ‘Growth promotion of plants by plant growth‐promoting rhizobacteria under greenhouse and two different field soil conditions’, Soil Biol. Biochem., 2006, 38, pp. 1482 –1487 (doi: 10.1016/j.soilbio.2005.09.019) [DOI] [Google Scholar]
- 11. Bock E. Sundermeyer‐Klinger H. Stackebrandt E.: ‘New facultative lithoautotrophic nitrite‐oxidizing bacteria’, Arch. Microbiol., 1983, 136, pp. 281 –284 (doi: 10.1007/BF00425217) [DOI] [Google Scholar]
- 12. Aamand J. Ahl T. Spieck E.: ‘Monoclonal antibodies recognizing nitrite oxidoreductase of Nitrobacter hamburgensis ’, Appl.ied and Environ. Microbiol., 1996, 67, pp. 2352 –2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bauer A.W. Kirby W.M. Sherris J.C. et al.: ‘Antibiotic susceptibility testing by a standardized single disk method’, Am. J. Clin. Pathol., 1966, 45, pp. 493 –496 [PubMed] [Google Scholar]
- 14. Serpen A. Capuano E. Fogliano V. et al.: ‘A new procedure to measure the antioxidant activity of insoluble food components’, J. Agric. Food Chem., 2007, 55, pp. 7676 –7681 (doi: 10.1021/jf071291z) [DOI] [PubMed] [Google Scholar]
- 15. Kalimuthu K. Deepak V. Ramkumarpandian S. et al.: ‘Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis ’, Mater. Lett., 2014, 62, pp. 4411 –4413 [Google Scholar]
- 16. Ahmad A. Mukherjee P. Senapati S. et al.: ‘Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum ’, Colloids Surf. B, 2003, 28, pp. 313 –318 (doi: 10.1016/S0927-7765(02)00174-1) [DOI] [Google Scholar]
- 17. Morones J.R. Elechiguerra J.L. Camacho A. et al.: ‘The bactericidal effect of silver nanoparticles’, Nanotechnology, 2005, 16, pp. 2346 –2353 (doi: 10.1088/0957-4484/16/10/059) [DOI] [PubMed] [Google Scholar]
- 18. Kalimuthu K. Suresh Babu R. Venkataraman D. et al.: ‘Biosynthesis of silver nanocrystals by Bacillus licheniformis ’, Colloids Surf. B Biointerfaces, 2008, 65, pp. 150 –153 (doi: 10.1016/j.colsurfb.2008.02.018) [DOI] [PubMed] [Google Scholar]
- 19. Kiruba Daniel S.C.G. Abirami J. Kumaran S. et al.: ‘Microbicidal tissue paper using green synthesized silver nanoparticles’, Current Nanosci., 2015, 11, pp. 64 –68 (doi: 10.2174/1573413710666140909211129) [DOI] [Google Scholar]
- 20. Jeeva K. Thiyagarajan M. Elangovan V. et al.: ‘ Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens’, Ind. Crops Prod., 2014, 52, pp. 714 –720 (doi: 10.1016/j.indcrop.2013.11.037) [DOI] [Google Scholar]
- 21. Nabikhan A. Kathiresan K. Anburaj K. et al.: ‘Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L.’, Coll. Surf. B Biointer., 2010, 79, pp. 488 –493 (doi: 10.1016/j.colsurfb.2010.05.018) [DOI] [PubMed] [Google Scholar]
- 22. Das B. Dash S.K. Mandal D. et al.: ‘Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage’, Arab. J. Chem., 2015, Article in press, 10.1016/j.arabjc.2015.08.008 [DOI] [Google Scholar]
- 23. Saravanakumar A. Ganesh M. Jayaprakash J. et al.: ‘Biosynthesis of silver nanoparticles using Cassia tora leaf extract and its antioxidant and antibacterial activities’, J. Ind. Eng. Chem., 2015, 28, pp. 277 –281 (doi: 10.1016/j.jiec.2015.03.003) [DOI] [Google Scholar]
- 24. Jayachandra Reddy N. Nagoor Vali D. Rani M. et al.: ‘Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit’, Mater. Sci. Eng. C, 2014, 34, pp. 115 –122 (doi: 10.1016/j.msec.2013.08.039) [DOI] [PubMed] [Google Scholar]
- 25. Shrivastava S. Bera T. Roy A. et al.: ‘Characterization of enhanced antibacterial effects of novel silver nanoparticles’, Nanotechnology, 2007, 18, pp. 225103 –225111 (doi: 10.1088/0957-4484/18/22/225103) [DOI] [PubMed] [Google Scholar]
- 26. Abbaszadegan A. Ghahramani Y. Gholami A. et al.: ‘The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram‐positive and gram‐negative bacteria: a preliminary study’, J. Nanomaterials, 2015, pp. 1 –8, ID 720654 (doi: 10.1155/2015/720654) [DOI] [Google Scholar]
- 27. Paul S. Saikia J.P. Samdarshi S.K. et al.: ‘Investigation of antioxidant property of iron oxide particlesby 1′‐1′ diphenylpicryl‐hydrazyle (DPPH) method’, J. Magn. Magn. Mater., 2009, 321, pp. 3621 –3623 (doi: 10.1016/j.jmmm.2009.07.004) [DOI] [Google Scholar]
- 28. Saikia J.P. Paul S. Konwar B.K. et al.: ‘Nickel oxide nanoparticles: a novel antioxidant’, Colloids Surf. B., 2010, 78, pp. 146 –148 (doi: 10.1016/j.colsurfb.2010.02.016) [DOI] [PubMed] [Google Scholar]
- 29. Watal G. Watal A. Rai P.K. et al.: ‘Biomedical applications of nano‐antioxidant’, Methods Mol. Biol., 2013, 1028, pp. 147 –151 (doi: 10.1007/978-1-62703-475-3_9) [DOI] [PubMed] [Google Scholar]
- 30. Pham‐Huy L.A. He H. Pham‐Huy C.: ‘Free radicals, antioxidants in disease and health’, Int. J. Biomed. Sci., 2008, 4, pp. 89 –96 [PMC free article] [PubMed] [Google Scholar]


