Abstract
Bio‐ synthesis of silver nanoparticles (AgNPs) was made by using the aqueous leaf extract of Ardisia solanacea. Rapid formation of AgNPs was observed from silver nitrate upon treatment with the aqueous extract of A. solanacea leaf. The formation and stability of the AgNPs in the colloidal solution were monitored by UV–visible spectrophotometer. The mean particle diameter of AgNPs was calculated from the DLS with an average size ∼4 nm and ∼65 nm. ATR‐FTIR spectroscopy confirmed the presence of alcohols, aldehydes, flavonoids, phenols and nitro compounds in the leaf which act as the stabilizing agent. Antimicrobial activity of the synthesized AgNPs was performed using agar well diffusion and broth dilution method against the Gram‐positive and Gram‐negative bacteria. Further, robust anti‐oxidative potential was evaluated by DPPH assay. The highest antimicrobial activity of synthesized AgNPs was found against Pseudomonas aeruginosa (28.2 ± 0.52 mm) whereas moderate activity was found against Bacillus subtilis (16.1 ± 0.76), Candida kruseii (13.0 ± 1.0), and Trichophyton mentagrophytes (12.6 ± 1.52). Moreover, the potential wound healing activity was observed against the BJ‐5Ta normal fibroblast cell line. Current research revealed that A. solanacea was found to be a suitable source for the green synthesis of silver nanoparticles.
Inspec keywords: antibacterial activity, nanoparticles, silver, nanomedicine, wounds, microorganisms, X‐ray diffraction, ultraviolet spectra, visible spectra, Fourier transform infrared spectra, transmission electron microscopy
Other keywords: phyto‐assisted synthesis, biofunctionalised silver nanoparticles, antioxidant antimicrobial wound healing activities, silver nanoparticle biosynthesis, aqueous leaf extract, Ardisia solanacea, silver nitrate, UV–visible spectroscopy, dynamic light scattering, Fourier transform infra‐red spectroscopy, X‐ray diffraction, electron microscopy, attenuated total reflection Fourier transform infra‐red spectroscopy, dilution method, Gram‐positive bacteria, Gram‐negative bacteria, radical scavenging method, Pseudomonas aeruginosa, Trichophyton mentagrophytes, Bacillus subtilis, Candida kruseii, BJ‐5Ta normal fibroblast cell line, SEM, alcohols, aldehydes, flavonoids, phenols, nitro compounds, Ag
1 Introduction
In recent time, synthesis of free metal nanoparticles is a great challenge. To materialise this challenge, the primary aim is to maximise the usage of environmental and eco‐friendly biological resources in the generation of metal nanoparticles. Various chemical and physical methods have been employed to synthesise the silver nanoparticles (AgNPs) such as chemical reduction [1, 2, 3], UV irradiation [4, 5], microwave irradiation [6, 7], photochemical method [8, 9], electron irradiation [10, 11] and sono‐electrochemical method [12].
However, the chemical and physical methods involve more than one step, high‐energy requirement, low material conversions, difficulty in purification and residual hazardous chemicals. A major drawback associated with the chemical synthesis method is that a substantial amount of residual toxic radicals and molecules are generated. These tend to adsorb to the top of the particle that may possess adverse impacts in its application. In contrast to the chemical means, biological synthesis of nanoparticle has proved to be a better solution for overcoming such problems. Moreover, the biologically active molecules involved in the biological synthesis of nanoparticles act as a functionalising ligand, making them more suitable for biomedical applications [13]. Biological synthesis by implicating the plant extracts have emerged as a simple and alternative method to chemical synthesis. The biological synthesis has provided an advancement over the chemical methods as it is environmental friendly, cost‐effective and easily scaled up for large‐scale synthesis. AgNPs are universally used as a strong anti‐microbial agent in the biomedical field due to their strong biocidal effect [14]. Besides the anti‐microbial activity, AgNPs are also found to be potent anti‐fungal [15], anti‐inflammatory [16], anti‐viral [17], anti‐angiogenesis [18] and anti‐platelet agent [19]. Due to such potential activities, the AgNPs are now used in textile, food and pharmaceutical industries. The US food and drug administration have already approved some applications of AgNPs in the biomedical field [20].
Ardisia solanacea (Family – Myrsinaceae) is a shrub widely used as traditional medicine for the treatment of a wide range of diseases. This plant has stimulant and carminative properties [21]. The plant is applied in the treatment of diarrhoea, dysmenorrhoea, gout, mental disorder, rheumatic arthritis, skin sore and vertigo [22]. Recently, the plant has been reported for hepato‐protective properties [23]. Other species of the Ardisia have been reported for their cytotoxic, thrombolytic and anti‐oxidant properties [21]. In the Similipal Biosphere Reserve (SBR), the plants are widely distributed and the juice of the ripen fruit is a favourite drink of tribal people and leaves are consumed mostly as fodder for cows and goats.
There is no report on the synthesis of AgNPs utilising an aqueous leaf extract of A. solanacea. In this experiment, we have taken A. solanacea leaf extract in a concentrated aqueous solution of silver nitrate for the reduction of silver ions and the formation of silver nanoparticles. These green‐synthesised AgNPs were characterised by the ultraviolet–visible (UV–Vis) spectroscopy, Fourier transform infra‐red spectroscopy (FTIR), dynamic light scattering (DLS) and scanning electron microscopy (SEM) to determine their size and shape. Besides, the characterisations, anti‐microbial, anti‐oxidant and wound healing activities were also evaluated for sustainable use of AgNPs.
2 Materials and methods
Silver nitrate (AgNO3), 1,1‐diphenyl‐2‐picryl‐hydrazyl (DPPH) radical and butylated hydroxytoluene (BHT) were purchased from Sigma‐Aldrich. Gallic acid, Mueller‐Hinton agar, Mueller‐Hinton broth, potato dextrose agar (PDA), gentamicin and clotrimazole were purchased from HiMedia. The bacterial strains Bacillus subtilis (MTCC 736), Staphylococcus aureus (MTCC 737), Pseudomonas aeruginosa (MTCC 424), Escherichia coli (MTCC 443) and fungal strains Candida albicans (MTCC 227), Candida kruseii (MTCC 9215), Candida viswanathii (MTCC 1929) and Trichophyton mentagrophytes (MTCC 8476) were purchased from ‘microbial type culture collection’ (MTCC), Chandigarh, India.
2.1 Collection and identification of plant
Procurement of a considerable amount of leaves of A. solanacea (Roxb.) was done from the Similipal Biosphere Reserve, Mayurbhanj, Odisha, India. The plant was identified by the taxonomist of P.G. Department of Botany, North Orissa University and the voucher specimen (NOU 2080) was deposited. The healthy leaves were first dried in shade and later powdered using a mechanical grinder followed by passing through a sieve to maintain homogeneity in the size of the granules.
2.2 Plant extracts preparation
Fifty millilitre of deionised water was poured into a conical flask containing 5 g of leaf powder. Thereafter, it was subjected to sonication for 5 min at 5000 rpm. The sonicated mixture was filtered through Whatman® grade no.1 filter paper. The filtrate was refrigerated at 4°C for further use.
2.3 Biosynthesis of AgNPs
A reaction mixture was prepared by combining 0.5 ml of aqueous leaf extract and 4.5 ml of 1 mM AgNO3 solution in a test tube for the biosynthesis of AgNPs. Simultaneously, control was taken by addition of 4.5 ml sterile deionised water with 0.5 ml of leaf extract. The chemical reactions were allowed to take place in a dark environment at 60°C for 12 h. The solution turns from pale yellow to dark brown indicating the completion of the reaction process for the formation of AgNPs. AgNPs were separated and purified by continuous centrifugation with sterile distilled water. In the post purification process, AgNPs were taken for anti‐microbial, anti‐oxidant and wound healing activity studies.
2.4 Characterisation of biosynthesised silver nanoparticles
2.4.1 UV–Vis spectroscopy
Monitoring of silver ions reduction to corresponding AgNPs in the aqueous solution was carried out using a UV–Vis spectrophotometer (Lambda 35® PerkinElmer, USA). The output was recorded in the range of 400–600 nm wavelengths at the room temperature (25°C).
2.4.2 DLS spectroscopy
The particle size and zeta potential of the AgNPs were calculated by a Zetasizer (Zs 90, Malvern, UK) at the room temperature. The dried samples were sufficiently diluted with phosphate buffer saline (PBS, 0.15 M, pH 7.2). The aliquots were subjected to further analysis in the DLS instrument. The particle size distribution was examined at 90° scattering angle.
2.4.3 X‐ray diffraction study
The X‐ray diffraction (XRD) patterns of AgNPs were obtained using an X‐ray diffractometer (PANalytical X'Pert, Almelo, the Netherlands) equipped with Ni filter and Cu Kα (l = 1.54056 Å) as the radiation source. The diffraction angle varied in the range of 20–80° while the scanning rate was 0.05°/s.
2.4.4 Field emission scanning electron microscopy
The field emission scanning electron microscope (FE‐SEM; Jeol 6480LV JSM, USA) was used to observe the nano‐dimension structure of the AgNPs. The microscope was operated at a vacuum pressure of 10−5 torr and accelerating voltage in the range of 10–20 kV.
2.4.5 High‐resolution transmission electron microscopy
The prepared AgNPs were sonicated for 15 min for a complete dispersion of the particles. The drop of solutions was later taken in a syringe and put into the carbon‐coated copper grids and subjected to air‐drying. Post drying, the copper grids were subjected to analysis by HR‐TEM (FEI‐TECNAI G2 20S‐TWIN, the Netherlands) at 80 Hz.
2.4.6 Attenuated total reflection Fourier transform infra‐red spectroscopy
The attenuated total reflection Fourier transform infra‐red spectroscopy (ATR‐FTIR) spectroscopy analysis of AgNPs was employed to substantiate the possible role of the various phytochemical found in the plant extracts that are responsible for the surface modification of the biosynthesised AgNPs. The ATR‐FTIR was performed on a Bruker ALPHA spectrophotometer (Ettlinger, Germany) at a resolution of 4 cm−1. The samples were scanned in the spectral region ranging from 4000 to 500 cm−1 by an average of 25 scans per sample. One drop of sample was kept on the sample holder and those samples were subsequently scanned and the obtained result was analysed by means of OPUS software.
2.5 Qualitative phytochemical analysis
The qualitative phytochemical analysis of A. solanacea (Roxb.) extracts was performed following the standard methods [24, 25]. The obtained results were qualitatively expressed as positive (+) or negative (−) [26]. The chemicals and reagents used for the study were purchased from Sigma‐Aldrich (India).
2.6 Quantitative phytochemical analysis and anti‐oxidant properties
2.6.1 Total phenolics content determination
Total phenolic quantity in the leaf extract was measured by the Folin‐Ciocalteu method with slight modifications [27]. All the experiments were conducted in triplicates. The TPC was expressed as gallic acid equivalent (GAE) in the mg/g sample.
2.6.2 Total flavonoids content determination
Total flavonoids were estimated by a modified aluminium chloride method [28]. All estimations were carried out in triplicates. The total flavonoids content (TFC) was expressed as GAE in the mg/g sample.
2.6.3 Quantification of radical scavenging activity (DPPH)
The potential anti‐oxidant activity was determined by the DPPH assay with sufficient modification wherever it seemed necessary [27]. The results were expressed as percentage (%) radical scavenging activity utilising BHT as a standard reagent. All the experiments were repeated in triplicates and results were expressed in mean ± SD.
2.7 Anti‐microbial activity
2.7.1 Microbial strains
Bacterial strains viz. B. subtilis (MTCC 736), S. aureus (MTCC 737), P. aeruginosa (MTCC 424), E. coli (MTCC 443) and fungal strains viz. C. albicans (MTCC 227), C. kruseii (MTCC 9215), C. viswanathii (MTCC 1929) and T. mentagrophytes (MTCC 1929) were exploited for implementing anti‐bacterial and anti‐fungal tests, respectively. In case of the bacterial strains, colonies were inoculated in Mueller‐Hinton (MH; 1.75% casamino acids, 0.015% soluble starch, 0.2% beef extract and MHA) plates while for fungi colonies, inoculation was done in PDA (10% potato and 2% dextrose), and after optimum growth was achieved, both were stored in a cold room (at 4°C).
2.7.2 Preparation of pre‐culture
Single colony from each of the test strains was picked from PDA media for fungus and MHA for bacteria and inoculated into two different culture tubes containing 1 ml of respective broths under aseptic conditions. The reaction tubes were kept for overnight incubation in a shaker incubator running at 200 rpm at 37°C for bacteria and 28°C for fungi.
2.7.3 Agar well diffusion method
The agar well diffusion method was used to evaluate the anti‐microbial property of the synthesised AgNPs based on the standard protocols [29, 30]. To test the anti‐microbial activity, MH broth culture (100 µl) of each test bacteria was seeded over the MHA plates, while for fungus 200 µl of overnight grown potato dextrose broth was seeded over PDA plates. Wells of ∼6 mm in diameter and 2.5 mm depth were made on the surface of the solid medium using a sterile borer. Each well was loaded with 40 µl of bio‐genically synthesised AgNPs. The same amount of leaf extract was used as a control while standard antibiotics – gentamicin and clotrimazole – were used as reference controls for the bacteria and fungi, respectively. All the plates were incubated at 37°C for 24 h. After the optimum growth, the plates were taken out and clear zones were measured using a HiMedia antibiotic scale and subsequent results were tabulated. Wells loaded with AgNPs having zones of inhibition (ZOI) greater or equal to 8 mm diameter were regarded as positive. Later, we conducted study with broth dilution test by taking the positive result. Every set of experiment was carried out thrice. The mean ± SD of the zone of inhibitions were used to calculate the anti‐microbial activity of different extracts.
2.7.4 Broth dilution method (anti‐bacterial)
AgNPs refrigerated at 4°C were brought to room temperature prior to the experiment. By means of sterile 96‐well flat‐bottom micro‐dilution plates, twofold serial dilutions were prepared with sterile Millipore water. A standardised inoculum was retrieved by growing the test organisms overnight in MH broth and later re‐inoculated in a fresh MH broth to obtain the turbidity with OD = 0.003 at 600 nm wavelength on a Perkin Elmer UV–Vis spectrophotometer (∼100‐fold). Every well of a micro‐dilution plate was supplied with 190 µl of the inoculum and 10 µl of the AgNPs. The control wells were prepared with 190 µl MH broth and 10 µl AgNPs, so as to minimise any absorption caused due to extract components. After mixing, the plates were properly sealed with parafilms.
The micro‐dilution trays were kept in a shaker incubator at 37°C for 24 h, and later readings were noted on a Biorad microplate reader at 590 nm. The extent of growth in the wells was compared to that of the control. For a test to be considered valid, appreciable growth (≥0.5 OD) must take place in the positive control, and none (OD < 0.05) in the negative control (medium only). The experiments were repeated for twice. The relative inhibition percentage of the test sample was calculated using the following formula:
2.7.5 Anti‐fungal activity using broth dilution method
Anti‐fungal activity was tested against Candida species and T. mentagrophytes in a similar manner to that in bacteria. Potato dextrose broth was used with a smaller quantity of AgNPs. To assess anti‐fungal activity, each well of the micro‐dilution plate was loaded with 190 µl of inoculum and 10 µl of AgNPs solution. Control wells were prepared with 190 µl PD broths and 10 µl AgNPs to rectify any absorption errors.
2.8 Wound healing assay
The wound healing activity of AgNPs was performed by the cell scratch assay [31, 32]. Normal fibroblast cell lines (BJ‐5Ta) were used to assay the wound healing capacity of green‐synthesised AgNPs for surface wound. For the assay, the cells of density 2 × 105 cells/ml were seeded in normal cell culture medium DMEM (Dulbecco's modified eagle medium) supplemented with 10% FBS and M199 medium. Later, the cells were incubated for 22–28 h in a CO2 incubator. With conformation of ∼70–80% confluence as a monolayer, the cells are scratched by sharp tips in the mid of the culture well. Later the ruptured cells were removed by repeated washing with growth medium. Subsequently, the test samples (AgNPs) were added into the scratched wells. Allantoin (50 µg/ml) (Sigma‐Aldrich) was added as a positive control and only Hanks’ balanced salt solution and leaf extract as negative control. The culture plates were again incubated for 22–24 h till appropriate growth was recorded. Later the cells were fixed and stained to observe the wound healing activity and the photographs were taken using a phase contrast microscope (Zeiss, Germany).
3 Results and discussion
3.1 Green synthesis and UV–Vis spectra study of silver (Ag) nanoparticles
UV–Vis spectroscopy is the most simple and indirect method to evaluate the bioreduction of the Ag ion to Ag nanoparticles. For this purpose, 4.5 ml of 1 mM AgNO3 solution was taken and 0.5 ml of the aqueous leaf extract was added. After two hours of incubation, a visible colour change (from pale yellow to dark brown) was observed. The intensity of the colour increased with the increase in incubation time. In the present study, we observed the appearance of the absorption peak of AgNPs at ∼432 nm. Earlier, it was reported that AgNPs give absorption band in the range of 420–450 nm due to its surface plasmon resonance behaviour [33]. In this study, the observed absorption peak at 432 nm confirms the green synthesis of Ag nanoparticles (Fig. 1).
Fig. 1.
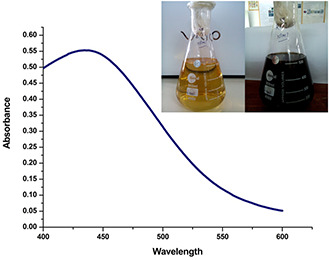
UV–Vis spectra of AgNPs synthesised by A. solanacea leaf extracts
3.2 DLS analysis
Investigation of the hydrodynamic size and surface zeta potential of AgNPs present in colloidal aqueous environment was carried out by the DLS method. From the size distributions, it has been observed that the AgNPs show bimodal distribution with an average size of ∼4 and ∼65 nm (Fig. 2 a). As shown in Fig. 2 a, the low‐end size distribution was relatively asymmetric and the peak highlighting particles of bigger dimension exhibited normal distribution. Additionally, it was observed that the mean size and surface charge of the AgNPs were ∼27 nm and −21.7 mV, respectively (Figs. 2 a and b). The magnitude of the zeta potential provides incipient unstable condition of the particles in the media; however, the value is fair enough to avoid aggregation. The average size and potential contribute strong characteristics of the Ag nanoparticles to be used in biomedical sciences as a drug carrier and biosensor [34]. Smaller size of the particles contributes a distinct property of permeability to the plasma membranes of the cell. Particle with size <100 nm is considered to be an effective tool for diverse applications such as drug delivery, biosensor and development of biomedical devices and others [34, 35]. Besides the hydrodynamic size, surface charges of the nanoparticles are considered as an important factor for the interaction with different macromolecules to the target pathways and cells [33].
Fig. 2.
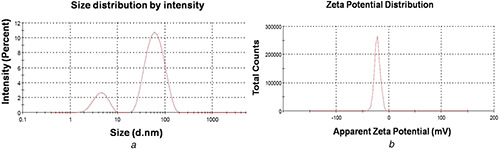
DLS spectra on hydrodynamic size distribution of synthesised AgNPs
(a) The x‐axis and y‐axis represent size (nm) and intensity (%); respectively; (b) The characteristic response of zeta potential (mV)
3.3 XRD analysis
In order to examine the physico‐chemical make‐up of a typical material, the XRD method is employed. The size, shape, lattice parameter determination and phase fraction analyses of the unit cell of any compound can be determined by the XRD study. Information about the translational symmetry size and shape of the unit cell are also obtained from the diffraction pattern [36] (Fig. 3).
Fig. 3.
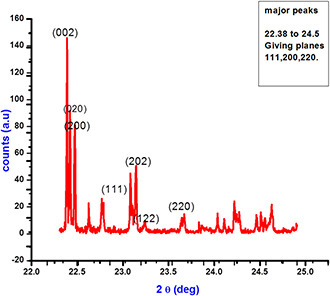
XRD diffractogram of the bio synthesised silver nanoparticles from the plant extracts of A. solanacea
The structural properties of bio‐synthesized AgNPs studied from XRD (Rigaku, Miniflex) reveal four different peaks at 2θ = 22.5, 23, 24.8 and 25. The lattice parameters, average crystallite size and micro‐strain were calculated by the Williamson‐Hall model given by β cos θ = 0.9 λ /D + 4ɛ sin θ, where β is the full width at half maxima, λ is the wavelength of X‐rays (=1.54 Å), ɛ is the microstrain and D is the average crystallite size. The estimated lattice parameters were in agreement with the previous report [37]. The average crystallite size and microstrain of AgNPs was found to be 27.29 nm and 1.60 × 10−3, respectively. Fig. 1 depicts three prime peaks with characteristic improvement of 220, 111, 200 and 311. It explains the monoclinic‐like structure of AgNPs [38]. A considerable broadening in XRD lines occurs when the particle size was found to be <100 nm [39]. The broadening was due to the particle size and strain from the diffraction pattern. This broadening was used to calculate the average particle size [39].
3.4 FE‐SEM study
Fig. 4 shows the SEM micrograph of AgNPs. The sphere‐shaped morphology along with the monodispersive nature of synthesised AgNPs capped by its biomoieties can be determined from the SEM micrograph.
Fig. 4.
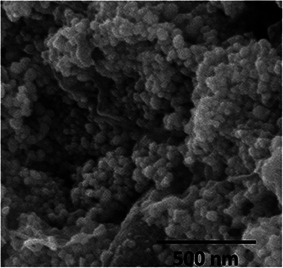
Scanning electron microscopy image of biosynthesised silver nanoparticles
3.5 High‐resolution transmission electron microscopy analysis
High‐resolution transmission electron microscopy (HR‐TEM) is a non‐destructive imaging technique that provides morphology and crystallographic details with a resolution from sub‐nanometer to atomic scale [40]. We have performed HR‐TEM studies on BioAgNPs and the results were presented at 10 nm (Fig. 5 a) and at 2 nm (Fig. 5 b). The nanoparticles were found to be polyhedral and oblate shape. Along the major axis of the oblate‐shaped particle, the particle size found to be 29 nm. The predicted dimension of the crystallites was bit smaller than the XRD studies made on the powder specimen. The characteristic d‐spacing showed a value of 0.21 nm. Selected area electron diffraction shown in Fig. 5 c showed prominent diffraction rings from the planes that corresponded to the monoclinic crystal structure. The central ring corresponds to superimposed response due to 111, 220 and 200 planes. The second and third rings corresponded to 202 and 122 planes, respectively. These planes are characteristics of the monoclinic structure and confirmed with the peaks observed in XRD studies.
Fig. 5.
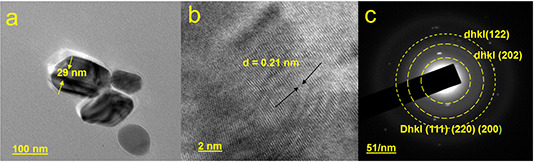
HR‐TEM image of biosynthesised silver nanoparticles
3.6 Attenuated total reflection Fourier transform infra‐red spectroscopy
The FTIR spectra of the AgNPs were captured for identifying the nature of the functional groups involved in the green synthesis and stabilisation of the nanoparticles. It is clear from the spectrum that eight prominent absorption peaks were observed at ∼3870, 3751, 3623, 2346, 1713, 1538 and 611 cm−1 (Fig. 6). Among the peaks, the strongest absorption was found at ∼3870, 3751 and 3623 cm−1, suggesting the binding of the silver ion with hydroxyl group which referred to the stretching of the –OH group or free hydroxyl group, indicating the presence of polyphenols. The band present at ∼2346 cm−1 takes place on account of the vibrational stretching of H–C=O:C–H, indicating the presence of aldehydes. The stretching vibrations of C=O were due to strong peak at ∼1713 cm−1 that confirms the presence of α, β ‐unsaturated aldehydes and ketones. The sharp band at ∼1538 cm−1 can be possible due to N–O asymmetric stretching, which indicates the involvement of nitro compounds. Another strong band at ∼611 cm−1 indicates the presence of alkynes which are very common in the phytochemicals. The characteristic FTIR bands represent the functional groups such as alcohols, aldehydes, flavonoids, phenols and nitro compounds which are the phyto‐constituents present in the leaves of A. solanacea play the key role in the biological synthesis of AgNPs.
Fig. 6.
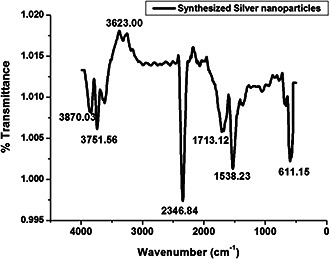
FTIR result analysis of AgNPs synthesised by A. solanacea leaf. The x‐axis represents spectra (cm−1) and y‐axis represents transmission (%)
3.7 Qualitative and quantitative assessment of phytochemicals and corresponding anti‐oxidative activities
Qualitative and quantitative phytochemical examinations of the aqueous leaf extracts are summarised in Tables 1 and 2 and Fig. 7. Phytochemical analysis revealed the existence of alkaloids, tannins, phenolic compounds and flavonoids, whereas glycoside, steroids, sterols and triterpenoids were found to be absent. The phytochemical study of the leaf extract of A. solanacea showed that alkaloids, tannins, phenolic compounds and flavonoids were present in the extract which may be the principal chemicals responsible for the synthesis of AgNPs. Shankar et al. reported the possibility of the role of terpenoids from Geranium leaf in the synthesis of nano‐sized AgNPs [39]. Polyols such as terpenoids, flavones and polysaccharides in the Cinnamomum camphora leaf were reported to be the main cause of the bioreduction of silver and chloroaurate ions [41].
Table 1.
Qualitative phytochemical screening of aqueous extract of A. solanacea
| Name of the phyto‐constituents | Observation |
|---|---|
| alkaloids | +++ |
| tannins and phenolic compounds | ++ |
| glycoside | — |
| flavonoids | +++ |
| steroids and sterols | — |
| triterpenoids | — |
Table 2.
Quantitative phytochemical constituents of aqueous extract of A. solanacea
| Phytochemical constituent | mg/100 g dry weight (mean ± SD) |
|---|---|
| TPC | 660.00 ± 36.00 |
| TFC | 846.66 ± 25.10 |
Fig. 7.
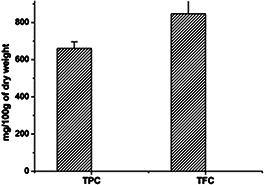
Total TPC and TFC content of A. solanacea
The current work does not report the presence of glycosides, steroids and sterols and triterpenoids which may be an outcome of selective qualitative test performed, and/or extraction procedures. As long as the hypothetical mechanism of AgNPs’ biosynthesis is concerned, cascades of complex anti‐oxidant enzymes might be involved [42].
The anti‐oxidant potential of A. solanacea exhibits a positive response towards the tentative involvement of anti‐oxidant molecules from the leaf extract during the biogenic synthesis of AgNPs. It is known that plants have a large collection of phenolics and flavonoids which may possess super anti‐oxidative capabilities and considered strong free radical scavengers. Significant anti‐oxidant activity was also observed by the DPPH radical scavenging assay (Fig. 8). The presence of moderate concentration of total phenolics and flavonoids in A. solanacea leaves indicated a notable anti‐oxidant activity. The high molecular weight and the proximity of many aromatic rings and hydroxyl groups are more important for the free radical scavenging activity of bioactive compounds [43]. Recently, Chandran et al. reported the in vitro anti‐oxidant potential of methanolic and aqueous extracts of A. solanacea Roxb. leaf through the DPPH radical scavenging assay that strongly supports our present result [44]. Luximon‐Ramma et al. investigated the entire phenolic, proanthocyanidin, flavonoids and the anti‐oxidant activities of vegetative and reproductive parts [45]. The anti‐oxidant activities were highly correlated with the total phenol levels. However, the result showed that the anti‐oxidant activities of reproductive parts surpassed the anti‐oxidant activities of the vegetative organs, including the pods that have the highest total phenolic and flavonoid contents [46]. Related findings were also reported in the present experiments.
Fig. 8.
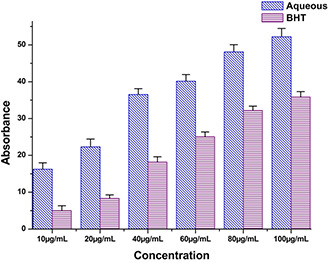
DPPH radical scavenging assay aqueous extract of A. Solanacea
It is also essential to evaluate the anti‐oxidant potential as some of the plant molecules still remain with AgNPs after purification as a capping agent, which should not be harmful to cells during cellular applications. Thus, the anti‐oxidant potential of A. solanacea established the green synthesis of AgNPs.
3.8 Anti‐microbial activity of AgNPs
Primary screening of anti‐microbial performance was carried out by the agar well diffusion method against four pathogenic bacterial and four pathogenic fungal strains (Table 3). In the agar well diffusion method, the highest zone of inhibition was noted against the Gram‐positive bacteria, B. subtilis, and Gram‐negative bacteria, P. aeruginosa, whereas no zones were evident when applied on S. aureus and E. coli (Figs. 9 a and b). Similarly, the zone of inhibition was recorded against C. kruseii and T. mentagrophytes (Fig. 9 c), while no zone of inhibition was observed against C. albicans and C. viswanathii.
Table 3.
Anti‐microbial activity by the agar well diffusion method
| Name of the test strain | Mean zone of inhibition ± SD (in mm) |
|---|---|
| Bacillus subtilis | 16.1 ± 0.76 |
| Staphylococcus aureus | — |
| Escherichia coli | — |
| Pseudomonas aeruginosa | 28.2 ± 0.52 |
| Candida albicans | — |
| Candida kruseii | 13.0 ± 1.0 |
| Candida viswanathii | — |
| Trichophyton mentagrophytes | 12.6 ± 1.52 |
Fig. 9.
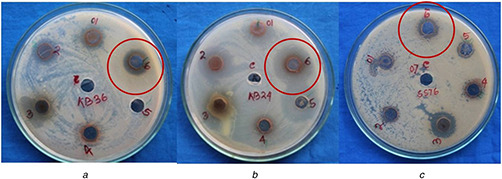
Anti‐microbial activity of AgNPs synthesised by A. solanacea against
(a) B. subtilis, (b) P. aeruginosa, (c) T. mentagrophytes
The micro‐dilution broth assay was followed to ensure the anti‐microbial activity of AgNPs and (Table 4). All the four strains showed growth inhibition above 88%.
Table 4.
Anti‐microbial activity in the broth dilution method
| Name of the test strains | Growth of inhibition (%) comparison with AgNPs, OD at 590 nm |
|---|---|
| Bacillus subtilis | 96.20 |
| Pseudomonas aeruginosa | 102.0 |
| Candida kruseii | 90.40 |
| Trichophyton mentagrophytes | 88.20 |
Several eminent researchers investigated on the anti‐bacterial activity of AgNPs against different bacterial strains [47, 48, 49]. Silver has been widely used for the treatment of bacterial infection adequately before the introduction of penicillin in 1940 [50]. After successful invention and execution of penicillin, the use of silver has been reduced, whereas the use of AgNO3 possesses very good anti‐bacterial property against S. aureus, P. aeruginosa and E. coli [51].
Feng et al. reported on the mechanistic study of silver ions inhibition against two strains of bacteria, S. aureus and E. coli [52]. Several scientists thoroughly investigated the effect of AgNPs and compared the activities with antibiotics, and concluded that the bio‐functionalised AgNPs in the size range of 10–15 nm has an excellent antibiotic effect on both Gram‐positive and Gram‐negative microorganisms [53]. Moreover, critical investigations have also been carried out on anti‐bacterial properties of AgNPs of different size and shapes and found that the anti‐bacterial efficacy of AgNPs is also size and shape dependent [37]. The studies of AgNPs of spherical shaped nanoparticles (20 nm) showed efficient anti‐bacterial activity against E. coli and S. aureus [54].
In the present scenario, AgNPs have made an outstanding comeback as a highly potent anti‐microbial agent during the acute development of resistance against various antibiotics by different pathogenic microorganisms. AgNPs have been emerged up as novel and potent anti‐microbial agents due to its high surface‐to‐volume ratio and unique physico‐chemical properties [15, 55]. Currently, the main interest of many emerging scientists is to develop novel metal ions having microbial resistance, against the common and emerging antibiotics resistant strains. AgNPs have been established to be the most effective anti‐microbial agent against bacteria, fungi, viruses and other eukaryotic microorganisms [56, 57].
3.9 Wound healing assay
The wound healing activity of the AgNPs was observed with positive effect (Fig. 10). The wound healing potential strengthens the application of AgNPs as medicine or ointment. Earlier, it was reported about the anti‐bacterial activity of AgNPs [58]. Jin et al. (2013) has reported the therapeutic applications of plant‐extract‐based scaffolds for wound healing and skin reconstitution studies [59]. There are very few reports on wound healing activity of AgNPs. Hence, the current research finding of potential wound healing capacity of plant‐extract‐based AgNPs will be a positive additive for the biomedical applications.
Fig. 10.
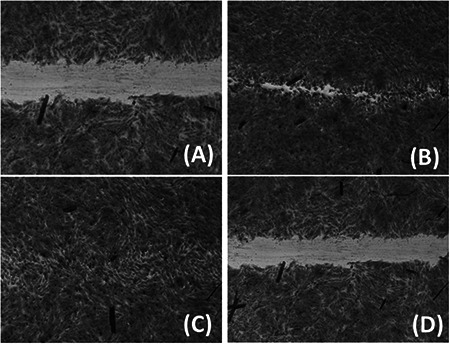
(a) BJ5‐Ta cells treated with leaf extract, (b) BJ5‐Ta cells treated with silver nanoparticles, (c) BJ‐5Ta cells treated with Allantoin (+ve control), (d) BJ‐5Ta cells treated with HBSS (−ve control)
4 Conclusion
The present study demonstrates the phyto‐assisted production of AgNPs from the aqueous extract of A. solanacea, being the simplest and efficient step to obtain AgNPs without utilising any harmful chemical as a reducing and dispersing agent. Water‐soluble organic molecules present in the leaf played a major role in the reduction of Ag+ ions to nano‐sized Ag0 particles. FTIR and anti‐oxidant results clearly revealed regarding the involvement of polyphenols and flavonoids as reducing and stabilising agent. The anti‐oxidant efficacy of A. solanacea gives a safety measure of using the leaf materials as reducing agent for biosynthesis and various biomedical applications of AgNPs. In future, selection of such plants may create a new platform for realising the potential of herbal medicines in nano‐science for drug delivery and biomedical application. In addition, this report provides a theoretical and experimental foundation for investigations of the biosynthesis of other metal nanoparticles.
5 Acknowledgments
North Orissa University, Baripada, Maulana Abul Kalam Azad University of Technology, West Bengal, and National Institute of Technology, Rourkela, India, are highly acknowledged for providing necessary research facility.
6 References
- 1. Suber L. Sondi I. Matijević E. et al.: ‘Preparation and the mechanisms of formation of silver particles of different morphologies in homogeneous solutions’, J. Colloid Interface Sci., 2005, 288, (2), pp. 489 –495 [DOI] [PubMed] [Google Scholar]
- 2. Golubeva O.Y. Shamova O.V. Orlov D.S. et al.: ‘Study of antimicrobial and hemolytic activities of silver nanoparticles prepared by chemical reduction’, Glas. Phys. Chem., 2010, 36, (5), pp. 628 –634 [Google Scholar]
- 3. Song K.C. Lee S.M. Park T.S. et al.: ‘Preparation of colloidal silver nanoparticles by chemical reduction method’, Korean J. Chem. Eng., 2009, 26, (1), pp. 153 –155 [Google Scholar]
- 4. Le A.‐T. Tam L.T. Tam P.D. et al.: ‘Synthesis of oleic acid‐stabilized silver nanoparticles and analysis of their antibacterial activity’, Mater. Sci. Eng. C, 2010, 30, (6), pp. 910 –916 [Google Scholar]
- 5. Huang H. Yang Y.: ‘Preparation of silver nanoparticles in inorganic clay suspensions’, Compos. Sci. Technol., 2008, 68, (14), pp. 2948 –2953 [Google Scholar]
- 6. Yin H. Yamamoto T. Wada Y. et al.: ‘Large‐scale and size‐controlled synthesis of silver nanoparticles under microwave irradiation’, Mater. Chem. Phys., 2004, 83, (1), pp. 66 –70 [Google Scholar]
- 7. Nadagouda M.N. Speth T.F. Varma R.S.: ‘Microwave‐assisted green synthesis of silver nanostructures’, Acc. Chem. Res., 2011, 44, (7), pp. 469 –478 [DOI] [PubMed] [Google Scholar]
- 8. Harada M. Kawasaki C. Saijo K. et al.: ‘Photochemical synthesis of silver particles using water‐in‐ionic liquid microemulsions in high‐pressure CO2’, J. Colloid Interface Sci., 2010, 343, (2), pp. 537 –545 [DOI] [PubMed] [Google Scholar]
- 9. Harada M. Kimura Y. Saijo K. et al.: ‘Photochemical synthesis of silver particles in tween 20/water/ionic liquid microemulsions’, J. Colloid Interface Sci., 2009, 339, (2), pp. 373 –381 [DOI] [PubMed] [Google Scholar]
- 10. Li K. Zhang F.‐S.: ‘A novel approach for preparing silver nanoparticles under electron beam irradiation’, J. Nanoparticle Res., 2010, 12, (4), pp. 1423 –1428 [Google Scholar]
- 11. B K.A. D S.D. Bhoraskar V.N.: ‘Silver nanoparticles: synthesis and size control by electron irradiation’, Nanotechnology, 2006, 17, (13), p. 3204 [DOI] [PubMed] [Google Scholar]
- 12. Zhu J. Liu S. Palchik O. et al.: ‘Shape‐controlled synthesis of silver nanoparticles by pulse sonoelectrochemical methods’, Langmuir, 2000, 16, (16), pp. 6396 –6399 [Google Scholar]
- 13. Lu A.‐H. Salabas E.L. Schüth F.: ‘Magnetic nanoparticles: synthesis, protection, functionalization, and application’, Angew. Chemie Int. Ed., 2007, 46, (8), pp. 1222 –1244 [DOI] [PubMed] [Google Scholar]
- 14. Oei J.D. Zhao W.W. Chu L. et al.: ‘Antimicrobial acrylic materials with in situ generated silver nanoparticles’, J. Biomed. Mater. Res. Part B Appl. Biomater., 2012, 100B, (2), pp. 409 –415 [DOI] [PubMed] [Google Scholar]
- 15. Kim K.‐J. Sung W.S. Suh B.K. et al.: ‘Antifungal activity and mode of action of silver nano‐particles on Candida albicans ’, BioMetals, 2009, 22, (2), pp. 235 –242 [DOI] [PubMed] [Google Scholar]
- 16. Nadworny P. Wang J. Tredget E. et al.: ‘Anti‐inflammatory activity of nanocrystalline silver‐derived solutions in porcine contact dermatitis’, J. Inflamm. (Lond.), 2010, 7, p. 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lara H.H. Ayala‐Nuñez N.V. Ixtepan‐Turrent L. et al.: ‘Mode of antiviral action of silver nanoparticles against HIV‐1’, J. Nanobiotechnol., 2010, 8, (1), pp. 1 –10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalishwaralal K. Banumathi E. Pandian S.B.R.K. et al.: ‘Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells’, Colloid Surf. B Bioint., 2009, 73, (1), pp. 51 –57 [DOI] [PubMed] [Google Scholar]
- 19. Shrivastava S. Bera T. Singh S.K. et al.: ‘Characterization of antiplatelet properties of silver nanoparticles’, ACS Nano, 2009, 3, (6), pp. 1357 –1364 [DOI] [PubMed] [Google Scholar]
- 20. Atiyeh B.S. Costagliola M. Hayek S.N. et al.: ‘Effect of silver on burn wound infection control and healing: review of the literature’, Burns, 2016, 33, (2), pp. 139 –148 [DOI] [PubMed] [Google Scholar]
- 21. Khatun A. Rahman M. Kabir S. et al.: ‘Phytochemical and pharmacological properties of methanolic extract of Ardisia humilis vahl. (Myrsinaceae)’, Int. J. Res. Ayurveda Pharm., 2013, 4, (1), pp. 38 –41 [Google Scholar]
- 22. Amin M. Banik S. Ibrahim M. et al.: ‘A study on Ardisia solanacea for evaluation of phytochemical and pharmacological properties’, Int. J. Pharmacogn. Phytochem. Res., 2015, 7, (1), pp. 8 –15 [Google Scholar]
- 23. Samal P.: ‘Hepatoprotective activity of Chara Parpam in Ccl4 induced rats’, Asian J. Pharm. Sci, 2013, 3, pp. 79 –82 [Google Scholar]
- 24. Parekh J. Chanda S.V.: ‘In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants’, Turk. J. Biotechnol., 2008, 31, pp. 53 –58 [Google Scholar]
- 25. Arunachalam K.D. Suhashani S. Sathesh K.A.: ‘Wound healing and antigenotoxic activities of Aegle marmelos with relation to its antioxidant properties’, J. Pharm. Res., 2012, 5, (3), pp. 1492 –1502 [Google Scholar]
- 26. Guruvaiah P. Arunachalam A. Velan L.P.T.: ‘Evaluation of phytochemical constituents and antioxidant activities of successive solvent extracts of leaves of Indigofera caerulea Roxb. using various in vitro antioxidant assay systems’, Asian Pacific J. Trop. Dis., 2012, 2, pp. S118 –S123 [Google Scholar]
- 27. McDonald S. Prenzler P.D. Antolovich M. et al.: ‘Phenolic content and antioxidant activity of olive extracts’, Food Chem., 2001, 73, (1), pp. 73 –84 [Google Scholar]
- 28. Chang C.‐C. Yang M.‐H. Wen H.‐M. et al.: ‘Estimation of total flavonoid content in propolis by two complementary colorimetric methods’, J. Food Drug Anal., 2002, 10, (3), pp. 178 –182 [Google Scholar]
- 29. Panda S.K.: ‘Ethno‐medicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha, India’, J. Ethnopharmacol., 2014, 151, (1), pp. 158 –175 [DOI] [PubMed] [Google Scholar]
- 30. Mohanta T. Patra J. Rath S.: ‘Evaluation of antimicrobial activity and phytochemical screening of oils and nuts of Semicarpus anacardium ’, Sci. Res. Essay, 2007, 2, (November), pp. 486 –490 [Google Scholar]
- 31. Chen Y. Lu B. Yang Q. et al.: ‘Combined Integrin phosphoproteomic analyses and small interfering RNA‐based functional screening identify key regulators for cancer cell adhesion and migration’, Cancer Res., 2009, 69, (8), pp. 3713 –3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yarrow J.C. Perlman Z.E. Westwood N.J. et al.: ‘A high‐throughput cell migration assay using scratch wound healing, a comparison of image‐based readout methods’, BMC Biotechnol., 2004, 4, p. 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nayak D. Ashe S. Rauta P.R. et al.: ‘Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma’, Mater. Sci. Eng. C, 2016, 58, pp. 44 –52 [DOI] [PubMed] [Google Scholar]
- 34. Ge L. Li Q. Wang M. et al.: ‘Nanosilver particles in medical applications: synthesis, performance, and toxicity’, Int. J. Nanomed., 2014, 9, pp. 2399 –2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohanta Y. Singdevsachan S. Parida U. et al.: ‘Green synthesis and antimicrobial activity of silver nanoparticles using wild medicinal mushroom Ganoderma applanatum (Pers.) Pat. from Similipal Biosphere Reserve, Odisha, India’, IET Nanobiotechnol., 2016, 10, (4), pp. 184 –189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kalimuthu K. Suresh Babu R. Venkataraman D. et al.: ‘Biosynthesis of silver nanocrystals by Bacillus licheniformis’, Colloids Surf. B. Biointerfaces, 2008, 65, (1), pp. 150 –153 [DOI] [PubMed] [Google Scholar]
- 37. Nayak D. Minz A.P. Ashe S. et al.: ‘Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: characterization and cytotoxic effect on MCF‐7 breast cancer cell lines’, J. Colloid Interface Sci., 2016, 470, pp. 142 –152 [DOI] [PubMed] [Google Scholar]
- 38. Lanje A. Sharma S. Pode R.: ‘Synthesis of silver nanoparticles: a safer alternative to conventional antimicrobial and antibacterial agents’, J. Chem. Pharm. Res., 2010, 2, pp. 478 –483 [Google Scholar]
- 39. Shankar S. Ahmad A. Sastry M.: ‘Geranium leaf assisted biosynthesis of silver nanoparticles’, Biotechnol. Prog., 2003, 19, (6), pp. 1627 –1631 [DOI] [PubMed] [Google Scholar]
- 40. Lu J. Yang J. Wang J. et al.: ‘One‐pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids’, ACS Nano, 2009, 3, (8), pp. 2367 –2375 [DOI] [PubMed] [Google Scholar]
- 41. Huang J. Li Q. Sun D. et al.: ‘Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf’, Nanotechnology, 2007, 18, (10), p. 105104 [Google Scholar]
- 42. Prasad R.: ‘Synthesis of silver nanoparticles in photosynthetic plants’, J. Nanoparticles, 2014, 2014, pp. 1 –8 [Google Scholar]
- 43. Hagerman A.E. Riedl K.M. Jones G.A. et al.: ‘High molecular weight plant polyphenolics (Tannins) as biological antioxidants’, J. Agric. Food Chem., 1998, 46, (5), pp. 1887 –1892 [DOI] [PubMed] [Google Scholar]
- 44. Pratap Chandran R. Manju S. Vysakhi M.V. et al.: ‘In vitro antioxidant potential of methanolic and aqueous extracts of Ardisia solanacea Roxb. leaf’, J. Pharm. Res., 2013, 6, (5), pp. 555 –558 [Google Scholar]
- 45. Luximon‐Ramma A. Bahorun T. Soobrattee M.A. et al.: ‘Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula ’, J. Agric. Food Chem., 2002, 50, (18), pp. 5042 –5047 [DOI] [PubMed] [Google Scholar]
- 46. Abdel‐Aziz M.S. Shaheen M.S. El‐Nekeety A.A. et al.: ‘Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract’, J. Saudi Chem. Soc., 2014, 18, p. 356 [Google Scholar]
- 47. Kotakadi V.S. Gaddam S.A. Venkata S.K. et al.: ‘New generation of bactericidal silver nanoparticles against different antibiotic resistant Escherichia coli strains’, Appl. Nanosci., 2015, 5, (7), pp. 847 –855 [Google Scholar]
- 48. Kim J.S. Kuk E. Yu K.N. et al.: ‘Antimicrobial effects of silver nanoparticles’, Nanomed. Nanotechnol. Biol. Med., 2007, 3, (1), pp. 95 –101 [DOI] [PubMed] [Google Scholar]
- 49. Railean‐Plugaru V. Pomastowski P. Wypij M. et al.: ‘Study of silver nanoparticles synthesized by acidophilic strain of Actinobacteria isolated from the of Picea sitchensis forest soil’, J. Appl. Microbiol., 120, 2016, pp. 1 –14 [DOI] [PubMed] [Google Scholar]
- 50. Chopra I.: ‘The increasing use of silver‐based products as antimicrobial agents: a useful development or a cause for concern?’, J. Antimicrob. Chemother., 2007, 59, pp. 587 –590 [DOI] [PubMed] [Google Scholar]
- 51. Moyer C.: ‘Some effects of 0.5% silver nitrate and high humidity upon the illness associated with large burns’, J. Natl. Med. Assoc., 1965, 57, pp. 95 –100 [PMC free article] [PubMed] [Google Scholar]
- 52. Feng Q. Wu J. Chen G. et al.: ‘A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus ’, J. Biomed. Mater. Res., 2000, 52, pp. 662 –668 [DOI] [PubMed] [Google Scholar]
- 53. Shahverdi A.R. Minaeian S. Shahverdi H.R. et al.: ‘Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach’, Process Biochem., 2007, 42, (5), pp. 919 –923 [Google Scholar]
- 54. Rai M. Yadav A. Gade A.: ‘Current trends in phytosynthesis of metal nanoparticles’, Crit. Rev. Biotechnol., 2008, 28, (4), pp. 277 –284 [DOI] [PubMed] [Google Scholar]
- 55. Morones J.R. Elechiguerra J.L. Camacho A. et al.: ‘The bactericidal effect of silver nanoparticles’, Nanotechnology, 2005, 16, (10), pp. 2346 –2353 [DOI] [PubMed] [Google Scholar]
- 56. Mohanta Y.K. Panda S.K. Bastia A.K. et al.: ‘Biosynthesis of silver nanoparticles from Protium serratum and investigation of their potential impacts on food safety and control’, Front. Microbiol., 2017, 8, (April), pp. 1 –10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mohanta Y.K. Panda S.K. Jayabalan R. et al.: ‘Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.)’, Front. Mol. Biosci., 2017, 4, (March), pp. 1 –9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mohanta Y. Panda S. Biswas K. et al.: ‘Biogenic synthesis of silver nanoparticles from Cassia fistula (Linn.): in vitro assessment of their antioxidant, antimicrobial and cytotoxic activities’, IET Nanobiotechnol., 2016, 10, (6), pp. 428 –444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jin G. Prabhakaran M.P. Kai D. et al.: ‘Tissue engineered plant extracts as nanofibrous wound dressing’, Biomaterials, 2013, 34, (3), pp. 724 –734 [DOI] [PubMed] [Google Scholar]


