Abstract
Carbon nanotubes represent one of the best examples of novel nanostructures, exhibit a range of extraordinary physical properties, strong antimicrobial activity and can pierce bacterial cell walls. This investigation handles the antimicrobial activity of functionalised multiwall carbon nanotubes (F‐MWNTs) as an alternative antimicrobial material compared to the commercial antibiotics. Antibacterial activities of F‐MWNTs are investigated through two different kinds of bacteria, E. coli and S. aureus. The results demonstrate that the best concentration of F‐MWNTs for the maximum inhibition and antibacterial functionality is 80 and 60 μg/ml for E. coli and S. aureus, respectively. The transmission electron microscope reveals the morphological changes damage mechanism for the cellular reliability on these microorganisms. F‐MWNTs are capable of biologically isolating the cell from their microenvironment, contributing to the development of toxic substances and placing the cell under oxidative stress leading to cellular death. The efficiency of F‐MWNTs is compared with the common antibiotics and shows an enhancement in the inhibitory effect with percentages reaches 85%. To account for the bactericidal performance of F‐MWNTs towards these pathogens, the dielectric conductivity and the bacterial growth measurements are conducted. The present study endeavour that F‐MWNTs could be exploited in biomedical devices and altering systems for hospital and industrial cleaning applications.
Inspec keywords: antibacterial activity, biomedical materials, microorganisms, cellular biophysics, toxicology, nanomedicine, multi‐wall carbon nanotubes, transmission electron microscopy, electrical conductivity, permittivity
Other keywords: F‐MWNTs, pathogenic microorganisms, antimicrobial activity, bacterial cell walls, functionalised multiwall carbon nanotubes, antibacterial activity, E. coli, S. aureus, antimicrobial material, physical properties, transmission electron microscopy, morphological changes, damage mechanism, cellular reliability, microenvironment, toxic substances, oxidative stress, cellular death, bactericidal performance, dielectric conductivity, bacterial growth measurements, biomedical devices, C
1 Introduction
Carbon nanotubes (CNTs) represent one of the best examples of novel nanostructures [1], exhibit a range of extraordinary physical properties, such as extremely small size and high aspect ratio (>1000), a rich spectrum of extraordinary electrical, thermal and mechanical properties [2]. Also CNTs exhibit a strong antimicrobial activity and can pierce bacterial cell walls. These properties allow CNTs to be one of the most prospective materials for use in various fields of science and technologies, such as nanoscale electronic devices [3], tissue engineering [4], polymer composites [5] and wastewater treatment filters [6, 7, 8]. CNTs have attracted increasing attention for their potential applications in the biomedical field as diagnostic and therapeutic nano‐tools, so that the effects induced by CNTs, on the biological environment is considered one of the important research fields in the world of biomedical applications of nanoparticles [9, 10]. CNTs can be considered as multifunctional nano‐tools for future therapeutic and diagnostic purposes [11, 12]. CNTs possess antibacterial properties, different mechanisms and cytotoxic effects against pathogenic organisms such as bacteria [13]. The antibacterial activity of CNTs influenced by the same factors that usually affect the behaviour of CNTs when in contact with different cell types: their diameter, length, aggregation, concentration, surface functional groups, buffer solution as well as contact time, intensity and the cell type [14].
CNTs cause damage to the cell wall and membrane of the microorganism [15]. Furthermore, CNTs are capable of placing the cell under oxidative stress leading to the biological death. The antimicrobial activity of the material increases as its size reached to nanoscale range because of their larger surface area per unit volume, excellent electrical conductivity [16], high transparency and structural stability [17, 18]. Many microorganisms are accountable for serious human infections, including E. coli (gram‐negative bacteria) and S. aureus (gram‐positive bacteria) [19]. The application of antimicrobial agents is recognised as the common medical treatment for such infections, such as antibiotics and chemotherapeutic agents. However, the microbial pathogens, owing to the regular and inappropriate use of antibiotics became resistant to the standard antimicrobial treatments, triggering an increase in the health risks. Therefore, it is crucial to discover efficient alternative approaches, which should not be harmful to healthy cells and living organisms, for the treatment of such antimicrobial resistant strains. In this study, the use of commercial multiwall CNTs is due to their low cost and their lenience massive production compared to the single‐walled CNTs [20].
The main principle of the bactericidal action of CNTs is achieved by combining physical and chemical mechanisms [21]. Physically, CNTs may be the cause of substantial structural damage to the cell wall and membrane of the microorganism. In addition, they have the ability to biologically isolate cell from their microenvironments, leading eventually to the production of toxic substances, such as reactive oxygen species, and placing the cell under oxidative stress leading to the biological death. It is definitely important to determine the length of the nanotubes during interactions with the cell membrane. The shorter tube is founded to exert higher bactericidal performance in comparison to longer tubes [22]. The interaction of CNTs with cells in a liquid medium is quite different compared to the solid surface. The diameter of a tube also plays an important role in the bacterial inactivation process. Smaller diameters can induce damage to cell membrane through the cell‐surface interaction [23].
Although different studies investigated the toxicological effect of both types single‐wall CNTs (SWNTs) and multiwall CNTs (MWNTs), towards a variety of microorganisms and the interactions between CNTs and microbial cells, this field remains still poorly understood. In this manuscript, we concerned with functionalised MWNTs (F‐MWNTs) and their antimicrobial effects on living microorganisms. We study the toxic effect of F‐MWNTs on bacterial biofilms and investigate the impact of two types of bacteria, one type is gram‐negative bacteria such as E. coli and the other is gram‐positive bacteria such as S. aureus. Firstly, F‐MWNTs interact with bacterial cells to biofilm formation and inhibit their growth and biofilm formation. Secondly, CNTs could disturb the mature biofilm leading to its detachment. Our investigations were supported by the dielectric measurements.
2 Materials and methods
2.1 Characterisation and modification of the MWNTs
The material of the present study is the commercial CNT (Taunit‐M), produced by ‘Nanotech Center’ (Tambov, Russia, http://www.nanotc.ru/producrions/87‐cnm‐taunit) in a form of powder, composed of grainy agglomerates of MWNTs with a length of several micrometres, external diameter (20–300) nm, specific surface area 232.6 m2 /g. To perform the purification of MWNT, a 100 mg of Taunit‐M was mixed directly with 10 ml of 5 M hydrochloric acid and 10 ml of 50% H2 O2 in 100 ml open flask [7]. In the second treatment for functionalisation, the treated MWNTs were dispersed in 100 ml of nitric acid 65% in 250 ml flask [7]. The F‐MWNTs samples were prepared by combining these treatments. F‐MWNTs were analysed using transmission electron microscope (TEM), scanning electron microscope equipped with energy dispersive X‐ray spectroscopy to clearly investigate the effectiveness of the purification and oxidation processes. Fourier‐transform infrared (FTIR) spectra of pristine and F‐MWNTs were recorded using (IFS 66 V/S) FTIR spectrometer. The specific surface area of F‐MWNTs was determined using nitrogen adsorption method. Samples for TEM (CM12 Philips) were prepared by the grinding of the powder and successive ultrasonic treatment in isopropyl alcohol for 1 min. A drop of the suspension was dried on the standard TEM sample grid covered with holey carbon film [24]. F‐MWNTs were incubated overnight at 37°C and then placed into the primary fixative and microwaved (MW) under vacuum conditions in a PelcoBiowave (Ted Pella, Inc., Redding, CA, USA) at 120 W. The stained samples were observed in JEOL 1400 (JEOL, Ltd, Tokyo, Japan) operating at 80 kV [25].
2.2 Bacterial culture media and gram staining
A strain of S. aureus (ATCC# 25923) and E.coli (O157:H7) was used during this investigation provided from the Microbiological Laboratory Damanhur oncology centre, Ministry of Health. S. aureus and E. coli was preserved on nutrient agar slants at 8°C for short periods in glycerol at −20°C. Bacterial strains were grown in LB broth at 37°C for (18–20) h. For incubation of bacteria in liquid broth, 1.3 g nutrient broth powder was dissolved in 100 ml distilled water and boiling in a 250 ml conical flask stoppered by cotton plug [1], its final pH was adjusted to 7.4, then the content was sterilised by autoclaving [26] for 15 min at 131°C. A disinfected wire loop size stock microorganisms was transferred into the cold medium under laminar flow which was disinfected by cleaning thoroughly with absolute alcohol followed by UV irradiation for 20 min [1]. Gram staining was investigated in order to differentiate between gram‐positive and gram‐negative bacteria through the morphological shape of microorganism under microscope by the chemical and physical properties of cell wall [27]. Fifty microlitres of the cell suspension were diluted with 500 µl of different concentrations of F‐MWNTs (0, 20, 40, 60, 80 and 100 μg/ml dissolved in ethanol) and allowed to incubate at 37°C and 150 rpm for a designed treatment time.
2.3 Growth curve measurements
The culture of S. aureus and E. coli was left to grow then a part of the sample was taken to mix with clean broth medium. After mixing process, the new culture was divided into six groups, one control, the others were treated with different concentrations of F‐MWNTs at 0, 20, 40, 60, 80 and 100 μg/ml. About 100 µl aliquots were taken from the solutions every 30 min for the next 5 h and tested for optical density. The remaining solutions continued to incubate in the shaker at 37°C and 200 rpm. Cell growth was measured using spectrophotometer model (UV/VIS spectrophotometer, 180 watt‐AC115/230v‐50/60 Hz) at 600 nm. Growth curves were created by plotting OD values versus time [28]. About 106 cells per ml of bacterial strains were incubated in PBS at 37°C (E. Coli and S. aureus) with different concentrations of F‐MWNTs at 0, 20, 40, 60, 80 and 100 μg/ml under shaking for 24 h [13].
2.4 Antibiotic sensitivity test (AST)
The antibiotics used in this study were chosen to be with different modes of action and these were: Amicacin [AK (30 µg)], Gentamicin [CN (10 µg)], Streptomycin [S (10 µg)], Kanamycin [K (30 µg)], Penicillin [P (10 µg)], Ampicillin [AM (10 µg)] and chloramphenicol [C (30 µg)] Ciprofloxacin [CIP (5 µg)], trimethoprim‐sulfamethoxazole [SXT (25 µg)], Levofloxacin [LEV(5 µg)] and Norfloxacin [NOR (10 µg)], Ceftazidime [CAZ (30 µg)], Amoxicillin/Clavulanic acid [AMC (30 µg)], Nitrofurantoin [F (300 µg)] and Rifampin [RA(5 µg)]. The AST was carried out using Mueller‐Hinton agar (MHA) medium as described in [29, 30]. About 7.6 g of MHA and 1.2 g agar were dissolved in 200 ml distilled water in a capped bottle. The agar solution was sterilised by autoclaving at 15 lbs pressure at 131°C for 15 min. The medium was then poured into Petridishes with almost equal agar thickness (2.5 mm) in a laminar hood, which was disinfected by cleaning it with absolute alcohol followed by UV irradiation for 20 min. The dishes were cooled for sufficient time to solidify the agar medium. The disc dipped in a solution of carbon nanoparticles was applied in the launder plate. The covered dishes were kept in an incubator oven at 37°C for 24 h to test. After the test inhibition zone diameter was measured from the clear zone of agar dish [1]. The method used to measure the sensitivity of the bacterial cells towards different antibiotics was disc method by Bauer‐Kirby technique [31]. After incubation, the plates are examined and the diameter of the zones of inhibition is measured by using a ruler.
2.5 Antimicrobial activity of F‐MWNTs
Antimicrobial activity of antibiotics is normally tested using a disk diffusion test, employing antibiotic disks. A similar test with F‐MWNTs instead of antibiotics was performed with a little change in this study. For unicellular bacterial system, a culture of E. coli and another of S. aureus were grown overnight on a nutrient broth medium at 37°C to provide optimum temperature for growth in a rotary shaker at 150 rpm to provide proper aeration, as these are aerobic microbes. The cultures (100 µl of 106 CFU/ml) were uniformly plated on nutrient agar plates, and six wells 2 mm in diameter were made with the sterile core borer. The aqueous solution of CNT of 100 µl each (0, 20, 40, 60, 80 and 100 μg /ml) was loaded to the wells and the plates were incubated at about 37°C. After ∼24 h of incubation, there resulted a zone of inhibition on the plates. The average diameter of the inhibition zone surrounding the disks was measured [32].
2.6 Conductivity measurements for the bacterial cells
For the conductivity measurements, 1 ml of the bacterial sample was placed into sterile micro‐centrifuge tube and centrifuged at 14,000 rpm at 4°C for 15 min. The pellet was then harvested and resuspended in a 1 ml volume of sterile deionised water. The tube was then centrifuged and the pellet was washed with deionised water twice more, before finally being re‐suspended in sterile deionised water. A fixed concentration of bacterial cells ((1 ± 0.08) × 106 CFU/ml) was used for all samples which were controlled through the use of the spectrophotometer. The dielectric measurements were carried out for the samples in the frequency range 42 Hz–5 MHz using a loss Factor Meter type HIOKI 3532 LCR Hi TESTER; version 1.02, Japan, and cell types (PW 950/60) manufactured by Philips. The measured values of capacitance, C, and resistance, R, were used to calculate the real dielectric constant and the imaginary dielectric loss of the complex permittivity [33]. The AC conductivity σ was calculated from
| (1) |
3 Results
3.1 Surface analysis of pristine and F‐MWNTs
Figs. 1 a and b show SEM images of the pristine and F‐MWNTs.
Fig. 1.
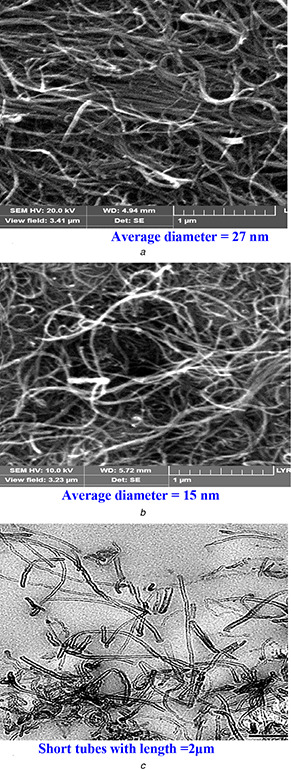
Surface analysis of pristine and F‐MWNTs using SEM and TEM as a powerful characterization tools
(a) SEM images of MWNTs for pristine, (b) F‐MWNTs, (c) TEM images of MWNTs
A considerable decrease in nanotube diameters and length was observed in F‐MWNTs after functionalisation as shown in Fig. 1.
Interestingly, it was observed that after functionalisation the average length of MWNTs decreased from 20 μm to about 2 μm. The specific surface area of MWNTs after ozone treatment was about 312 g/m2 which are high comparable with pristine one. The EDS analysis offers a quantitative study of the oxygen and metal content of the pristine and F‐MWNTs. The particle morphologies for F‐MWNTs are examined by TEM and presented in Fig. 1 c. Pristine MWNTs contain a fraction of iron metal impurity and tiny oxygen content due to the synthesis procedure (Figs. 2 A and B). After treating pristine MWNTs, with (HCl + H2 O2) and nitric acid solutions the metal impurity was removed. This is followed by enhancement of the oxygen content due to the oxidation effect. Fig. 2 C summarises the behaviour of the functional groups observed via FTIR spectroscopy of the pristine and F‐MWNTs samples before adjustment (Fig. 2 C (a)) and after adjustment (Fig. 2 C (b)). The F‐MWNTs show new peaks in comparison with the FTIR spectrum of the untreated samples, which lack the hydroxyl and carbonyl groups [34]. This shows that hydroxyl and carbonyl groups have been introduced on the nanotube surface [35, 36]. From a qualitative point of view these samples mostly consist of multiwalled nanotubes with a hollow internal channel bearing at the tip, infrequently at an intermediate length position.
Fig. 2.
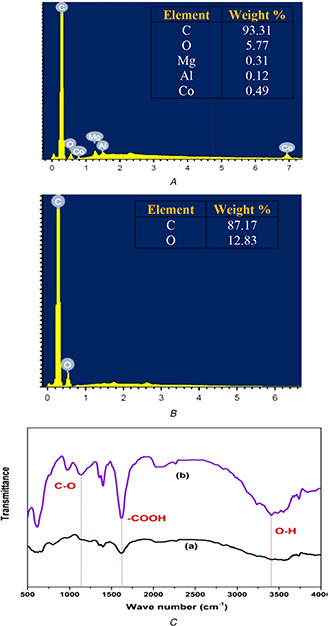
Investigation of the MWNTs content and the functional oxygenated groups using EDS and FTIR spectroscopy
(A) EDS analysis shows the element content for pristine, (B) F‐MWNTs, (C) FTIR spectra show the pristine (a), and F‐MWNTs (b)
3.2 Antibacterial effects of F‐MWNTs and the conductivity measurements
Figs. 3 a and b indicated the growth characteristic curve for E. coli and S. aureus, respectively, to find the best concentration of F‐MWNTs. Growth characteristics curve of bacteria showed the bacterial count as a function of incubation time with treated bacteria for different concentrations of F‐MWNTs from C1, C2, C3, C4 and C5 as 20, 40, 60, 80 and 100 μg/ml, respectively, as compared with control bacteria. The results showed that the best concentrations of F‐MWNTs which gives the maximum inhibition or minimum inhibitory concentration (MIC) is at C4 (80 μg/ml) with inhibition percentage 75.92% for E. coli and C3 (60 μg/ml) with inhibition percentage 63.88% for S. aureus (Fig. 4).
Fig. 3.
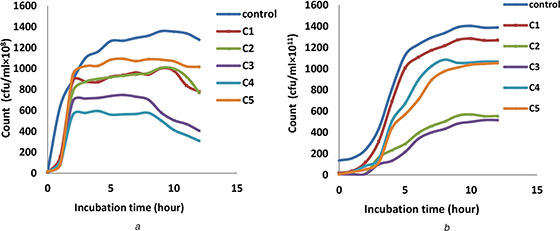
Growth characteristics curve of bacteria shows the bacterial count as a function of incubation time with treated bacteria for different concentrations of F‐MWNTs
(a) Growth characteristics curve for E. coli bacteria, (b) S. aureus after treating with different concentrations of F‐MWNTs
Fig. 4.
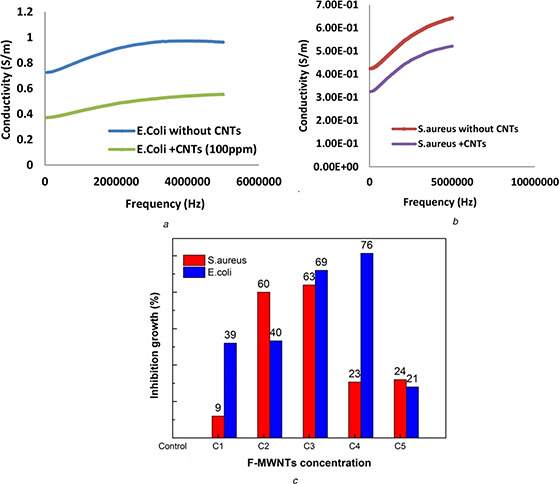
The variation of conductivity plotted as a function of frequency, for E. coli and S. aureus bacterial cells and treated with F‐MWNTs at the most effective concentration
(a) Variation of the conductivity with frequency for E. coli, (b) S. aureus without and with F‐MWNTs, (c) Inhibition growth percentage
Figs. 4 a and b illustrate the variation of conductivity plotted as a function of frequency, for E. coli and S. aureus bacterial cells and treated with F‐MWNTs at the most effective concentration. We concluded that the conductivity of the E. coli and S. aureus treated with F‐MWNTs is lower values as compared with control cells.
In order to determine the MIC of F‐MWNTs which gives the best inhibition for microbial growth of E. coli and S. aureus, five concentrations of F‐MWNTs (20, 40, 60, 80 and 100 μg/ml) were investigated to study their effects on the microbial activity. From Table 1 it was found that MIC was at 80 μg for E. coli and 60 μg for S. aureus which gives inhibition zone diameter 33 and 30 mm, respectively (Figs. 5 a and 6 a).
Table 1.
Inhibition zone diameter for E. coli and S. aureus after treated with F‐MWNTs
| Concentrations of CNTs, μg/ml | 0 | 20 | 40 | 60 | 80 | 100 | |
|---|---|---|---|---|---|---|---|
| inhibition zone diameter in, mm | E. coli | — | — | 11 | 23 | 30 | — |
| S. aureus | — | — | 30 | 33 | 15 | 12 | |
Fig. 5.
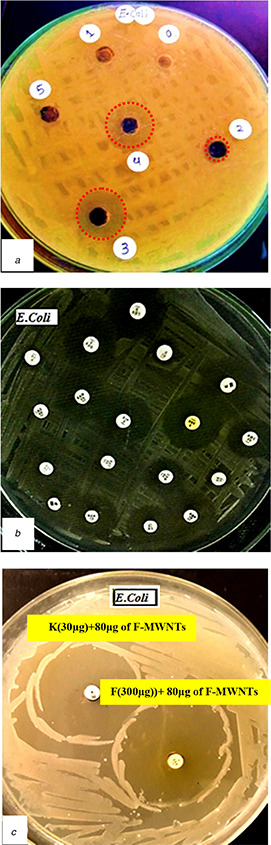
The diameter of the inhibition zone for microbial growth of E. coli at five concentrations of F‐MWNTs (20, 40, 60, 80 and 100 μg/ml) compared with commercial antibiotics
(a) Antibacterial effects of F‐MWNTs, (b) Different types of antibiotics, (c) Combination of antibiotics and F‐MWNTs investigated for E. coli
Fig. 6.
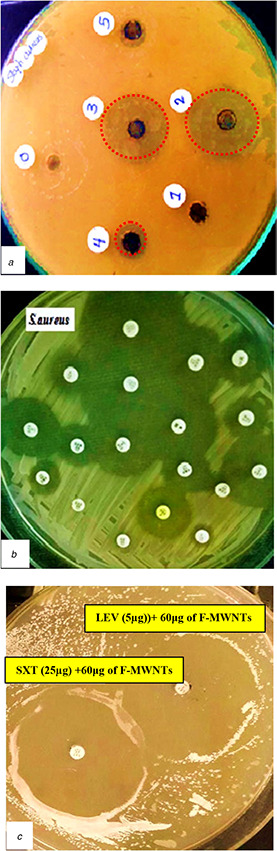
The diameter of the inhibition zone for microbial growth of S. aureus at five concentrations of F‐MWNTs (20, 40, 60, 80 and 100 μg/ml) compared with commercial antibiotics
(a) Antibacterial effects of F‐MWNTs, (b) Different types of antibiotics, (c) Combination of antibiotics and F‐MWNTs investigated for S. aureus
As a comparison with F‐MWNTs, the effects of 13 different types of antibiotics on the two strains of bacteria E. coli and S. aureus were investigated as shown in Figs. 5 b and 6 b and Table 2. We found that the best antibiotics which gives the maximum inhibition for E. coli are K (30 µg) and F (300 µg), with 27 mm zone diameter. However, for S. aureus, the inhibition zone diameter was 35 and 48 mm for LEV (5 µg) and SXT (25 µg), respectively.
Table 2.
AST of E. coli and S. aureus
| Antibiotics | Code, µg | Inhibition zone diameter, mm | |
|---|---|---|---|
| E. coli | S. aureus | ||
| CN | 10 | 25 | 22 |
| AMP | 10 | 26 | 21 |
| CXM | 30 | 20 | 18 |
| F | 300 | 27 | 27 |
| AK | 30 | 12 | 11 |
| AM | 30 | 21 | 19 |
| K | 30 | 27 | 34 |
| S | 10 | 12 | 20 |
| CIP | 5 | 26 | 30 |
| LEV | 5 | 25 | 35 |
| OFX | 5 | 22 | 3 |
| SXT | 25 | — | 48 |
| C | 30 | 26 | 21 |
| NOR | 10 | 26 | 28 |
| CAZ | 30 | — | — |
| P | 12 | 10 | 12 |
| SAM | 20 | 22 | 20 |
As shown in Table 3, the diameter of inhibition zone for E. coli was 27 mm for both two antibiotics [K (30 µg)] and [F (300 µg)]. We found that the sensitivity for E. coli bacteria increased by the combination between MIC of F‐MWNTs which found to be at concentration 80 μg and the best antibiotics which gives us the best sensitivity as [K (30 µg) + 80 μg CNTs] and [F(300 µg) + 80 μg CNTs]. The inhibition zone diameter increased from 27 to 42 mm with percentage 55.6% for [K(30 µg) + 80 μg of CNTs] and increased to 50 mm and percentage 85.2% for [F(300 µg) + 80 μg of CNTs] (Table 3, Fig. 5 c). However, the bacterial sensitivity of S. aureus will be increased by combination between MIC of F‐MWNTs (60 μg) and the best antibiotics which gives the best sensitivity as [LEV(5 µg) + 60 μg CNTs] and [SXT (25 µg) + 60 μg CNTs]. The inhibition zone diameter increased from 35 to 55 mm for [LEV(5 µg) + 60 μg CNTs] and from 48 to 70 mm for [SXT (25 µg) + 60 μg], this increases the inhibition by percentages 57.1 and 45.8%, respectively (Table 3, Fig. 6 c).
Table 3.
Antibacterial activity of CNTs and inhibition zone diameter (IZD) for E. coli and S. aureus at MIC of CNTs and the most sensitive antibiotic
| Effective antibiotics and MIC of CNTs for E. coli | IZD (mm) for E. coli | Effective antibiotics and MIC of CNTs for S. aureus | IZD (mm) for S. aureus |
|---|---|---|---|
| CNTs (80 µg) | 33 | CNTs (80 µg) | 30 |
| K (30 µg) | 27 | SXT (25 µg) | 48 |
| F (300 µg) | 27 | LEV (5 µg) | 35 |
| K + CNTs | 42 | SXT + CNTs | 70 |
| F + CNT | 50 | LEV + CNTs | 55 |
3.3 Morphology study by TEM
TEM is a complementary technique, useful to investigate the samples inner structure, composition and a suitable set of correlated instruments for this multi‐facet investigation [37, 38]. We used TEM to obtain distinct images in order to investigate the morphological changes in bacteria after treatment with F‐MWNTs. Micrographs of untreated E. coli cells show that they retained the rod shape with normal cell walls and nuclei smooth and continuous double membrane structure (Fig. 7 a). However, remarkable morphological changes were observed in E.coli cells after treatment with F‐MWNTs (Figs. 7 b –d). E. coli cells showed a rough cell wall and a blurry cell membrane (Fig. 7 b) with a decomposing inner structure. Some cells were disrupted (Fig. 7 c). In Fig. 7 d, the cytoplasmic material component of the cell was released to the extracellular medium and the cell becomes empty from its entire component. The insoluble CNTs compounds were seen directly in the cell membrane and inside the cell membrane as small black granules. The obtained results are correlated with the ultrasound effect that showed a rough cell wall and a blurry cell membrane, with a decomposing inner structure [39].
Fig. 7.
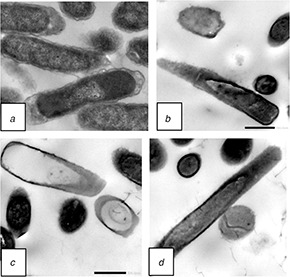
TEM images of E. coli
(a) Untreated cells of E.coli, (b)–(d) Treated samples with F‐MWNTs at concentration of 8 μg/ml after 24 h of incubation
S. aureus showed a normal cell shape with an undamaged structure of the membrane also shows very distinct outer membrane, plasma membrane and thick cell walls. Untreated S. aureus cells showed homogeneous electron density in the cytoplasm and presented continuous and smooth cell membranes (Fig. 8) compared to treated samples with F‐MWNTs at concentration 6 μg/ml after 24 h of incubation (Figs. 8 b –d). F‐MWNTs precipitate in the cell membrane and appear as small black particles inside the cells. F‐MWNTs caused alterations in the morphology of cells, rupturing of membrane and loss of cell organisation when compared with the control one (Figs. 8 b and c). F‐MWNTs treated cells presented thinner discontinuous or ruptured cell walls with leakage of cytoplasmic material. Furthermore, the inner structure of cells seemed to be strikingly affected also treated cells appeared to have decreased electron density in the cytoplasm (Fig. 8 d).
Fig. 8.
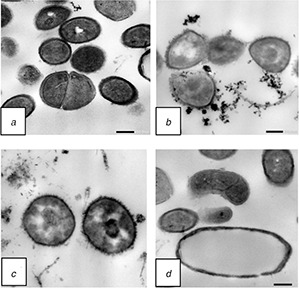
TEM images of S. aureus
(a) Untreated cells of S. aureus, (b)–(d) Compared to treated ones with F‐MWNTs at concentration 6 μg/ml after 24 h of incubation
4 Discussion
The functionalised MWNTs showed a decrease in nanotube diameter, the shorter length of the tube, high surface area and oxygen functional groups that enhanced their antibacterial activity. Compared with the most effective commercial antibiotics, F‐MWNTs increased the inhibitory performance that reached 85.2 and 57.1% for E. coli and S. aureus, respectively. The mechanism of CNT toxicity is highly influenced by several factors such as diameter, length, electronic structure and surface functional group [40]. Here we report in particular the more suitable suggestion for the antimicrobial activity mechanism. First, the length of nanotubes is significantly important during interactions with the cell membrane of bacteria. The shorter tube is found to exert superior bactericidal performance and may increase the chances for interaction between open ends of nanotubes and a microorganism (Figs. 7 and 8). A second factor that enhances the bacterial inactivation is the diameter of a tube, as smaller diameters can endorse damage to cell membrane through the cell‐surface interaction. Moreover, the small tubes made the bacteria interrelate closely with each other. Finally, important factor affecting the antimicrobial efficacy of F‐MWNTs is the functional groups (e.g. COOH). We surmise that van der Waals force between the molecules on F‐MWNTs and the cell membrane might be one possible foremost force that governs formation of the cell‐F‐MWNTs aggregates. The nanoparticles might cross the cell membranes, penetrating into the interior of the cell and interacting with intracellular sites, by preventing bacteria from dividing and multiplying. It induces cell lysis and kills the bacteria. The measured inhibition clearly demonstrated the potential activity of F‐MWNTs against E. coli and S. aureus. Conductivity measurements and the growth curve of two strains of bacteria were performed to account for the antimicrobial activity of F‐MWNTs. Dielectric measurements on suspensions of biological cells have provided valuable information about passive electrical properties of compartments of the cells, such as capacitances of cellular membranes and conductivities of interiors and cell walls [41]. The lower values of the conductivity give us an indication about the inhibition in the growth of the bacterial cells that treated by F‐MWNTs in agreement with previous report [42]. The bacterial membrane interacts with F‐MWNTs and cause structural changes and cell death. As the size of the functionalised carbon particles decreases down to nano‐scale range their antimicrobial activity increases because of their larger surface area per unit volume. These observations are important to explain the antibacterial mechanism of CNTs which contains a high percentage of strong antimicrobial agents that have the ability to penetrate the cell wall of bacterial. In conclusion, F‐MWNTs showed the best antibacterial activities compared to commercial antibiotics. TEM was used to characterise the bactericidal action of F‐MWNTs on E. coli and S. aureus cultures. We found that impairment of the cell membrane, inactivation of enzymatic activity and inhibition of metabolic performance were involved in the process of sterilisation of E. coli and S. aureus. The dielectric conductivity and the bacterial growth measurements confirmed the antibacterial efficiency of F‐MWNTs that could be exploited in the biomedical applications.
5 Conclusion
In conclusion, functionalised MWNTs represented antimicrobial activities against both gram‐negative and gram‐positive bacteria. In fact, F‐MWNTs showed the best antibacterial activities compared to commercial antibiotics. TEM was used to ensure the bactericidal action of F‐MWNTs on E. coli and S. aureus cultures. We found that injury of the cell membrane, inactivation of enzymatic activity and inhibition of metabolic performance were involved in the process of sterilisation of E. coli and S. aureus. The dielectric conductivity and the bacterial growth measurements confirmed the antibacterial efficiency of F‐MWNTs that could be exploited in the biomedical applications. F‐MWNTs augmented the inhibitory performance that reached 85.2 and 57.1% for E. coli and S. aureus, respectively. The essential research on F‐MWNTs antimicrobial activity is reinforcement the basis to advance the potential application of CNTs as structure blocks for antimicrobial materials sanitisation of surfaces for medical purposes.
6 References
- 1. Varghese S. Kuriakose S. Jose S.: ‘Antimicrobial activity of carbon nanoparticles isolated from natural sources against pathogenic Gram negative and Gram‐positive bacteria’, J. Nanosci., 2013, 2013, pp. 1 –5 [Google Scholar]
- 2. Baughman R.H. Zakhidov A.A. de Heer W.A.: ‘Carbon nanotubes—the route toward applications’, Science, 2002, 297, pp. 787 –792 [DOI] [PubMed] [Google Scholar]
- 3. Stancu E.C. Stanciuc A.M. Vizireanu S. et al.: ‘Plasma functionalization of carbon nanowalls and its effect on attachment of fibroblast‐like cells’, J. Phys. D Appl. Phys., 2014, 47, pp. 1 –10 [Google Scholar]
- 4. Goenka S. Sant V. Sant S.: ‘Graphene‐based nanomaterials for drug delivery and tissue engineering’, J. Control. Release, 2014, 173, pp. 75 –88 [DOI] [PubMed] [Google Scholar]
- 5. Suzuki S.: ‘Physical and chemical properties of carbon nanotubes’, Nanotechnol. Nanomater. (InTech), 2013, p. 414 [Google Scholar]
- 6. Elsehly E.M. Chechenin N.G. Bukunov K.A. et al.: ‘Removal of iron and manganese from aqueous solutions using carbon nanotubes filters’, Water Sci. Technol., Water Supply, 2016, 16, pp. 347 –353 [Google Scholar]
- 7. Elsehly E.M. Chechenin N.G. Makunin A.V. et al.: ‘Functionalized carbon nanotubes based filters for chromium removal from aqueous solutions’, Water Sci. Technol., 2017, 75, (7), pp. 1564 –1571 [DOI] [PubMed] [Google Scholar]
- 8. Elsehly E.M. Chechenin N.G. Makunin A.V. et al.: ‘Enhancement of CNT‐based filters efficiency by ion beam irradiation’, Radiat. Phys. Chem., 2018, 146, pp. 19 –25 [Google Scholar]
- 9. Prato M. Kostarelos K. Bianco A.: ‘Functionalized carbon nanotubes in drug design and discovery’, Acc. Chem. Res., 2008, 41, pp. 60 –68 [DOI] [PubMed] [Google Scholar]
- 10. Taylor E. Webster T.J.: ‘Reducing infections through nanotechnology and nanoparticles’, Int. J. Nanomed., 2011, 6, pp. 1463 –1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang S. Pinault M. Pfefferle L.D. Elimelech M.: ‘Single‐walled carbon nanotubes exhibit strong antimicrobial activity’, Langmuir, 2007, 23, (17), pp. 8670 –8673 [DOI] [PubMed] [Google Scholar]
- 12. Kang S. Mauter M.S. Elimelech M.: ‘Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity’, Environ. Sci. Technol., 2008, 42, (19), pp. 7528 –7534 [DOI] [PubMed] [Google Scholar]
- 13. Olivi M. Zanni E. De Bellis G. et al.: ‘Inhibition of microbial growth by carbon nanotube networks’, Nanoscale, 2013, 5, (19), pp. 9023 –9029 [DOI] [PubMed] [Google Scholar]
- 14. Kang S. Mauter M.S. Elimelech M.: ‘Microbial cytotoxicity of carbon‐based nanomaterials: implications for river water and wastewater effluent’, Environ. Sci.Technol., 2009, 43, pp. 2648 –2653 [DOI] [PubMed] [Google Scholar]
- 15. Prasad K. Lekshmi G. Ostrikov K. et al.: ‘Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against gram‐positive and gram‐negative bacteria’, Sci. Rep., 2017, 7, p. 1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li H. He X. Liu Y. et al.: ‘One‐step ultrasonic synthesis of water‐soluble carbon nanoparticles with excellent photoluminescent properties’, Carbon., 2011, 49, pp. 605 –609 [Google Scholar]
- 17. Cao Q. Rogers J.A.: ‘Ultrathin. Films of single‐walled carbon nanotubes for electronics and sensors’, A Rev. Fundam. Appl. Asp. Adv. Mater., 2009, 21, pp. 29 –53 [Google Scholar]
- 18. Choi W. Lahiri I. Seelaboyina R. et al.: ‘Synthesis of graphene and its applications: A review’, Crit. Rev. Solid State Mater. Sci., 2010, 35, pp. 52 –71 [Google Scholar]
- 19. Wu J.Y. Li C.W. Tsai C.H. et al.: ‘Synthesis of antibacterial TiO2/PLGA composite biofilms’, Nanomed. Nanotechnol., 2017, 10, (5), pp. 1097 –1107 [DOI] [PubMed] [Google Scholar]
- 20. Mukherjee A. Majumdar S. Servin A.D. et al.: ‘Carbon nanomaterials in agriculture a critical review’, Front. Plant Sci., 2016, 7, p. 172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji H. Sun H. Qu X.: ‘Antibacterial applications of graphene‐based nanomaterials: recent achievements and challenges’, Adv. Drug Deliv. Rev., 2016, 105, pp. 176 –189 [DOI] [PubMed] [Google Scholar]
- 22. Rhiem S.: ‘End of life cycle assessment for carbon nanotube (CNT) containing composites: release of CNT and ecotoxicological consequences’, Hochschulbibliothek der Rheinisch‐Westfälischen Technischen Hochschule Aachen, 2014. Available at http://publications.rwth‐aachen.de/record/444979/files/5137.pdf
- 23. Smith S.C. Rodrigues D.F.: ‘Carbon‐based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications’, Carbon, 2015, 91, pp. 122 –143 [Google Scholar]
- 24. Branca C. Frusteri F. Magazu V. et al.: ‘Characterization of carbon nanotubes by TEM and infrared spectroscopy’, J. Phys. Chem. B, 2004, 108, pp. 3469 –3473 [Google Scholar]
- 25. Liu S. Wei L. Hao L.: ‘Sharper and faster ‘nano darts’ kill more bacteria: a study of antibacterial activity of individually dispersed pristine single‐walled carbon nanotube’, ACS Nano, 2009, 3, (12), pp. 3891 –3902 [DOI] [PubMed] [Google Scholar]
- 26. Pratik P.P. Shrenik K.S. Kalpen N.P. et al.: ‘A detailed review on sustained release drug delivery system’, Int. J. Med. Pharm. Res., 2013, 1, (1), pp. 145 –153 [Google Scholar]
- 27. Madigan M.T. Martinko J. Parker J.: ‘Brock biology of microorganisms’ (Lippincott Williams &Wilkins, Boston, 2004, 11th edn.), ISBN 0‐13‐066271‐2 [Google Scholar]
- 28. Lifeng D. Alex H. Christopher F.: ‘Antimicrobial Activity of Single‐Walled Carbon Nanotubes Suspended in Different Surfactants’, J. Nanotechnol., 2012, 2012, pp. 1 –7 [Google Scholar]
- 29. Ebie M.Y. Kandakai‐Olukemi Y.T. Ayanbadejo J. et al.: ‘Urinary tract infections in a Nigerian military hospital’, Nig. J. Microbiol., 2001, 15, (1), pp. 31 –37 [Google Scholar]
- 30. Coyle M.B. et al.: ‘Manual of antimicrobial susceptibility testing’, (American Society for Microbiology, Washington, USA, 2005) [Google Scholar]
- 31. Amin N.C. Huguette F. Marie‐Dominique B. Jérôme M. Michèle A.: ‘Determination of artemether and lumefantrine in antimalarial fixed dose combination tablets by microemulsion electro kinetic chromatography with short‐end injection procedure’, J. Malaria, 2013, 12, (202), p. 202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sunil C. Somashekar R.K. Nagaraja B.C.: ‘Riparian vegetation dynamics across two different landscapes along the river Cauvery in the Kodagu region of Western Ghats’, J. Mt. Sci., 2012, 9, pp. 351 –361 [Google Scholar]
- 33. Fadel M.A. Mohamed S.A. Abdelbacki A.M. et al.: ‘Inhibition of Salmonella typhi growth using extremely low frequency electromagnetic (ELF‐EM) waves at resonance frequency’, J. Appl. Microbiol., 2014, 117, (2), pp. 358 –365 [DOI] [PubMed] [Google Scholar]
- 34. Elsehly E.M. Chechenin N.G. Makunin A.V. et al.: ‘Characterization of functionalized multiwalled carbon nanotubes and application as an effective filter for heavy metal removal from aqueous solutions’, Chin. J. Chem. Eng, 2016, 24, pp. 1695 –1702 [Google Scholar]
- 35. Theodore M. Hosur M. Thomas J. Jeelani S.: ‘Influence of functionalization on properties of MWCNT–epoxy nanocomposites’, Mater. Sci. Eng. A, 2011, 528, pp. 1192 –1200 [Google Scholar]
- 36. Wu D.C. Shen L. Low J.E. et al.: ‘Multi‐walled carbon nanotube/polyimide composite film fabricated through electrophoretic deposition’, Polymer, 2010, 51, pp. 2155 –2160 [Google Scholar]
- 37. Didenko L.V. Avtandilov G.A. Shevlyagina N.V. et al.: ‘Nanoparticles production and inclusion in S. aureus incubated with olyurethane: an Electron microscopy analysis’, J. Med. Imaging, 2013, 3, pp. 69 –73 [Google Scholar]
- 38. Kentish S Feng H.: ‘Applications of power ultrasound in food processing’, Annu. Rev. Food. Sci. Technol., 2014, 5, pp. 263 –284 [DOI] [PubMed] [Google Scholar]
- 39. Li J. Ahn J. Liu D. et al.: ‘Evaluation of ultrasound‐induced damage to Escherichia coli and Staphylococcus aureus by flow cytometry and transmission electron microscopy’, Appl. Environ. Microbiol., 2016, 82, (6), pp. 1828 –1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jackson P. Jacobsen N.R. Baun A. et al.: ‘Bioaccumulation and ecotoxicity of carbon nanotubes’, Chem. Cent. J., 2013, 7, p. 154. doi: 10.1186/1752‐153X‐7‐154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwan H.P.: ‘Electrical properties of tissue and Cellsuspensions', in Lawrence V.J.H. Tobias C.A. (Eds.): ʻAdvance in biological and medical Physics' (Academic Press, Inc., NewYork, 1959), p. 147 [DOI] [PubMed] [Google Scholar]
- 42. Ingraham J. Low K.B. Magasanik B. et al.: ‘Escherichia coli and Salmonella typhimurium: cellular and molecular biology’, Am. Soc. Microbiol., 1987, 1, pp. 1444 –1452 [Google Scholar]


