Abstract
Nowadays, tissue engineering vascularisation has become an important means of organ repair and treatment of major traumatic diseases. Vascular endothelial layer regeneration and endothelial functionalisation are prerequisites and important components of tissue engineering vascularisation. The present researches of endothelial functionalisation mainly focus on tissue engineering scaffold preparation and implant surface modification. Few studies have reported the interaction of endothelial functionalisation and scaled materials, especially the nanomaterials. Magnesium (Mg), as an essential cytotropic active element in the human body, should promote the growth of endothelial cells. However, the authors’ previous work found that the Mg in the alloys had a defect of delayed endothelialisation, which may be attributed to the non‐uniform scales of the degradation products from Mg alloys. To validate this hypothesis and fabricate a novel nanomaterial for tissue engineering vascularisation, the authors prepared Mg‐doped hyaluronan (HA)/polyethyleneimine (PEI) nanoparticles for endothelial cells testing. Their data showed that the Mg‐doped HA/PEI nanoparticle with small scales (diameter <150 nm) presented better ability on improving endothelial cells growth, functionalisation and nitric oxide release.
Inspec keywords: diseases, tissue engineering, biomedical materials, contact angle, cellular biophysics, nanoparticles, filled polymers, nanocomposites, nanomedicine, magnesium compounds, nanofabrication
Other keywords: tissue engineering vascularisation, vascular endothelial layer regeneration, endothelial functionalisation, tissue engineering scaffold preparation, nitric oxide release, magnesium‐doped hyaluronan‐polyethyleneimine nanoparticle, endothelial cell testing, traumatic diseases, implant surface modification, human body, Mg‐doped hyaluronan‐polyethyleneimine nanoparticles, endothelial cell growth
1 Introduction
The intake of essential elements in the human body is related to the maintenance of normal metabolism and physiological function [1]. For instance, magnesium (Mg) can not only activate the activity of various enzymes in the body, but also regulate nerve function, maintain the stability of nucleic acid structure, participate in protein synthesis, regulate body temperature, and also affect people's mood [2, 3, 4]. Therefore, Mg is involved in almost all metabolic processes in the body. Although the content of Mg in the cell is less than that of potassium, it affects the ‘channels’ for the transfer of potassium, sodium and calcium ions inside and outside the cell, and maintains the function of biofilm potential [5, 6]. Mg deficiency is bound to cause harm to human health. In addition, Mg can also contribute to tissue regeneration and organ repair in the treatment of major diseases through a variety of pathways [7]. Moderate intake of Mg can effectively inhibit the physical discomfort caused by lactic acid accumulation and increase muscle toughness/strength [8]. Mg as a metal material has also been used in the development of various kinds of degradable absorbable vascular stents (including pure Mg stent and Mg alloy stents) [9, 10, 11]. However, both the pure Mg stent and Mg alloy stents had a defect on vascular endothelial layer regeneration, which is an important requirement for the vascular stent [12, 13]. Most researches attributed the delayed endothelialisation to the degradation of the pure Mg stent and Mg alloy stents and developed series of anticorrosive coatings which do promote the surface endothelialisation of Mg/Mg alloys to some extent [14, 15, 16]. For all that, few studies reported the influence of micro and/or nanoscales, especially the nanoscales, of the degradation products from the pure Mg stent or Mg alloy stents on the endothelial cells behaviour [17], although it's a very difficult study. Thus, in this contribution, we developed two Mg‐doped hyaluronan (HA)/polyethyleneimine (PEI) nanoparticles with different nanoscales to investigate the role of scale effect in Mg involvement in endothelial functionalisation.
HA is an acidic mucopolysaccharide with a linear single‐chain structure, which will form the secondary structure of double helix and tertiary structure of network according to specific concentrations [18]. HA constitutes the extracellular matrix and the intercellular matrix, which have the biological functions of influencing cell growth, survival and death, determining cell shape, controlling cell differentiation and participating in cell migration [19]. Under physiological conditions, carboxylic acids in hyaluronic acids are dissociated into carboxylic anions, which become negatively charged polyelectrolytes [20]. Monovalent metal positive ions are required for each disaccharide unit to maintain its electroneutrality. The interaction between polyanions and cations is an important factor affecting the supramolecular structure. Therefore, HA and its modification molecules have natural advantages as Mg carrier. PEI is usually applied for surface modification to endow the materials rich amino and positive charge [21, 22]. It is often used in conjunction with some negatively charged polymers to prepare polymer scaffolds [23, 24]. In summary, both the HA and PEI have good biocompatibility to ensure that the HA/PEI nanoparticles transport Mg to endothelial cells.
2 Materials and methods
2.1 Preparation and characterisation of Mg‐doped HA/PEI nanoparticles
Hyaluronic acid (HA, MW: 4000 Da or 100,000 Da, Bloomage Biotechnology Corporation Limited, China) was dissolved in deionised water (dH2 O) to prepare 0.5 mg/ml solution [25]. After activation of carboxyl groups in the HA molecular structure [26], 0.95 mg/ml magnesium chloride (MgCl2) solution (dissolved in dH2 O by ultrasonic for 5 min) was added to the HA solution with a volume ratio of 10:1 (V Mg : V HA = 10:1), and then the mixtures were placed onto a shaker and shaken at 60 r/min for 30 min. Next, 0.5 mg/ml polyethyleneimine (PEI, MW: 10,000 Da or 1800Da, Aladdin, China) was added to the HA/Mg mixture to form white colloids instantaneously. Finally, the white suspension solution was centrifuged at 3000 rpm for 15 min, thereafter the precipitates centrifugally cleaned with dH2 O for three times, and the cleaned precipitates were the Mg‐doped HA/PEI nanoparticles (both samples were labelled as MgNP). Wherein if the diameters of the MgNP ranged <150 nm, we named them as MgNPs; if the diameters of the MgNP ranged bigger than 150 nm, we named them as MgNPb. The pure HA/PEI nanoparticles and MgCl2 were also applied here as the reference samples to investigate the function of the two MgNPs.
Particle size and zeta potential of nanoparticles were measured by DLS particle size and potential analyser (ZS90, Malvern, UK), and the content of Mg in MgNPs and MgNPb was quantified by energy dispersion spectrometer (EDS, FEI Quanta 200, Eindhoven, Holland) [27, 28].
2.2 Endothelial functional tests
Human umbilical vein endothelial cells (HUVEC, purchased from Chengdu Hao Yi Biotechnology Co., Ltd., Chengdu, China) ranged from the third to the fifth passages were seeded on the 6‐well plate with concentration of 7 × 103 cells/well, and cultured with the 1640 medium containing MgNPs, MgNPb, HA/PEIs (small size), HA/PEIb (big size) and the pure MgCl2 at 37°C, with 5% CO2 [29]. After incubated for 1, 6, 12 h, 1 day, 3 days and 5 days, the HUVEC were cleaned with phosphate buffer solution (PBS, pH 7.4) and fixed with 4% paraformaldehyde [30]. The fixed HUVEC were fluorescence‐stained with CD31 antibody (Sigma, USA) and 4, 6‐diamino‐2‐phenyl indole (DAPI, Sigma, USA), and recorded by a fluorescence microscope (DMRX, Leica, Germany) [19]. The fluorescence intensity of CD31 on each sample was calculated by the software ipwin32 [31]. An MTT method was also performed to investigate the influence of the MgNP on HUVEC growth [32]. The nitric oxide (NO) released from the HUVEC was determined by the Griess reagent method [33]. The HUVEC apoptosis was stained with acridine orange (AO, green, Solarbio, China) and ethyl bromide (EB, orange red, Solarbio, China), and random 15 images were applied for calculating the vital ratios of HUVEC in each group [34]. The pure RPMI‐1640 medium (Solarbio, China) cultured HUVEC was also evaluated as the control sample.
2.3 Statistical analysis
Mean values ± SD are presented with their representative images. Statistical analysis was applied as one‐way analysis of variance followed by the Tukey test, while P ‐values <0.05 suggesting significant difference. The OriginPro 2018 software (OriginLab, Northampton, MA, USA) was used in the study. Each sample was detected at least three times in each characterisation.
3 Results and analysis
3.1 Characterisation of MgNP
Fig. 1 and Table 1 depict the sizes and charge property of the HA/PEIs, HA/PEIb, MgNPs and MgNPb: obviously both HA/PEIs and HA/PEIb possessed negative charge which ensures the Mg ion could be loaded in the nanoparticles via electrostatic interaction; the MgNPs ranged their diameters around 144.8 nm (<150 nm), which permitted them delivering the Mg into the interior of the cells by across cell membrane and further influencing cells behaviour; the MgNPb ranged their diameters around 913 nm (>150 nm), and particles at this scale cannot enter cells, so they can only affect the cells externally. The HA/PEIb particles and HA/PEI's particles were obtained by the interaction of HA and PEI with different molecular weights: HA of 4000 Da and PEI of 10,000 Da were prepared into HA/PEI's particles, and HA of 100,000 Da and PEI of 1800Da were prepared into HA/PEIb particles; MW of HA plays an important role in nanoparticle size, wherein higher MW contributes to larger particle size and lower MW contributes to smaller particle size. In addition, the EDS characterisation showed that MgNPb particle loaded more Mg compared with MgNPs (Table 2). It may be another reason that the MgNPb particle has a larger size than the MgNPs.
Fig. 1.
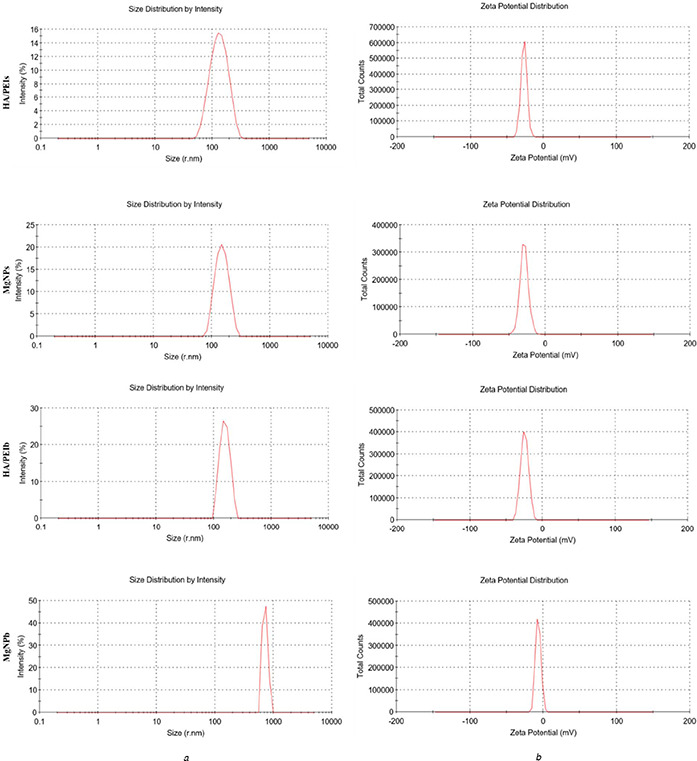
Scales and charge of the prepared nanoparticles
(a) Size measurements
(b) Charge property of HA/PEIs, MgNPs, HA/PEIb and MgNPb
Table 1.
Sizes and zeta potential values of HA/PEIs, MgNPs, HA/PEIb and MgNPb
| Group | Size, nm | ζ, mV |
|---|---|---|
| HA/PEIs | 122 | −23.5 |
| MgNPs | 144.8 | −34.4 |
| HA/PEIb | 155.6 | −24.7 |
| MgNPb | 913 | −6.8 |
Table 2.
Quantitative determination of magnesium in MgNPs and MgNPb
| Element | Wt% | At% |
|---|---|---|
| MgNPs | ||
| MgK | 01.34 | 00.83 |
| MgNPb | ||
| MgK | 01.94 | 01.20 |
3.2 Effect of MgNP on endothelial function
Fig. 2 depicts that the Mg‐doped samples showed an obvious improvement on HUVEC growth, the MgNPs, MgCl2 and MgNPb groups presented markedly higher HUVEC numbers compared with HA/PEIb and control groups (green fluorescence labelled cells).
Fig. 2.
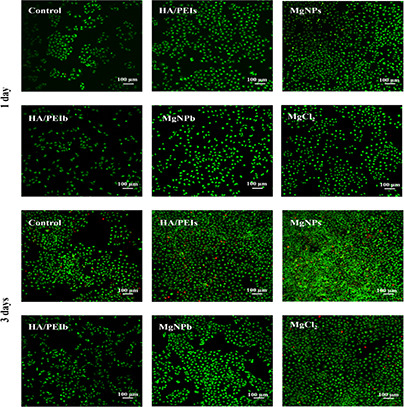
AO/EB staining images of HUVEC co‐cultured with culture medium containing HA/PEIs
This result indicates that the Mg played a significant role in promoting HUVEC adhesion and proliferation. Wherein the MgNPs group showed a higher HUVEC number than the pure MgCl2 group, while the MgNPb group presented a lower HUVEC number than the MgCl2 group. This may be attributed to the particle sizes difference between MgNPs and MgNPb: MgNPs owned its size smaller than 150 nm, which permitted the particle to cross the HUVEC membrane and deliver the Mg inside the cells; MgNPb possessed its size bigger than 150 nm, which prevented the particle from entering cells and delayed the Mg delivery [28]. In addition, the HA/PEIs group also presented higher HUVEC number, which may benefit from its small size (122 nm) and low molecular weight hyaluronic acid (LMW‐HA, 4000 Da). Lots of previous studies have proven LMW‐HA improves vascular endothelial cells growth, activity and functions [18, 19, 20]. The results of MTT (Fig. 3 a) and vital ratio (ratio of living cells to all cells) of HUVEC (Fig. 3 b) indicated the MgNPs significantly enhanced HUVEC activity and inhibited HUVEC apoptosis.
Fig. 3.
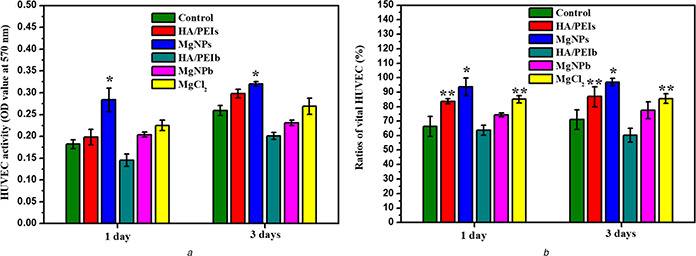
Quantitative characterisation of HUVEC in each group
(a) HUVEC activity in each group detected by a MTT assay (*p <0.05 compared with the other groups, mean ± SD, n = 3),
(b) Calculation of HUVEC vital ratios in each group (*p <0.05 compared with the other groups, **p <0.05 compared with MgNPb, HA/PEIb and control groups, mean ± SD, n = 15)
To investigate the Mg‐doped nanoparticles’ effect on vascular endothelial cells’ function, we stained the HUVEC with CD31 antibody (Fig. 4) and further calculated the CD31 expression via three‐dimensional imaging technology by ipwin32 software (Fig. 5). CD31 is a specific marker of vascular endothelial cells, and it's up‐regulated expression or down‐regulated expression represents the normal function or dysfunction of endothelial cells, respectively [26]. Figs. 4 and 5 show that the Mg groups (MgNPs, MgCl2 and MgNPb) and the small particle groups (MgNPs and HA/PEIs) expressed stronger CD31 compared with other groups. Wherein, MgNPs displayed stronger CD31 expression compared to other groups, suggesting a better ability to improve endothelial function.
Fig. 4.
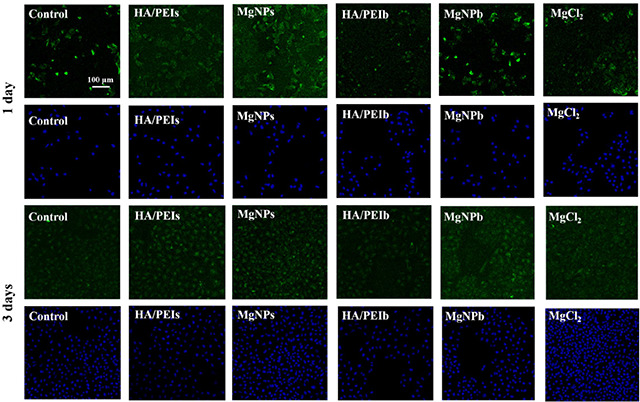
Immunofluorescence staining images of CD31 (green colour, vascular endothelial cell marker) and DAPI (blue colour, DNA marker) of the HUVEC after being co‐cultured with the HA/PEIs, MgNPs, HA/PEIb, MgNPb and MgCl2 for 1 day and 3 days, respectively
Fig. 5.
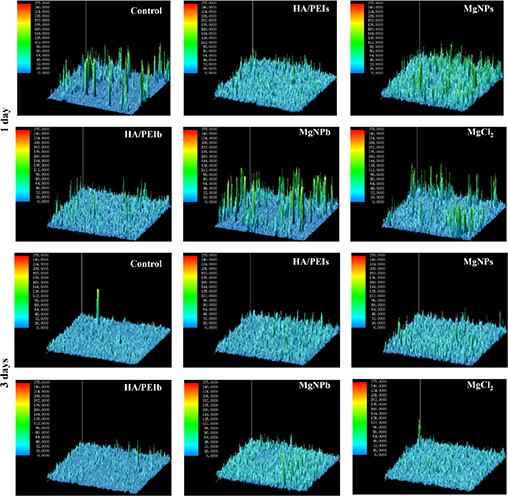
Expression quantity of CD31 on HUVEC after being co‐cultured with the HA/PEIs, MgNPs, HA/PEIb, MgNPb and MgCl2 for 1 day and 3 days by calculating fluorescence intensity using ipwin32
NO is an important characteristic and a functional factor of vascular endothelial cells [35]. It regulates vasodilation and is involved in the suppression of thrombosis and hyperplasia [36]. NO is also a key signalling molecule involved in the cellular cascade signalling pathways [37]. In this study, we detected the NO released from the HUVEC that being co‐cultured with HA/PEIs, MgNPs, HA/PEIb and MgNPb to investigate the influence of the Mg‐doped particles on the anti‐thrombosis and anti‐hyperplasia functions of HUVEC (Fig. 6). Fig. 6 shows that the MgNPs group released significantly more NO compared with the other group, which indicated both the Mg amount and the smaller particle size (<150 nm) contributed to the anti‐thrombosis and anti‐hyperplasia functions of the vascular endothelial cells.
Fig. 6.
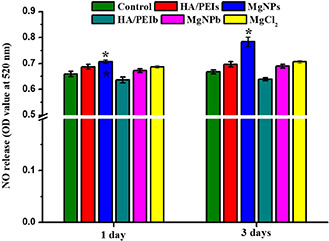
Detecting NO release of HUVEC after being co‐cultured with the HA/PEIs, MgNPs, HA/PEIb, MgNPb and MgCl2 for 1 day and 3 days, respectively (*p <0.05 compared with other samples, mean ± SD, n = 3)
4 Conclusion
In this study, we prepared Mg‐doped HA/PEI nanoparticles with two different diameters (144.8 and 913 nm), aiming at improving endothelial functionalisation. Wherein the MgNP with small diameters (<150 nm) was proved to enhance the vascular endothelial cells’ growth, CD31 expression, NO release, and meanwhile inhibit cell apoptosis. Our data also indicated that the novel nanoparticle's function simultaneously depended on the Mg element and the particle sizes. We hope our study may provide theoretical basis for the action pathway of Mg‐containing drugs or biomaterials in vivo, also offer inspiration for new drug design.
5 Funding
This research was funded by the National Natural Science Foundation of China, grant no. NSFC51671175. The Fostering Talents of National Natural Science Foundation of China and Henan Province, grant no. U1804251. Key Scientific and Technological Research Projects in Henan Province, grant no. 182102310076. Top Doctor Program of Zhengzhou University, grant no. 32210475.
6 References
- 1. Texada M.J. Jørgensen A.F. Christensen C.F. et al.: ‘A fat‐tissue sensor couples growth to oxygen availability by remotely controlling insulin secretion’, Nat. Commun., 2019, 10, p. 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andreini C. Bertini I. Cavallaro G. et al.: ‘Metal ions in biological catalysis: from enzyme databases to general principles’, J. Biol. Inorg. Chem., 2008, 13, (8), pp. 1205 –1218 [DOI] [PubMed] [Google Scholar]
- 3. Wang Y. Li X.R. Wang Q. et al.: ‘Preliminary biocompatibility and neurotoxicity assessment of silk fibroin coated magnesium alloys for peripheral nerve repairs’, Basic Clin. Pharmacol., 2019, 124, pp. 46 –47 [Google Scholar]
- 4. Li X.P. Manz A.: ‘Precise definition of starting time by capillary‐based chemical initiation of digital isothermal DNA amplification’, Sens. Actuat. B‐Chem., 2019, 288, pp. 678 –682 [Google Scholar]
- 5. Ben‐Ari Y. Gaiarsa J.L. Tyzio R. et al.: ‘GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations’, Physiol. Rev., 2007, 87, (4), pp. 1215 –1284 [DOI] [PubMed] [Google Scholar]
- 6. Schlingmann K.P. Weber S. Peters M. et al.: ‘Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family’, Nat. Genet., 2002, 31, (2), pp. 166 –170 [DOI] [PubMed] [Google Scholar]
- 7. Kraus T. Fischerauer S.F. Hanzi A.C. et al.: ‘Magnesium alloys for temporary implants in osteosynthesis: in vivo studies of their degradation and interaction with bone’, Acta Biomater., 2012, 8, (3), pp. 1230 –1238 [DOI] [PubMed] [Google Scholar]
- 8. Chamberlain B.M. Cheng M. Moore D.R. et al.: ‘Polymerization of lactide with zinc and magnesium beta‐diiminate complexes: stereocontrol and mechanism’, J. Am. Chem. Soc., 2011, 123, (14), pp. 3229 –3238 [DOI] [PubMed] [Google Scholar]
- 9. Wang J. Giridharan V. Shanov V. et al.: ‘Flow‐induced corrosion behavior of absorbable magnesium‐based stents’, Acta Biomater., 2014, 10, (12), pp. 5213 –5223 [DOI] [PubMed] [Google Scholar]
- 10. Chen L. Wang S. Zhu S.J. et al.: ‘Surface modification of the biodegradable cardiovascular stent material Mg‐Zn‐Y‐Nd alloy via conjugating REDV peptide for better endothelialization’, J. Mater. Res., 2018, 33, (23), pp. 4123 –4133 [Google Scholar]
- 11. Chen L. Li J.A. Chang J.W. et al.: ‘Mg‐Zn‐Y‐Nd coated with citric acid and dopamine by layer‐by‐layer self‐assembly to improve surface biocompatibility’, Sci. China Technol. Sci., 2018, 61, (8), pp. 1228 –1237 [Google Scholar]
- 12. Li H. Peng F. Wang D.H. et al.: ‘Layered double hydroxide/poly‐dopamine composite coating with surface heparinization on Mg alloys: improved anticorrosion, endothelialization and hemocompatibility’, Biomater. Sci., 2018, 6, (7), pp. 1846 –1858 [DOI] [PubMed] [Google Scholar]
- 13. Zhang E.L. Shen F.: ‘A ferulic acid (FA)‐eluting system for biodegradable magnesium stent: cells response of HUVECs’, J. Biomed Mater. Res. A, 2015, 103, (8), pp. 2758 –2769 [DOI] [PubMed] [Google Scholar]
- 14. Zhang E.L. Shen F.: ‘Blood compatibility of a ferulic acid (FA)‐eluting PHBHHx system for biodegradable magnesium stent application’, Mater. Sci. Eng. C‐Mater., 2015, 52, pp. 37 –45 [DOI] [PubMed] [Google Scholar]
- 15. Wei Z.L. Tian P. Liu X.Y. et al.: ‘Hemocompatibility and selective cell fate of polydopamine‐assisted heparinized PEO/PLLA composite coating on biodegradable AZ31 alloy’, Colloid. Surface B, 2014, 121, pp. 451 –460 [DOI] [PubMed] [Google Scholar]
- 16. Zhao N. Workman B. Zhu D.H.: ‘Endothelialization of novel magnesium‐rare earth alloys with fluoride and collagen coating’, Int. J. Mol. Sci., 2014, 15, (4), pp. 5263 –5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang S. Zhu S.J. Zhang X.Q. et al.: ‘Effects of degradation products of biomedical magnesium alloys on nitric oxide release from vascular endothelial cells’, Med. Gas. Res., 2019, 9, (3), pp. 153 –159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J.G. Li G.C. Zhang K. et al.: ‘Co‐culture of vascular endothelial cells and smooth muscle cells by hyaluronic acid micro‐pattern on titanium surface’, Appl. Surf. Sci., 2013, 273, pp. 24 –31 [Google Scholar]
- 19. Li J.G. Wu F. Zhang K. et al.: ‘Controlling molecular weight of hyaluronic acid conjugated on amine‐rich surface: toward better multifunctional biomaterials for cardiovascular implants’, ACS Appl. Mater. Interfaces, 2017, 9, (36), pp. 30343 –30358 [DOI] [PubMed] [Google Scholar]
- 20. Li J.A. Zhang K. Xu Y. et al.: ‘A novel coculture model of HUVECs and HUASMCs by hyaluronic acid micropattern on titanium surface’, J. Biomed Mater. Res. A, 2014, 102, (6), pp. 1950 –1960 [DOI] [PubMed] [Google Scholar]
- 21. Zhang K. Bai Y.X. Wang X.F. et al.: ‘Surface modification of esophageal stent materials by a polyethylenimine layer aiming at anti‐cancer function’, J. Mater. Sci. ‐Mater. M, 2017, 28, p. 125 [DOI] [PubMed] [Google Scholar]
- 22. Bai Y.X. Zhang K. Xu R. et al.: ‘Surface modification of esophageal stent materials by a drug‐eluting layer for better anti‐restenosis function’, Coatings, 2018, 8, (6), p. 215 [Google Scholar]
- 23. Wang S. Li J.A. Zhou Z.X. et al.: ‘Micro/nano scales direct cell behaviour on biomaterials surface’, Molecules, 2019, 24, (1), p. 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu R. Su C. Cui L.L. et al.: ‘Preparing sodium alginate/polyethyleneimine microspheres via layer‐by‐layer cross‐linking method for potential application of killing tumor cells by reducing the concentration of copper ions in the lesions of colon cancer’, Materials (Basel), 2019, 12, (9), p. 1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu F. Li J.A. Zhang K. et al.: ‘Multifunctional coating based on hyaluronic acid and dopamine conjugate for potential application on surface modification of cardiovascular implanted devices’, ACS Appl. Mater. Interfaces, 2016, 8, (1), pp. 109 –121 [DOI] [PubMed] [Google Scholar]
- 26. Li J.A. Zou D. Zhang K. et al.: ‘Strong multi‐functions based on conjugating chondroitin sulfate onto an amine‐rich surface will direct the vascular cell fate for cardiovascular implanted devices’, J. Mater. Chem. B, 2017, 5, (42), pp. 8299 –8313 [DOI] [PubMed] [Google Scholar]
- 27. Li J.A. Zhang K. Yang P. et al.: ‘Human vascular endothelial cell morphology and functional cytokine secretion influenced by different size of HA micro‐pattern on titanium substrate’, Colloid. Surface B, 2013, 110, pp. 199 –207 [DOI] [PubMed] [Google Scholar]
- 28. He D. Zhao A.S. Su H. et al.: ‘An injectable scaffold based on temperature responsive hydrogel and factors loaded nano‐particles for potential application of vascularization in tissue engineering’, J. Biomed. Mater. Res.: A, 2019, 107A, pp. 2123 –2134 [DOI] [PubMed] [Google Scholar]
- 29. Yao H. Li J.A. Li N. et al.: ‘Surface modification of cardiovascular stent material‐316L SS with estradiol loaded poly (trimethylene carbonate) film for better biocompatibility’, Polymers (Basel), 2017, 9, p. 598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han C.Z. Luo X. Zou D. et al.: ‘Nature‐inspired extracellular matrix coating produced by micro‐patterned smooth muscle and endothelial cells endows cardiovascular materials better biocompatibility’, Biomater. Sci., 2019, 7, pp. 2686 –2701 [DOI] [PubMed] [Google Scholar]
- 31. Zou D. Luo X. Han C.Z. et al.: ‘Preparation of a biomimetic ECM surface on cardiovascular biomaterials via a novel layer‐by‐layer decellularization for better biocompatibility’, Mater. Sci. Eng. C‐Mater., 2019, 96, pp. 509 –521 [DOI] [PubMed] [Google Scholar]
- 32. Li L.H. Xu Y. Zhou Z. et al.: ‘The effects of Cu‐doped TiO2 thin films on hyperplasia, inflammation and bacterial infection’, Appl. Sci. ‐Basel, 2015, 5, (4), pp. 1016 –1032 [Google Scholar]
- 33. Yang Z.L. Yang Y. Zhang L. et al.: ‘Mussel‐inspired catalytic selenocystamine‐dopamine coatings for long‐term generation of therapeutic gas on cardiovascular stents’, Biomaterials, 2018, 178, pp. 1 –10 [DOI] [PubMed] [Google Scholar]
- 34. Zhang K. Wang X.F. Guan F.X. et al.: ‘Immobilization of ophiopogonin D on stainless steel surfaces for improving surface endothelialization’, RSC Adv., 2016, 6, pp. 113893 –113898 [Google Scholar]
- 35. Yang Z.L. Yang Y. Xiong K.Q. et al.: ‘Metal‐phenolic surfaces for generating therapeutic nitric oxide gas’, Chem. Mater., 2018, 30, pp. 5220 –5226 [Google Scholar]
- 36. Zhang F. Zhang Q. Li X.Y. et al.: ‘Mussel‐inspired dopamine‐CuII coatings for sustained in situ generation of nitric oxide for prevention of stent thrombosis and restenosis’, Biomaterials, 2019, 194, pp. 117 –129 [DOI] [PubMed] [Google Scholar]
- 37. Li X.Y. Gao P. Tan J.Y. et al.: ‘Assembly of metal − phenolic/catecholamine networks for synergistically anti‐inflammatory, antimicrobial, and anticoagulant coatings’, ACS Appl. Mater. Interfaces, 2018, 10, pp. 40844 –40853 [DOI] [PubMed] [Google Scholar]


