Abstract
Nanotechnology is an emerging field of science that applies particles between 1 and 100 nm in size for a range of practical uses. Nano‐technological discoveries have opened novel applications in biotechnology and agriculture. Many reactions involving nanoparticles (NPs) are more efficient compared to those of their respective bulk materials. NPs obtained from plant material, denoted as biogenic or phytosynthesised NPs, are preferred over chemically synthesised NPs due to their low toxicity, rapid reactions and cost‐effective production. NPs impart both positive and negative impacts on plant growth and development. NPs exhibit their unique actions as a function of their size, reactivity, surface area and concentration. An insight into NP biosynthesis and translocation within the plant system will shed some light on the roles and mechanisms of NP‐mediated regulation of plant metabolism. This review is a step towards that goal.
Inspec keywords: nanofabrication, nanoparticles, nanobiotechnology, particle size, reviews, botany, biochemistry
Other keywords: chemically synthesised NPs, low toxicity, rapid reactions, cost‐effective production, positive impacts, plant growth, translocation, plant system, plant metabolism, nanotechnological discoveries, biotechnology, agriculture, plant material, biogenic NPs, phytosynthesised NPs, bulk materials, nanoparticles, biosynthesis, surface area, review, size 1.0 nm to 100.0 nm
1 Introduction
Nanotechnology is a branch of technology that focuses on nanomaterials and their application in various fields. Nanoparticles (NPs) exist at the nanoscale, having at least one dimension between 1 and 100 nm. NPs are derived from their bulk counterparts but offer distinct properties [1]. Since the inception of nanotechnology in recent decades, market investment has increased substantially due to its applications in environmental science and agriculture [2]. Global investment for nanotechnology applications in environmental management to date, and that expected in the near‐future, is given in Fig. 1.
Fig. 1.
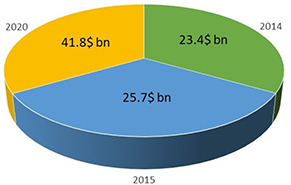
Investment in the global nanotechnology market for environmental applications
The role of NPs in commercial products and industrial applications has increased significantly. A large quantity and variety of naturally occurring NPs are distributed throughout the atmosphere, oceans, aquatic ecosystems, soil and most living organisms [3, 4, 5]. Interaction between NPs and biological systems requires extensive molecular study, which is a relatively new area of nanotechnology. NPs possess myriad functions and infinite uses due to their varying conformation, composition and behaviour; hence, nanotechnology has become a rapidly expanding field of investigation and development [6].
The exceptional characteristics and high reactivity of NPs are due to their small size and large surface area that play a critical role in enhancing their performance as compared to their conventional bulk materials. The unique features of NPs are exploited by a wide array of industries. Applications of NPs have been examined in several fields of bioscience and biomedicine with an increasing number of commercial applications [7]. By evaluating the bioavailability and potential toxicity of engineered nanomaterials, their beneficial effects can be further explored.
Research has been conducted to determine the possible roles of NPs in plant growth and development. It has been found that NPs are capable of generating both positive and negative response in plants. In addition, NPs play an important role in phytoremediation, i.e. plant‐based cleanup of contaminated soil and water. Currently, a vast proportion of nanotechnology in industry is devoted to the development of new functional materials, product development and design of methods and instrumentation for food safety and biosecurity [8].
In this review, the sources, biosynthesis, uptake and translocation of NPs, and their plausible role(s) in regulating plant metabolism are summarised.
2 Sources of NPs
Common sources of NPs are shown in Fig. 2; sources are both natural and anthropogenic. Diverse natural processes can indirectly generate NPs; these include photochemical reactions, volcanic eruptions and forest fires. Even wind‐ and water‐based soil erosion result in NP formation [9]. Anthropogenic activities are also responsible for NP production. NPs are produced inadvertently; they may be byproducts of simple combustion and food cooking or manufacturing processes such as welding, refining and smelting [6]. NPs which are intentionally manufactured are used for specialised applications in industrial, agricultural and other purposes; these are termed engineered NPs and include carbon NPs, metal oxide NPs, zero valence metal NPs and so on [10].
Fig. 2.
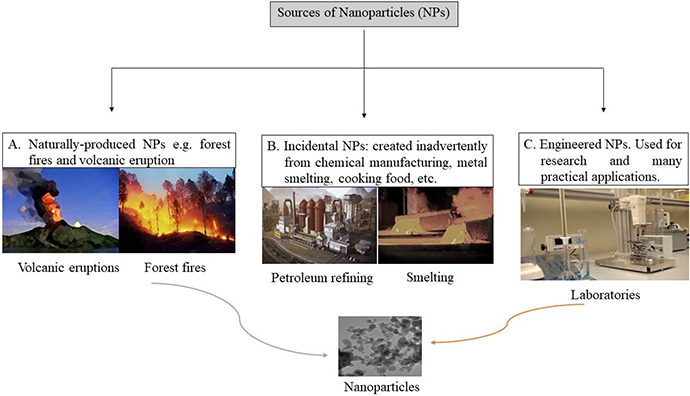
Sources of NPs: A. natural; B. incidental (created during manufacturing and other processes); and C. engineered NPs
3 Classification of NPs
NPs have been classified into different groups on the basis of their inherent characteristics. Scientists often modify NPs to render them more applicable to various uses and end‐products. Klaine et al. [11] divide NPs into different groups on the basis of their origin and chemical nature.
(i) NPs derived from carbon‐based materials are known as carbon nanotubes (CNTs) including fullerenes, single‐walled CNTs (SWCNT) and multiwalled CNTs (MWCNT). Fullerenes, also called buckyballs (due to resemblance to a ball) are hollow spheres containing 60 carbons obtained from graphite [12].
(ii) Oxides of metal NPs [11]. The most commonly used NP oxides in plants are TiO2 [13, 14], ZnO [15], CuO [16, 17, 18], Al2 O3 and CeO2.
(iii) Dendrimers are nano‐sized polymers formed by the branching of large unit NPs; its core and/or branches are formulated to perform specific chemical functions [11].
(iv) Biological materials attached to NMs comprise the fourth class. Attachment of DNA to titanium is an example (U.S. EPA2007).
(v) Zero‐valent metal (zV). This particle serves to reduce a number of elements and compounds in situ. For example, zero valent iron is used to treat groundwater to remove nitrate, and also for the dehalogenation of organochlorine pesticides and polychlorinated biphenyls [19].
4 Plants can be used as a ‘green’ source for NP synthesis
NPs are synthesised using varied chemical approaches. Scientists can modify NP chemical, physical and surface properties in order to utilise them optimally to perform a specific function. NPs produced by standardised chemical and physical processes in laboratories and industry tend to be more popular and can consistently produce NPs having desired characteristics [20, 21, 22]; however, such NPs may be directly hazardous to the environment and human health. Chemical synthesis methods are also expensive and often time consuming [23, 24].
It is important to understand how NPs are synthesised in a simple and reproducible manner and still possess the desired characteristics. In recent years, scientists have identified alternative methods to produce NPs which are eco‐friendly and less costly.
Fig. 3 reveals that NPs can be synthesised by a vast range of precursors beyond plants.
Fig. 3.
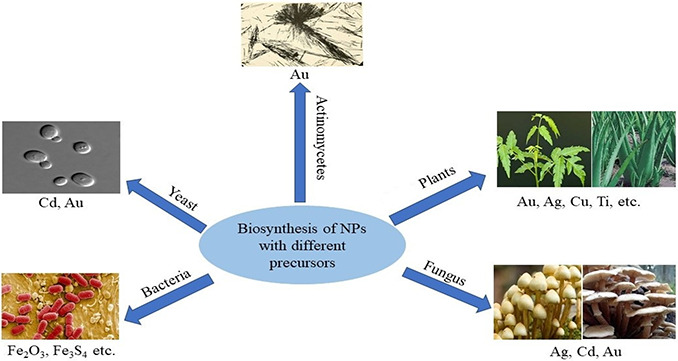
NP biosynthesis using different biological precursors
It has been reported that algae [25], diatoms [26], bacteria [27], yeast [28] and fungi [29] transform bulk materials into their respective NPs due to the reductive nature of proteins and metabolites present in these organisms [30].
Plants have become popular ‘green factories’ for the synthesis of NPs, which are often non‐toxic [31]. A wide spectrum of plants can be cultivated and processed through which ‘green NPs’ are extracted; NPs derived from plant extracts and microorganisms were more pharmacologically active than chemically synthesised NPs. Among these, NPs produced by medicinal plants were found to be the most pharmacologically active, possibly due to the attachment of several pharmacologically active residues [32].
In plants, the basic synthesis mechanism involves the accumulation of the metal within tissue and, after sequential physiologic processes, NPs are generated which can be extracted. An example is the synthesis of silver NPs (AgNPs) – Brassica juncea and Medicago sativa use silver nitrate as a substrate, transform it and accumulate it as 50 nm AgNPs [33]. Gold icosahedral NPs, 4 nm in size, were observed in M. sativa; copper particles, semi‐spherical in shape and 2 nm in size were detected in Iris pseudacrus [34, 35]. Extracts of aloe vera leaves were used for the production of cubic In2 O3 NPs measuring 5–50 nm [36]. Waghmare et al. [37] synthesised extracellular AgNPs from Candida utilis NCIM 3469. The authors characterised the NPs by UV–visible spectroscopy and scanning electron microscopy and found that the AgNPs are spherical. Extracts from lemon grass (Cymbopogon flexuosus) was used for the production of gold nanospheres and nanotriangles with a size range of 0.05–18 µm [38]. NPs and the respective plants from which they are synthesised are provided in Table 1.
Table 1.
Selected examples of plants used in NPs synthesis
| Plant | Nanoparticles (NPs) | Reference |
|---|---|---|
| Emblica officinalis | Ag/Au triangle form | [39] |
| Avena sativa | Au | [40] |
| Aloe barbadensis | Au (nanotriangle)/Ag | [41] |
| Cinnamomum camphora | Au/Ag | [42] |
| Medicago sativa | Ti/Ni bimetallic | [43] |
| Iris pseudacorus | Cu | [35] |
| Pelargonium graveolens | Ag | [44] |
| Lavandula intermedia | Ag | [45] |
| Ixiro coccinea | CuO | [46] |
| Aloe vera | Cu | [47] |
| Ginkgo biloba | Cu | [48] |
| Nigella sativa | Ag | [49] |
| Zea mays L. | Ag | [50] |
| Musa sp. | CdS | [51] |
| Glycyrrhiza uralensis | Au/AgCl | [52] |
| Syzygium cumini | Ag | [53] |
| Juglans regia | Au | [54] |
| Eichhornia crassipes | ZnO | [55] |
| Kalopanax pictus | MnO2 | [56] |
| Diopyros kaki | Pt | [57] |
| Vitis vinifera | Se | [58] |
| Brassica oleracia | CaO | [59] |
| Elettaria cardamomum | Au | [60] |
| Suaeda aegyptiaca | ZnO | [61] |
| Sageretia thea | ZnO | [62] |
| Cymbopogon citratus | Ag | [63] |
| Butea monosperma | Ag | [64] |
5 Uptake, accumulation and translocation into plant cells
Engineered nanomaterials are present in soil, water and the atmosphere, so plants readily encounter them. Plant‐accumulated NPs can subsequently become important routes of exposure for higher species, i.e. bioaccumulation through the food chain [65]. Entry of NPs into the plant cell is oftentimes not simple, however, as the cell wall resists entry of foreign materials. The mode of entry of NPs in plants depends on plant species, size of NPs and NP chemical composition. Stability of NPs also affects entry into the plant [66].
Carbon‐based materials and metal oxide NPs enter plants via distinct modes. Carrier proteins, aquaporins and endocytosis ion channels in cells comprise entry points for NPs [66]. An endocytosis‐like structure in the plasma membrane of Arabidopsis thaliana leaves allowed for entry of SWCNTs as reported by Shen et al. [67]. Transporters are proteins which mediate the shunting of ions across the cell membrane. In several studies, metal‐based NPs were moved across membranes with the assistance of transporters [68]. Once NPs enter plant tissue they can be transported elsewhere by apoplastic or symplastic methods. NPs can be transported from one cell to another with the help of plasmodesmata, which are located between the two cells [66].
Liu et al. reported that SWCNTs penetrate intact cell walls and cell membranes via a fluid phase of endocytosis in Nicotiana tabacum cv bright yellow (BY‐2) [69].
In tomato seedlings, MWCNTs enter cells through seeds and the root system [70]. It was found that MWCNTs enter wheat seedlings by piercing the epidermal and root hair cell wall and by penetrating the root cap [71]. Some CNTs are unable to penetrate plant cells; this is especially true for MWCNTs, due to difficulty in penetrating the rigid cell wall. Tan et al. [72] showed that cell walls of rice in suspension culture limited entry of MWCNTs into the cellular cytoplasm.
In natural ecosystems, uptake of nanomaterials by plants depends strongly upon soil properties such as chemical composition, organic matter content and colloidal properties [66]. Natural organic matter (NOM), a collection of heterogeneous organic substances derived from decomposed organisms, is a key factor affecting NP exposure to, and uptake by, plants [73]. Uptake, accumulation, and translocation of fullerene C70 (CNTs) and MWCNTs in rice plants with the support of a NOM suspension was reported by Lin et al. [74].
In several studies, different modes of uptake and translocation of metal oxide and metallic NPs have been reported. Kurepa et al. showed that uptake and translocation of conjugated TiO2 NPs (size ≤5 nm) with Alizarin red S by seedlings of Arabidopsis thaliana showed cell‐ and tissue‐specific distribution [75]. Uptake and translocation of ZnO NPs were investigated by López‐Moreno et al. in Glycine max (soybean) seedlings where ZnO NPs accumulated in roots and affected root growth in concentration‐dependent manner; at lower concentration it increased the root length whereas, at higher concentration a reduction in root length was observed [76]. In ryegrass, adsorption and aggregation of ZnO NPs to root surfaces, observed by scanning electron microscopy, was reported by Lin et al. [74]. Other metal oxide NPs can be translocated and accumulated in plant cells; however, few references are available. At this time only one report is available about Ni hydroxide NP translocation: when mesquite plants were treated with Ni(OH)2 NPs of size 8.7 nm, the NPs were absorbed by roots and translocated to shoots. Plants were treated with both uncoated and citrate‐coated Ni(OH)2 NPs and the results observed with XANES spectra. The uncoated Ni NPs were present in roots and shoots, whereas coated NPs were confined solely to the root region [77]. Lee et al. examined possible uptake and translocation of Cu NPs by Phaseolus radiate and Triticum aestivum using agar as the growth medium [78]. Ag NPs taken up by Cucurbita pepo accumulated more in the shoots in comparison to their bulk materials.
Some NPs are present only at the site of application and nowhere else in the cells; this demonstrates that transport of some NPs is severely restricted. For example, when leaf petioles of live pumpkin plants were treated with carbon‐coated Fe NPs, the NPs were detected only in the epidermal cells, close to the point of application [79].
6 Role of NPs in plants
The main objective of nanotechnology in field agriculture is to enhance the efficiency and sustainability of agricultural practices using fewer inputs, and generate less waste than conventional products and approaches [80].
The most commonly used NPs in plant science have been Au, Zn, Ti, Ag and Si; all have demonstrated promising effects on plant growth and development. Other NPs show positive results with plants; however, more study is required to clarify mechanisms of uptake, translocation and effects on metabolism. A number of studies have revealed that NPs impart positive effects on the morphological and physiological condition of plants. Some NPs are toxic to plants (discussed below). A simplified view depicting the uses of NPs in the field of agriculture is shown in Fig. 4.
Fig. 4.
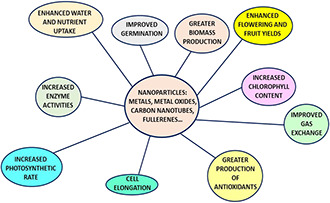
NPs can be used to perform different functions in agriculture
6.1 Role of carbon‐based nanomaterials
Due to their distinct characteristics, CNTs play important roles in plant growth and development. One unique property is their ability to penetrate the cell wall and cell membrane and act as a delivery system of chemicals to the cells. SWCNTs can be used as nano‐transporters for delivery of DNA and dye molecules into plant cells. The positive response of plants to CNTs and other carbon‐based nanomaterials like fullerenes (C 70) and fullerols C 60 (OH)2 have been investigated in many studies. Khodakovskaya et al. [70] reported that CNTs penetrate tomato seeds and improve germination and growth rates. Germination was found to be dramatically higher for seeds placed in a medium containing CNTs (10–40 g/ml) compared to the control. Various analytical methods have indicated that CNTs penetrate the thick seed coat and support water uptake into seeds, a process which influences seed germination and growth of tomato seedlings. Water and essential nutrients, for example, Ca and Fe, are important for increasing germination and plant growth and development. It is reported that MWCNT enhanced the uptake efficiency of water and nutrients in maize and tomato [81, 82]. Lahiani et al. [83] have reported that MWCNTs regulated genes encoding the expression of several types of water channel proteins in soybean, corn and barley seed coats.
Enhancement of root cell elongation and increased dehydrogenase activity in the presence of oxidised MWCNTs has been reported [84]. Water‐soluble CNTs were present in wheat plants and confirmed by scanning electron and fluorescence microscopy. The presence of CNTs in the cells induced root and shoot growth under both light and dark conditions [85]. The influence of MWCNTs on plants is diverse – they improve a number of growth parameters and overall development in plants. Improvement in water retention capacity and biomass, flowering and fruit yield and increased medicinal properties of plants with MWCNTs have been reported by Khodakovskaya et al. [86] and Husen and Siddiqi [87].
6.2 Role of metal oxide and metal‐based NPs
A number of reports have focused on the impact of metal and metal oxide NPs on plant growth and development as compared to their corresponding bulk NPs or as ions. It was reported that spinach (Spinacia oleracea) grown in 2.5% TiO2 NPs (rutile) solution showed more rapid germination and vigor than did plants in contact with bulk TiO2 ‐exposed seeds [14]. In canola seedlings, TiO2 NPs improved germination and promoted radicle and plumule growth [88]. The effect of foliar application of Ti NPs to wheat plants (Triticum aestivum) was observed under stressed conditions with normal irrigation. Titanium NPs applied to the plants resulted in significantly enhanced stem elongation and flowering relative to plants exposed to the corresponding bulk Ti particles [89].
The positive effects of AgNPs to plants have been reported. Savithramma et al. [90] reported enhanced seed germination and seedling growth of the tree Boswellia ovaliofoliolata using biologically synthesised AgNPs. Increased shoot and root length and leaf area of Brassica juncea, common bean and corn were reported using AgNPs. Treated plants also experienced an increase in biochemical parameters including chlorophyll, carbohydrate and protein contents, and activities of antioxidant enzymes [31, 91]. Induction of root growth in Crocus sativus by AgNPs was due to the blocking of ethylene signalling in plants [92].
Gold NPs (AuNPs) are reported to play an important role in plant growth. AuNPs improved seed germination and the antioxidant system in Arabidopsis thaliana. AuNPs also altered levels of microRNA (miRNA) expression that regulates various morphological, physiological and metabolic processes in the plants. Neomycin phosphotransferase II gene was introduced into the soybean genome with the assistance of AuNPs coated with DNA [93].
A number of experiments have been conducted using ZnO NPs in plants, and their benefits for plant growth and development have been documented. Pearl millet (Pennisetum americanum) grown in soil received 10 mg l−1 as foliar application of both bulk and biosynthesised Zn NPs. The Zn NPs were synthesised from a ZnO solution using fungal cell‐free filtrate (Rhizoctonia bataticola TFR‐6). In the ZnO NP treatment, there were considerable increases in shoot length (10.8%), root area (18.4%), dry biomass (12.0%) and grain yield (29.5%) as compared to that in the bulk Zn particle treatment [94]. Prasad et al. [95] treated peanut seeds with ZnO NPs and chelated bulk ZnSO4; with the NPs, improvement in germination, chlorophyll content and stem and root growth were reported in comparison to bulk Zn salts. Higher biomass was reported in Cicer arietinum L var. HC‐1 (chickpea) when treated with ZnO NPs in aqueous solution as compared with the salt of a bulk ZnSO4 solution [96]. A low concentration of ZnO NPs improved seed germination in soybean, wheat and onion [97, 98, 99]. ZnO NPs enhanced root and shoot length and root and shoot biomass in seedlings of Vigna radiata and Cicer arietinum [100]. Positive results of ZnO NPs were also reported in plant tissue culture. MS media prepared with ZnO NPs improved somatic embryogenesis, shooting and regeneration of plantlets [101].
Improvement in seed germination was reported in tomato using a low concentration of nano‐SiO2 [102]. Exogenous application of nano‐SiO2 to Changbai larch (Larix olgensis) seedlings yielded beneficial results on seedling growth and quality, including mean height, root collar diameter, main root length and number of lateral roots of seedlings [103]. SiO2 NPs applied to plants under stressed conditions also mediated improved plant growth and development. Nano‐SiO2 improved seed germination in tomato and squash and enhanced the antioxidant system under NaCl (salt stress) conditions [104, 105]. Nitrate reductase activity was increased by the application of nano‐SiO2 and nano‐TiO2, which improved seed germination of soybean [106].
6.3 Role of NPs in photosynthesis and biochemical activity
Photosynthesis is a process in which plants absorb light energy and convert it to chemical energy. Enzymes play a critical role in photosynthesis. Nanotechnology has included attempts at increasing the photosynthetic rate with the help of NPs. NPs influence photosynthesis by acting upon the enzyme Rubisco, which is the primary enzyme involved in photosynthesis and the most abundant protein on earth. Siddiqui and Al‐Whaibi [102] and Xie et al. [107] found that SiO2 NPs improved carbonic anhydrase (an enzyme that converts CO2 to bicarbonate) activity and increased photosynthetic pigments which enhance photosynthetic rate. TiO2 NPs act as a scavenger by protecting chloroplasts from extreme light, which can be achieved by increasing the activities of antioxidant enzymes such as catalase, peroxidase and superoxide dismutase (SOD) [108]. TiO2 NPs influence Rubisco activity, chlorophyll formation and increase photosynthetic rate, which enhance plant growth and development [109]. Lei et al. worked with nano‐anatase and suggested that these nanomaterials promote the ETS, PS II and photophosphorylation of chlorophyll activity under both visible and UV conditions [110]. A three‐fold greater photosynthetic rate was reported with SWCNTs embedded in isolated chloroplasts as compared to controls. The SWCNTs also enhanced the rate of electron transport in plants [111]. When Spinacia oleracea was treated with TiO2 NPs and bulk‐TiO2, the NPs enhanced chlorophyll formation, Rubisco activity and overall photosynthetic rate in comparison to bulk TiO2 [14]. Nano‐mesoporous silica compound (SBA) bonded with PSII and maintained the stability of the oxygen‐evolving reaction in the photosynthetic system [112]. Pradhan et al. [113] worked on mung bean with MnNPs in Hoagland solution and compared their results with bulk MnSO4 salt; they reported that MnNPs increased chlorophyll content, carotene level, photophosphorylation and oxygen evolution in plants. Increased conductance of water and increased transpiration rate, and an improved net photosynthetic rate was reported in Lycopersicon esculentum when TiO2 NPs were applied exogenously [114].
ZnNPs increase the activity of acid phosphatase, alkaline phosphatase, phytase and dehydrogenase in pearl millet when compared to bulk‐Zn metals [94]. MWCNTs mediated a positive response in peroxidase and dehydrogenase activities [84]. MS media supplemented with ZnO NPs had positive effects on proline synthesis activity, SOD, catalase and peroxidase (POX); hence plants could recover more efficiently under biotic stress [101]. Increase in plant growth and development have been reported using nano‐SiO2 via escalating gas exchange and chlorophyll fluorescence parameters such as net photosynthetic rate, transpiration rate, stomatal conductance, PSII, effective photochemical efficiency, actual photochemical efficiency, electron transport rate and photochemical quench [105, 107]. Wang et al. [115] studied the physiological effects of magnetite (Fe3 O4) NPs on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta cv. white cushaw) plants under hydroponic conditions. They reported that Fe3 O4 NPs enhance catalase (CAT) activity in ryegrass and pumpkin roots and shoots.
7 Nanotoxicology: NPs may be toxic to plants
A range of beneficial properties of NPs to plants have been demonstrated thus far; however, many NPs exert detrimental effects on plants. Environmental factors which alter dissolution of metal‐containing NPs and their bioavailability play important roles in determining toxicity, especially for metals such as Cu, that are both essential for cellular function yet exhibit very sharp toxic thresholds [116].
7.1 Impact of toxicity at various levels
NPs may impart more negative effects to plants (phytotoxicity) as compared with positive responses. It is imperative to determine at which levels NPs affect plant health directly. Plant nanotoxicology was introduced as a discipline to explore the effects and toxicity mechanisms of NPs in plants, including transport, surface interactions and material‐specific responses [117]. Toxicity of AgNPs at low concentrations has been reported in seedlings of Arabidopsis thaliana plants [118]. Phytotoxicity of different NPs (MWCNTs, Al, Zn and ZnO) has been reported for seed germination and root growth in six higher plant species [119]. Phytotoxicity of NPs at cytogenic levels has also been investigated. Production of reactive oxygen species (ROS) and their accumulation in the cell wall and plasma membrane cause apoptotic cell death in leaves of A. thaliana when CNT suspension is injected into plants [67]. Toxicity at the genetic level (genotoxicity) has been reported with AgNPs on Allium cepa (onion) root tip cells, where the chromosome sequence in the cells was altered [120]. No negative effects were detected on seed germination and root elongation in zucchini plants when exposed to MWCNTs. With SWCNTs, however, a decrease in plant biomass was observed [121]. Toxic effects of Cu NPs on mung bean and wheat plants were observed by Lee et al. [122]. Plants were treated with different concentrations of Cu NPs and inhibition in seedling growth was reported in both species but mung bean was more sensitive than wheat.
TiO2 inhibited germination and growth of onion at high concentrations and also induced the activities of hydrolytic and antioxidant enzymes [123]. Atha et al. [17] were the first to report toxicity at the molecular level by copper oxide (CuO) NPs. CuO NPs stimulated DNA damage in agricultural and grassland plants. Oukarroum et al. [124] studied the effect of AgNPs on the aquatic plant Lemna gibba and found that NPs reduced the plant growth, cellular viability and production of intracellular ROS. López‐Moreno et al. [125] reported toxicity due to CeO2 NPs in alfalfa, corn, cucumber and tomato. Rico et al. [126] reported NP‐mediated alteration in antioxidant enzyme activities, sugar accumulation and fatty acid content in a concentration‐dependent manner where toxicity generated by nano‐CeO2 resulted in membrane damage and photosynthetic stress in shoots of rice. Lei et al. [110] found that nano‐anatase TiO2 under UV‐B radiation mitigated the stress effects by decreasing the accumulation of superoxide radicals, H2 O2 and malondialdehyde content, and increasing the activities of SOD, catalase, ascorbate peroxidase and guaiacol peroxidase in chloroplasts of spinach.
8 Conclusion
Nanotechnology, a new and intriguing field of science, permits advanced research in many areas that could be used for novel applications in improving crop physiology. Plants can be used as ‘green factories’ for NP biosynthesis as they produce non‐toxic, rapid and cost‐effective particles. The role of NPs in promoting photosynthetic rate, pigment production and enzyme function could be exploited to increase crop productivity. The stress mitigating properties of NPs should be explored further for better understanding of their application to field situations. The toxicity symptoms generated by NPs must be investigated at the molecular level to better understand optimal methods for synthesis of NPs that would be more efficient for plant growth and development. NPs are capable of entering food chains which may be harmful to humans, livestock and other organisms. Hence, uptake and translocation of NPs from the soil by plants should be focused upon in future studies.
9 Acknowledgments
The authors thank Aligarh Muslim University and the University Grants Commission for financial assistance. They also acknowledge all the authors for their support and assistance in writing this review.
10 References
- 1. Nel A. Xia T. Mädler L. et al.: ‘Toxic potential of materials at the nanolevel’, Science, 2006, 311, (5761), pp. 622 –627 [DOI] [PubMed] [Google Scholar]
- 2. Rajput V.D. Minkina T. Suskova S. et al.: ‘Effects of copper nanoparticles (CuO NPs) on crop plants: a mini review’, Bionanoscience, 2018, 8, (1), pp. 36 –42 [Google Scholar]
- 3. Colvin V.L.: ‘The potential environmental impact of engineered nanomaterials’, Nat. Biotechnol., 2003, 21, (10), p. 1166 [DOI] [PubMed] [Google Scholar]
- 4. Hochella M.F. Lower S.K. Maurice P.A. et al.: ‘Nanominerals, mineral nanoparticles, and earth systems’, Science, 2008, 319, (5870), pp. 1631 –1635 [DOI] [PubMed] [Google Scholar]
- 5. Wiesner M.R. Lowry G. V Jones K.L. et al.: ‘Decreasing uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials’, Environ. Sci. Technol., 2009, 43, (17), pp. 6458 –6462 [DOI] [PubMed] [Google Scholar]
- 6. Remédios C. Rosário F. Bastos V.: ‘Environmental nanoparticles interactions with plants: morphological, physiological, and genotoxic aspects’, J. Bot., 2012, 2012, pp. 1 –8 [Google Scholar]
- 7. Salata O.V: ‘Applications of nanoparticles in biology and medicine’, J. Nanobiotechnol., 2004, 2, (1), p. 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moraru C.I. Panchapakesan C.P. Huang Q. et al.: ‘Nanotechnology: a new frontier in food science’, Food Technol., 2003, 57, pp. 24 –29 [Google Scholar]
- 9. Buzea C. Pacheco I.I. Robbie K.: ‘Nanomaterials and nanoparticles: sources and toxicity’, Biointerphases, 2007, 2, (4), p. 17 [DOI] [PubMed] [Google Scholar]
- 10. Barceló D. Farré M.: ‘Characterization, analysis and risks of nanomaterials in environmental and food samples’, Trends Analyt. Chem., 2011, 30, (3), pp. 517 –527 [Google Scholar]
- 11. Klaine S.J. Alvarez P.J.J. Batley G.E. et al.: ‘Nanomaterials in the environment: behavior, fate, bioavailability, and effects’, Environ. Toxicol. Chem., 2008, 27, (9), pp. 1825 –1851 [DOI] [PubMed] [Google Scholar]
- 12. Kroto H.W. Heath J.R. O'Brien S.C. et al.: ‘C60: buckminsterfullerene’, Nature, 1985, 318, pp. 162 –163 [Google Scholar]
- 13. Larue C. Laurette J. Herlin‐Boime N. et al.: ‘Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): influence of diameter and crystal phase’, Sci. Total Environ., 2012, 431, pp. 197 –208 [DOI] [PubMed] [Google Scholar]
- 14. Zheng L. Hong F. Lu S. et al.: ‘Effect of nano‐TiO2 on strength of naturally aged seeds and growth of spinach’, Biol. Trace Elem. Res., 2005, 104, (1), pp. 83 –91 [DOI] [PubMed] [Google Scholar]
- 15. Hernandez‐Viezcas J.A. Castillo‐Michel H. Servin A.D. et al.: ‘Spectroscopic verification of zinc absorption and distribution in the desert plant Prosopis juliflora‐velutina (velvet mesquite) treated with ZnO nanoparticles’, Chem. Eng. J., 2011, 170, (2–3), pp. 346 –352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saison C. Perreault F. Daigle J.‐C. et al.: ‘Effect of core–shell copper oxide nanoparticles on cell culture morphology and photosynthesis (photosystem II energy distribution) in the green alga, chlamydomonas reinhardtii’, Aquat. Toxicol., 2010, 96, (2), pp. 109 –114 [DOI] [PubMed] [Google Scholar]
- 17. Atha D.H. Wang H. Petersen E.J. et al.: ‘Copper oxide nanoparticle mediated DNA damage in terrestrial plant models’, Environ. Sci. Technol., 2012, 46, (3), pp. 1819 –1827 [DOI] [PubMed] [Google Scholar]
- 18. Nekrasova G.F. Ushakova O.S. Ermakov A.E. et al.: ‘Effects of copper (II) ions and copper oxide nanoparticles on Elodea densa planch’, Russ. J. Ecol., 2011, 42, (6), p. 458 [Google Scholar]
- 19. Zhang W.: ‘Nanoscale iron particles for environmental remediation: an overview’, J. Nanoparticle Res., 2003, 5, (3–4), pp. 323 –332 [Google Scholar]
- 20. Tsuji T. Kakita T. Tsuji M.: ‘Preparation of nano‐size particles of silver with femtosecond laser ablation in water’, Appl. Surf. Sci., 2003, 206, (1–4), pp. 314 –320 [Google Scholar]
- 21. Kundu S. Wang K. Liang H.: ‘Size‐controlled synthesis and self‐assembly of silver nanoparticles within a minute using microwave irradiation’, J. Phys. Chem. C., 2008, 113, (1), pp. 134 –141 [Google Scholar]
- 22. Okitsu K. Mizukoshi Y. Yamamoto T.A. et al.: ‘Sonochemical synthesis of gold nanoparticles on chitosan’, Mater. Lett., 2007, 61, (16), pp. 3429 –3431 [Google Scholar]
- 23. Narayanan K.B. Sakthivel N.: ‘Biological synthesis of metal nanoparticles by microbes’, Adv. Colloid Interface Sci., 2010, 156, (1–2), pp. 1 –13 [DOI] [PubMed] [Google Scholar]
- 24. Gan P.P. Ng S.H. Huang Y. et al.: ‘Green synthesis of gold nanoparticles using palm oil mill effluent (POME): a low‐cost and eco‐friendly viable approach’, Bioresour. Technol., 2012, 113, pp. 132 –135 [DOI] [PubMed] [Google Scholar]
- 25. Govindaraju K. Kiruthiga V. Kumar V.G. et al.: ‘Extracellular synthesis of silver nanoparticles by a marine alga, sargassum wightii grevilli and their antibacterial effects’, J. Nanosci. Nanotechnol., 2009, 9, (9), pp. 5497 –5501 [DOI] [PubMed] [Google Scholar]
- 26. Scarano G. Morelli E.: ‘Characterization of cadmium‐and lead‐phytochelatin complexes formed in a marine microalga in response to metal exposure’, Biometals, 2002, 15, (2), pp. 145 –151 [DOI] [PubMed] [Google Scholar]
- 27. Lengke M.F. Fleet M.E. Southam G.: ‘Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex’, Langmuir, 2007, 23, (5), pp. 2694 –2699 [DOI] [PubMed] [Google Scholar]
- 28. Kowshik M. Ashtaputre S. Kharrazi S. et al.: ‘Extracellular synthesis of silver nanoparticles by a silver‐tolerant yeast strain MKY3’, Nanotechnology, 2002, 14, (1), p. 95 [Google Scholar]
- 29. Rautaray D. Ahmad A. Sastry M.: ‘Biosynthesis of CaCO3 crystals of complex morphology using a fungus and an actinomycete’, J. Am. Chem. Soc., 2003, 125, (48), pp. 14656 –14657 [DOI] [PubMed] [Google Scholar]
- 30. Makarov V.V Love A.J. Sinitsyna O.V et al.: ‘‘Green’ nanotechnologies: synthesis of metal nanoparticles using plants’, Acta Naturae (англоязычная версия), 2014, 6, (1), pp. 35 –44 [PMC free article] [PubMed] [Google Scholar]
- 31. Salama H.M.H.: ‘Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.)’, Int. Res. J. Biotechnol., 2012, 3, (10), pp. 190 –197 [Google Scholar]
- 32. Singh P. Kim Y.‐J. Zhang D. et al.: ‘Biological synthesis of nanoparticles from plants and microorganisms’, Trends Biotechnol., 2016, 34, (7), pp. 588 –599 [DOI] [PubMed] [Google Scholar]
- 33. Harris A.T. Bali R.: ‘On the formation and extent of uptake of silver nanoparticles by live plants’, J. Nanoparticle Res., 2008, 10, (4), pp. 691 –695 [Google Scholar]
- 34. Gardea‐Torresdey J.L. Parsons J.G. Gomez E. et al.: ‘Formation and growth of Au nanoparticles inside live alfalfa plants’, Nano Lett., 2002, 2, (4), pp. 397 –401 [Google Scholar]
- 35. Manceau A. Nagy K.L. Marcus M.A. et al.: ‘Formation of metallic copper nanoparticles at the soil − root interface’, Environ. Sci. Technol., 2008, 42, (5), pp. 1766 –1772 [DOI] [PubMed] [Google Scholar]
- 36. Maensiri S. Laokul P. Klinkaewnarong J. et al.: ‘Indium oxide (In2 O3) nanoparticles using aloe vera plant extract: synthesis and optical properties’, J. Optoelectron. Adv. Mater., 2008, 10, pp. 161 –165 [Google Scholar]
- 37. Waghmare S.R. Mulla M.N. Marathe S.R. et al.: ‘Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms’, 3 Biotech, 2015, 5, (1), pp. 33 –38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shankar S.S. Ahmad A. Pasricha R. et al.: ‘Bioreduction of chloroaurate ions by Geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes’, J. Mater. Chem., 2003, 13, (7), pp. 1822 –1826 [Google Scholar]
- 39. Ankamwar B. Damle C. Ahmad A. et al.: ‘Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution’, J. Nanosci. Nanotechnol., 2005, 5, (10), pp. 1665 –1671 [DOI] [PubMed] [Google Scholar]
- 40. Armendariz V. Herrera I. Peralta‐Videa J.R. et al.: ‘Size controlled gold nanoparticle formation by Avena sativa biomass: Use of plants in nanobiotechnology’, J. Nanoparticle Res., 2004, 6, (4), pp. 377 –382 [Google Scholar]
- 41. Chandran S.P. Chaudhary M. Pasricha R. et al.: ‘Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract’, Biotechnol. Prog., 2006, 22, (2), pp. 577 –583 [DOI] [PubMed] [Google Scholar]
- 42. Huang J. Li Q. Sun D. et al.: ‘Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf’, Nanotechnology, 2007, 18, (10), p. 105104 [Google Scholar]
- 43. Schabes‐Retchkiman P.S. Canizal G. Herrera‐Becerra R. et al.: ‘Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles’, Opt. Mater. (Amst), 2006, 29, (1), pp. 95 –99 [Google Scholar]
- 44. Shankar S.S. Ahmad A. Sastry M.: ‘Geranium leaf assisted biosynthesis of silver nanoparticles’, Biotechnol. Prog., 2003, 19, (6), pp. 1627 –1631 [DOI] [PubMed] [Google Scholar]
- 45. Elemike E.E. Onwudiwe D.C. Ekennia A.C. et al.: ‘Biosynthesis, characterization, and antimicrobial effect of silver nanoparticles obtained using Lavandula intermedia’, Res. Chem. Intermed., 2017, 43, (3), pp. 1383 –1394 [Google Scholar]
- 46. Kiran V. Krishnan A. Haribabu K. et al.: ‘Green synthesis of copper oxide nanoparticles using ixiro coccinea plant leaves and its characterization’, Bionanoscience, 2018, 8, (2), pp. 554 –558 [Google Scholar]
- 47. Pawlowska A. Sadowski Z.: ‘Biosynthesis of copper nanoparticles using aqueous extracts of aloe vera and geranium and bioleaching solutions’. 22nd Int. Biohydrometallurgy Symp., Freiberg, Germany, 2017, pp. 193 –196 [Google Scholar]
- 48. Nasrollahzadeh M. Mohammad Sajadi S.: ‘Green synthesis of copper nanoparticles using Ginkgo biloba L. Leaf extract and their catalytic activity for the Huisgen [3 + 2] cycloaddition of azides and alkynes at room temperature’, J. Colloid Interface Sci., 2015, 457, pp. 141 –147 [DOI] [PubMed] [Google Scholar]
- 49. Amooaghaie R. Saeri M.R. Azizi M.: ‘Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles’, Ecotoxicol. Environ. Saf., 2015, 120, pp. 400 –408 [DOI] [PubMed] [Google Scholar]
- 50. Tong X. Guo N. Dang Z. et al.: ‘In vivo biosynthesis and spatial distribution of Ag nanoparticles in maize (Zea mays L.)’, IET Nanobiotechnol., 2018, 12, (7), pp. 987 –993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou G.J. Li S.H. Zhang Y.C. et al.: ‘Biosynthesis of CdS nanoparticles in banana peel extract’, J. Nanosci. Nanotechnol., 2014, 14, (6), pp. 4437 –4442 [DOI] [PubMed] [Google Scholar]
- 52. Huo Y. Singh P. Kim Y.J. et al.: ‘Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications’, Artif. Cells, Nanomed. Biotechnol., 2018, 46, (2), pp. 303 –312 [DOI] [PubMed] [Google Scholar]
- 53. Ojo O.A. Oyinloye B.E. Ojo A.B. et al.: ‘Green‐route mediated synthesis of silver nanoparticles (AgNPs) from syzygium cumini (L.) Skeels polyphenolic‐rich leaf extracts and investigation of their antimicrobial activity’, IET Nanobiotechnol., 2018, 12, (3), pp. 305 –310 [Google Scholar]
- 54. Izadiyan Z. Shameli K. Hara H. et al.: ‘Cytotoxicity assay of biosynthesis gold nanoparticles mediated by walnut (Juglans regia) green husk extract’, J. Mol. Struct., 2018, 1151, pp. 97 –105 [Google Scholar]
- 55. Vanathi P. Rajiv P. Narendhran S. et al.: ‘Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach’, Mater. Lett., 2014, 134, pp. 13 –15 [Google Scholar]
- 56. Sathiyamoorthi E. Moon S.A. Alkotaini B. et al.: ‘Biological synthesis of manganese dioxide nanoparticles by Kalopanax pictus plant extract’, IET Nanobiotechnol., 2015, 9, (4), pp. 220 –225 [DOI] [PubMed] [Google Scholar]
- 57. Song J.Y. Kwon E.Y. Kim B.S.: ‘Biological synthesis of platinum nanoparticles using diopyros kaki leaf extract’, Bioprocess Biosyst. Eng., 2010, 33, (1), pp. 159 –164 [DOI] [PubMed] [Google Scholar]
- 58. Sharma G. Sharma A.R. Bhavesh R. et al.: ‘Biomolecule‐mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract’, Molecules, 2014, 19, (3), pp. 2761 –2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Osuntokun J. Onwudiwe D.C. Ebenso E.E.: ‘Aqueous extract of broccoli mediated synthesis of CaO nanoparticles and its application in the photocatalytic degradation of bromocrescol green’, IET Nanobiotechnol., 2018, 12, (7), pp. 888 –894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rajan A. Rajan A.R. Philip D.: ‘Elettaria cardamomum seed mediated rapid synthesis of gold nanoparticles and its biological activities’, OpenNano, 2017, 2, pp. 1 –8 [Google Scholar]
- 61. Rajabi H.R. Naghiha R. Kheirizadeh M. et al.: ‘Microwave assisted extraction as an efficient approach for biosynthesis of zinc oxide nanoparticles: synthesis, characterization, and biological properties’, Mater. Sci. Eng. C, 2017, 78, pp. 1109 –1118 [DOI] [PubMed] [Google Scholar]
- 62. Khalil A.T. Ovais M. Ullah I. et al.: ‘Sageretia thea (osbeck.) mediated synthesis of zinc oxide nanoparticles and its biological applications’, Nanomedicine, 2017, 12, (15), pp. 1767 –1789 [DOI] [PubMed] [Google Scholar]
- 63. Ajayi E. Afolayan A.: ‘Green synthesis, characterization and biological activities of silver nanoparticles from alkalinized Cymbopogon citratus stapf’, Adv. Nat. Sci. Nanosci. Nanotechnol., 2017, 8, (1), p. 015017 [Google Scholar]
- 64. Pattanayak S. Mollick M.M.R. Maity D. et al.: ‘Butea monosperma bark extract mediated green synthesis of silver nanoparticles: characterization and biomedical applications Butea monosperma bark extract mediated green synthesis of silver nanoparticles’, J. Saudi Chem. Soc., 2017, 21, (6), pp. 673 –684 [Google Scholar]
- 65. Miralles P. Church T.L. Harris A.T.: ‘Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants’, Environ. Sci. Technol., 2012, 46, (17), pp. 9224 –9239 [DOI] [PubMed] [Google Scholar]
- 66. Rico C.M. Majumdar S. Duarte‐Gardea M. et al.: ‘Interaction of nanoparticles with edible plants and their possible implications in the food chain’, J. Agric. Food Chem., 2011, 59, (8), pp. 3485 –3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shen C. Zhang Q. Li J. et al.: ‘Induction of programmed cell death in Arabidopsis and rice by single‐wall carbon nanotubes’, Am. J. Bot., 2010, 97, (10), pp. 1602 –1609 [DOI] [PubMed] [Google Scholar]
- 68. Hall S.R. Bolger H. Mann S.: ‘Morphosynthesis of complex inorganic forms using pollen grain templates’, Chem. Commun., 2003, 35, (22), pp. 2784 –2785 [DOI] [PubMed] [Google Scholar]
- 69. Le Van N. Rui Y. Cao W. et al.: ‘Toxicity and bio‐effects of CuO nanoparticles on transgenic Ipt‐cotton’, J. Plant Interact., 2016, 11, (1), pp. 108 –116 [Google Scholar]
- 70. Khodakovskaya M. Dervishi E. Mahmood M. et al.: ‘Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth’, ACS Nano, 2009, 3, (10), pp. 3221 –3227 [DOI] [PubMed] [Google Scholar]
- 71. Wild E. Jones K.C.: ‘Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants’, Environ. Sci. Technol., 2009, 43, (14), pp. 5290 –5294 [DOI] [PubMed] [Google Scholar]
- 72. Tan X. Lin C. Fugetsu B.: ‘Studies on toxicity of multi‐walled carbon nanotubes on suspension rice cells’, Carbon N. Y., 2009, 47, (15), pp. 3479 –3487 [DOI] [PubMed] [Google Scholar]
- 73. Chen R. Ratnikova T.A. Stone M.B. et al.: ‘Differential uptake of carbon nanoparticles by plant and mammalian cells’, Small, 2010, 6, (5), pp. 612 –617 [DOI] [PubMed] [Google Scholar]
- 74. Lin S. Reppert J. Hu Q. et al.: ‘Uptake, translocation, and transmission of carbon nanomaterials in rice plants’, Small, 2009, 5, (10), pp. 1128 –1132 [DOI] [PubMed] [Google Scholar]
- 75. Kurepa J. Paunesku T. Vogt S. et al.: ‘Uptake and distribution of ultrasmall anatase TiO2 alizarin red S nanoconjugates in Arabidopsis thaliana’, Nano Lett., 2010, 10, (7), pp. 2296 –2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. López‐Moreno M.L. de la Rosa G. Hernández‐Viezcas J.Á. et al.: ‘Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants’, Environ. Sci. Technol., 2010, 44, (19), pp. 7315 –7320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Parsons J.G. Peralta‐Videa J.R. Cruz‐Jiminez G. et al.: ‘Potential of chilopsis linearis for gold phytomining: using XAS to determine gold reduction and nanoparticle formation within plant tissues’, Int. J. Phytoremediat., 2009, 9, p. 133, (SLAC‐REPRINT‐2009–514) [DOI] [PubMed] [Google Scholar]
- 78. Lee W. An Y. Yoon H. et al.: ‘Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water‐insoluble nanoparticles’, Environ. Toxicol. Chem., 2008, 27, (9), pp. 1915 –1921 [DOI] [PubMed] [Google Scholar]
- 79. Corredor E. Testillano P.S. Coronado M.‐J. et al.: ‘Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification’, BMC Plant Biol., 2009, 9, (1), p. 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chinnamuthu C.R. Boopathi P.M.: ‘Nanotechnology and agroecosystem’, Madras Agric. J., 2009, 96, (1/6), pp. 17 –31 [Google Scholar]
- 81. Villagarcia H. Dervishi E. de Silva K. et al.: ‘Surface chemistry of carbon nanotubes impacts the growth and expression of water channel protein in tomato plants’, Small, 2012, 8, (15), pp. 2328 –2334 [DOI] [PubMed] [Google Scholar]
- 82. Tiwari D.K. Dasgupta‐Schubert N. Cendejas L.M.V. et al.: ‘Interfacing carbon nanotubes (CNT) with plants: enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture’, Appl. Nanosci., 2014, 4, (5), pp. 577 –591 [Google Scholar]
- 83. Lahiani M.H. Dervishi E. Chen J. et al.: ‘Impact of carbon nanotube exposure to seeds of valuable crops’, ACS Appl. Mater. Interfaces, 2013, 5, (16), pp. 7965 –7973 [DOI] [PubMed] [Google Scholar]
- 84. Wang X. Han H. Liu X. et al.: ‘Multi‐walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants’, J. Nanoparticle Res., 2012, 14, (6), p. 841 [Google Scholar]
- 85. Tripathi S. Sarkar S.: ‘Influence of water soluble carbon dots on the growth of wheat plant’, Appl. Nanosci., 2015, 5, (5), pp. 609 –616 [Google Scholar]
- 86. Khodakovskaya M.V Kim B. Kim J.N. et al.: ‘Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community’, Small, 2013, 9, (1), pp. 115 –123 [DOI] [PubMed] [Google Scholar]
- 87. Husen A. Siddiqi K.S.: ‘Carbon and fullerene nanomaterials in plant system’, J. Nanobiotechnol., 2014, 12, (1), p. 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mahmoodzadeh H. Nabavi M. Kashefi H.: ‘Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus)’, J. Ornamental Hortic. Plants, 2013, 3, pp. 25 –32 [Google Scholar]
- 89. Jaberzadeh A. Moaveni P. Moghadam H.R.T. et al.: ‘Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress’, Not. Bot. Horti Agrobot. Cluj‐Napoca, 2013, 41, (1), p. 201 [Google Scholar]
- 90. Savithramma N. Ankanna S. Bhumi G.: ‘Effect of nanoparticles on seed germination and seedling growth of Boswellia ovalifoliolata an endemic and endangered medicinal tree taxon’, Nano Vis., 2012, 2, (1), p. 2 [Google Scholar]
- 91. Sharma P. Bhatt D. Zaidi M.G.H. et al.: ‘Silver nanoparticle‐mediated enhancement in growth and antioxidant status of Brassica juncea’, Appl. Biochem. Biotechnol., 2012, 167, (8), pp. 2225 –2233 [DOI] [PubMed] [Google Scholar]
- 92. Rezvani N. Sorooshzadeh A. Farhadi N.: ‘Effect of nano‐silver on growth of saffron in flooding stress’, World Acad. Sci. Eng. Technol., 2012, 6, (1), pp. 517 –522 [Google Scholar]
- 93. Christou P. McCabe D.E. Swain W.F.: ‘Stable transformation of soybean callus by DNA‐coated gold particles’, Plant Physiol., 1988, 87, (3), pp. 671 –674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tarafdar J.C. Sharma S. Raliya R.: ‘Nanotechnology: interdisciplinary science of applications’, African J. Biotechnol., 2013, 12, (3), pp. 219 –226 [Google Scholar]
- 95. Prasad T. Sudhakar P. Sreenivasulu Y. et al.: ‘Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut’, J. Plant Nutr., 2012, 35, (6), pp. 905 –927 [Google Scholar]
- 96. Burman U. Saini M. Kumar P.: ‘Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings’, Toxicol. Environ. Chem., 2013, 95, (4), pp. 605 –612 [Google Scholar]
- 97. Ramesh M. Palanisamy K. Babu K. et al.: ‘Effects of bulk & nano‐titanium dioxide and zinc oxide on physio‐morphological changes in Triticum aestivum linn’, J. Glob. Biosci., 2014, 3, pp. 415 –422 [Google Scholar]
- 98. Sedghi M. Mitra H. Toluie S.G.: ‘Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress’, Ann. West Univ. Timisoara Ser. Biol., 2013, 16, (2), pp. 73 –78 [Google Scholar]
- 99. Raskar S.V Laware S.L.: ‘Effect of zinc oxide nanoparticles on cytology and seed germination in onion’, Int J Curr. Microbiol. App. Sci., 2014, 3, (2), pp. 467 –473 [Google Scholar]
- 100. Mahajan P. Dhoke S.K. Khanna A.S.: ‘Effect of nano‐ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method’, J. Nanotechnol., 2011, 2011, pp. 1 –7 [Google Scholar]
- 101. Helaly M.N. El‐Metwally M.A. El‐Hoseiny H. et al.: ‘Effect of nanoparticles on biological contamination of‘in vitro’cultures and organogenic regeneration of banana’, Aust. J. Crop. Sci., 2014, 8, (4), p. 612 [Google Scholar]
- 102. Siddiqui M.H. Al‐Whaibi M.H.: ‘Role of nano‐SiO2 in germination of tomato (Lycopersicum esculentum seeds mill.)’, Saudi J. Biol. Sci., 2014, 21, (1), pp. 13 –17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bao‐shan L. Chun‐hui L. Li‐jun F. et al.: ‘Effect of TMS (nanostructured silicon dioxide) on growth of changbai larch seedlings’, J. Res., 2004, 15, (2), pp. 138 –140 [Google Scholar]
- 104. Haghighi M. Afifipour Z. Mozafarian M.: ‘The effect of N‐Si on tomato seed germination under salinity levels’, J. Biol. Env. Sci., 2012, 6, (16), pp. 87 –90 [Google Scholar]
- 105. Siddiqui M.H. Al‐Whaibi M.H. Faisal M. et al.: ‘Nano‐silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L’, Environ. Toxicol. Chem., 2014, 33, (11), pp. 2429 –2437 [DOI] [PubMed] [Google Scholar]
- 106. Lu C. Zhang C. Wen J. et al.: ‘Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism’, Soybean. Sci., 2002, 21, (3), pp. 168 –171 [Google Scholar]
- 107. Xie Y. Li B. Zhang Q. et al.: ‘Effects of nano‐silicon dioxide on photosynthetic fluorescence characteristics of Indocalamus barbatus McClure’, J. Nanjing. Univ. (Natural Sci. Ed.), 2012, 2, pp. 59 –63 [Google Scholar]
- 108. Hong F. Yang F. Liu C. et al.: ‘Influences of nano‐TiO2 on the chloroplast aging of spinach under light’, Biol. Trace Elem. Res., 2005, 104, (3), pp. 249 –260 [DOI] [PubMed] [Google Scholar]
- 109. Yang F. Hong F. You W. et al.: ‘Influence of nano‐anatase TiO2 on the nitrogen metabolism of growing spinach’, Biol. Trace Elem. Res., 2006, 110, (2), pp. 179 –190 [DOI] [PubMed] [Google Scholar]
- 110. Lei Z. Mingyu S. Chao L. et al.: ‘Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination’, Biol. Trace Elem. Res., 2007, 119, (1), pp. 68 –76 [DOI] [PubMed] [Google Scholar]
- 111. Giraldo J.P. Landry M.P. Faltermeier S.M. et al.: ‘Plant nanobionics approach to augment photosynthesis and biochemical sensing’, Nat. Mater., 2014, 13, (4), p. 400 [DOI] [PubMed] [Google Scholar]
- 112. Noji T. Kamidaki C. Kawakami K. et al.: ‘Photosynthetic oxygen evolution in mesoporous silica material: adsorption of photosystem II reaction center complex into 23 nm nanopores in SBA’, Langmuir, 2010, 27, (2), pp. 705 –713 [DOI] [PubMed] [Google Scholar]
- 113. Pradhan S. Patra P. Das S. et al.: ‘Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: a detailed molecular, biochemical, and biophysical study’, Environ. Sci. Technol., 2013, 47, (22), pp. 13122 –13131 [DOI] [PubMed] [Google Scholar]
- 114. Qi M. Liu Y. Li T.: ‘Nano‐TiO2 improve the photosynthesis of tomato leaves under mild heat stress’, Biol. Trace Elem. Res., 2013, 156, (1–3), pp. 323 –328 [DOI] [PubMed] [Google Scholar]
- 115. Wang H. Kou X. Pei Z. et al.: ‘Physiological effects of magnetite (Fe3 O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants’, Nanotoxicology, 2011, 5, (1), pp. 30 –42 [DOI] [PubMed] [Google Scholar]
- 116. Gajjar P. Pettee B. Britt D.W. et al.: ‘Antimicrobial activities of commercial nanoparticles against an environmental soil microbe, Pseudomonas putida KT2440’, J. Biol. Eng., 2009, 3, (1), p. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dietz K.‐J. Herth S.: ‘Plant nanotoxicology’, Trends Plant Sci., 2011, 16, (11), pp. 582 –589 [DOI] [PubMed] [Google Scholar]
- 118. Ma X. Geiser‐Lee J. Deng Y. et al.: ‘Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation’, Sci. Total Environ., 2010, 408, (16), pp. 3053 –3061 [DOI] [PubMed] [Google Scholar]
- 119. Lin D. Xing B.: ‘Root uptake and phytotoxicity of ZnO nanoparticles’, Environ. Sci. Technol., 2008, 42, (15), pp. 5580 –5585 [DOI] [PubMed] [Google Scholar]
- 120. Kumari M. Mukherjee A. Chandrasekaran N.: ‘Genotoxicity of silver nanoparticles in Allium cepa’, Sci. Total Environ., 2009, 407, (19), pp. 5243 –5246 [DOI] [PubMed] [Google Scholar]
- 121. Stampoulis D. Sinha S.K. White J.C.: ‘Assay‐dependent phytotoxicity of nanoparticles to plants’, Environ. Sci. Technol., 2009, 43, (24), pp. 9473 –9479 [DOI] [PubMed] [Google Scholar]
- 122. Lee W.‐M. An Y.‐J. Yoon H. et al.: ‘Toxicity and bioavailability of copper nanoparticles to teh terrestrial plants mung bean and wheat’, Environ. Toxicol. Chem., 2008, 27, (9), pp. 1915 –1921 [DOI] [PubMed] [Google Scholar]
- 123. Laware S.L. Raskar S.: ‘Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion’, Int. J. Curr. Microbiol. App. Sci., 2014, 3, (7), pp. 749 –760 [Google Scholar]
- 124. Oukarroum A. Bras S. Perreault F. et al.: ‘Inhibitory effects of silver nanoparticles in two green algae, chlorella vulgaris and dunaliella tertiolecta’, Ecotoxicol. Environ. Saf., 2012, 78, pp. 80 –85 [DOI] [PubMed] [Google Scholar]
- 125. López‐Moreno M.L. de la Rosa G. Hernández‐Viezcas J.A. et al.: ‘X‐ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species’, J. Agric. Food Chem., 2010, 58, (6), pp. 3689 –3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rico C.M. Morales M.I. Barrios A.C. et al.: ‘Effect of cerium oxide nanoparticles on the quality of rice (Oryza sativa L.) grains’, J. Agric. Food Chem., 2013, 61, (47), pp. 11278 –11285 [DOI] [PubMed] [Google Scholar]


