Abstract
Consistent search of plants for green synthesis of silver nanoparticles (SNPs) is an important arena in Nanomedicine. This study focuses on synthesis of SNPs using bioreduction of silver nitrate (AgNO3) by aqueous root extract of Decalepis hamiltonii. The biosynthesis of SNPs was monitored by UV–vis analysis at absorbance maxima 432 nm. The fluorescence emission spectra of SNPs illustrated the broad emission peak 450–483 nm at different excitation wavelengths. The surface characteristics were studied by scanning electron microscope and atomic force microscopy, showed spherical shape of SNPs and dynamic light scattering analysis confirmed the average particle size 32.5 nm and the presence of metallic silver was confirmed by energy dispersive X‐ray. Face centred cubic structure with crystal size 33.3 nm was revealed by powder X‐ray diffraction. Fourier transform infrared spectroscopy indicated the biomolecules involved in the reduction mainly polyols and phenols present in root extracts were found to be responsible for the synthesis of SNPs. The stability and charge on SNPs were revealed by zeta potential analysis. In addition, on therapeutic forum, the synthesised SNPs elicit antioxidant and antimicrobial activity against Bacillus cereus, Bacillus licheniformis, Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus.
Inspec keywords: silver, nanoparticles, nanomedicine, antibacterial activity, biomedical materials, nanofabrication, particle size, microorganisms, ultraviolet spectra, visible spectra, fluorescence, scanning electron microscopy, atomic force microscopy, light scattering, X‐ray diffraction, X‐ray chemical analysis, Fourier transform infrared spectra, molecular biophysics, electrokinetic effects
Other keywords: phenols, zeta potential analysis, therapeutic forum, antioxidant activity, antimicrobial activity, Bacillus cereus, Bacillus licheniformis, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Ag, polyols, biomolecules, Fourier transform infrared spectroscopy, powder X‐ray diffraction, crystal size, face centred cubic structure, energy dispersive X‐ray analysis, metallic silver, particle size, dynamic light scattering analysis, spherical shape, atomic force microscopy, scanning electron microscopy, surface characteristics, excitation wavelengths, fluorescence emission spectra, UV‐visible analysis, biosynthesis, silver nitrate bioreduction, nanomedicine, Decalepis hamiltonii aqueous root extract, bioactivity, plant‐mediated silver nanoparticles, green synthesis
1 Introduction
Recent advancements in Nanobiotechnology led to a new avenue in the field of science and engineering mainly finding importance in contemporary material science. Metal nanoparticles (NPs) exhibit unique physico‐chemical properties and potential applications in bio‐labelling, targeted drug delivery, biosensors, antimicrobial and antiviral activity, gene therapy, DNA sequencing and detection of genetic disorders [1]. Significant exploitation of biological systems in NP synthesis and application has received attention during the last decades [2, 3]. From the prehistoric period, sliver is well known metal for its medicinal properties and silver NPs (SNPs) generally with the size 1–100 nm are studied for its biomedical applications [4]. On the therapeutic forum, SNPs found to exhibit antibacterial, antifungal, antiviral, and anti‐inflammatory activities [4, 5]. They are used in drug delivery, diagnosis, treatment, medical device coatings, medical textiles and contraceptive devices [6].
Synthesis of SNPs uses chemical and photochemical reduction methods utilise silver nitrate (AgNO3) as a salt; sodium citrate, sodium borohydride and alcohols as a reducing agent. Physical vapour condensation and arc‐discharge, thermal decomposition, irradiation, diffusion and so on are generally used in case of physical methods [7]. These methods require hazardous and toxic chemicals, maintenance of high temperature and pressure which may pose potential environmental and biological risk. Moreover, SNPs so produced are always been a concern with respect to stability and safety. Thus, these inherent drawbacks alarmed the need of alternative and safer approaches for SNPs synthesis. In biological methods, the use bacteria, fungi, and plant extracts created interest due to their easy, cost effective, non‐toxic, high purity, ease of mass production as well as an environmentally friendly synthesis of SNPs [4].
Use of plant extracts has advantages because by‐products formed are generally biocompatible, less prone to contamination [8]. SNPs have been synthesised earlier using different medicinal plants such as Ocimum sanctum [9], catharananthus roseus [10], Azadirachta indica [11], Aloe vera [12], Camellia sinensis [13], Origanum vulgare [14], Moringa oleifera [15], Piper nigrum [16] and Coriandrum sativum [17]. Decalepis hamiltonii (DH), native of Deccan peninsula and forest areas of the Western Ghats of India popularly known as swallow root belongs to the family Periploaceace (an offshoot of the Asclepiadaceae) [18]. The ethno‐medicinal property of DH is mainly due to a vanillin isomer present in the plant. The bioactive compounds in this plant possess potent antioxidant, antibacterial, antifungal, anti‐diabetic, antiulcer, anti‐inflammatory, antipyretic and gastro protective activities [19, 20].
This work describes the synthesis of SNPs using roots of DH at ambient temperature, without using any additive or chemical agents for the reduction and capping of NPs. The rapid reduction of silver ions in the presence of the aqueous root extract of DH provides valuable information for scale up of greener synthesis of SNPs and further nanomedicinal application of SNPs.
2 Materials and methods
2.1 Synthesis of SNPs
Fresh and healthy roots of DH were purchased from a local market, Mysuru, India. They were first washed with tap water followed by distilled water. The roots were cut into small pieces of 1 cm and sun dried for three days. Samples were powdered and the aqueous extract was prepared by mixing 5 g of dried root powder with 100 ml of sterile boiling water with continuous stirring for 30 min. The extract was allowed to cool, filtered using Whatman No. 1 filter paper and stored at 4°C. For synthesis of SNPs from the root extract, 90 ml of 1 mM AgNO3 solution was mixed with 10 ml of root extract and the flask was kept under bright light condition and incubated for 24 h with intermittent mixing for every 10 min. Time taken for the colour change from yellow to dark brown were recorded using UV–vis. spectrophotometry. The SNPs were first filtered using Whatman No.1 filter paper (0.45 µm) followed by 0.20 µm Micro‐Por, Sterile Minigen Syringe Filter. Purified SNPs were lyophilised and stored at 4°C [21].
2.2 Characterisation of SNPs
2.2.1 UV–vis and fluorescence spectroscopic analysis
The bio‐reduction of silver ions was monitored by UV–vis spectroscopy (Shimadzu UV 1800). The spectrum was recorded at time intervals of 0, 10, 30 min, 1, 2, 4 and 24 h with a nanometre range of 300–700. Twenty four hours incubated samples of SNPs were used for fluorescence measurements (F‐4600 Fluorescence Spectrophotometer, Hitachi). The fluorescence properties of SNPs were analysed under different excitation wavelengths 300–380 nm and emission spectrum was recorded. The excitation slit width was set at 10 nm and emission slit width was 25 nm with the process voltage temperature (PVT) voltage 400 V.
2.2.2 Scanning electron microscopy (SEM) and energy dispersive X‐ray (EDX) spectroscopy
The surface morphology of SNPs was characterised by SEM (Hitachi S‐3400N SEM) operated at an accelerating voltage between 5 and 30 kV. Samples of lyophilised and aqueous SNPs were placed on the carbon coated grids and the images of SNPs were obtained at different resolution. For EDX analysis, lyophilised SNPs were placed on the carbon coated grids and scanned from 0 to 10 keV.
2.2.3 Dynamic light scattering (DLS) and zeta potential (ZP) analysis
Hydrodynamic diameter of the SNPs was analysed by DLS measurements (DLS Microtrack Nanotrack). Purified SNPs were ultrasonicated for 30 min followed by the size measurements from 0.1 to 1000 nm at room temperature. ZP analysis was performed in both distilled water and acetone dispersed SNPs at room temperature and pH 7.
2.2.4 Powder X‐ray diffraction (XRD) measurements
For the determination of structure and size of the nanocrystals XRD analysis was performed (Rigaku XRD system). The diffractogram was recorded using Cu Kα target at 1.54060 Å. The diffracted intensities were recorded at 2θ angles between 10° and 80°. The crystal size of the SNPs was calculated using Debye–Scherrer equation
where K is the shape factor; λ is the X‐ray wavelength; θ is Bragg's angle and β is the full‐width half‐maxima in radians.
2.2.5 Fourier transform infrared (FTIR) spectroscopy
FTIR spectroscopy analysis (JASCO FT/IR 4100 spectroscopy) was performed using potassium bromide (KBr) pellet method and spectra were recorded between 4000 and 550 cm−1 at 8 cm−1 resolution.
2.2.6 Atomic force microscopy (AFM)
SNPs were first ultrasonicated for 30 min and a drop of the sample was placed on the cover slip and was allowed to dry at room temperature. The raster scan was performed under contact mode (A.P.E Research, A100).
2.3 Antioxidant activity by DPPH (1,1‐diphenyl‐2‐picrylhydrazyl) method
Antioxidant activity was performed according to Srivastava et al. (2006) with slight modifications. 3 ml of 100 µM methanolic DPPH was added to aliquots of 0.05 and 0.1 ml of root extracts and SNPs. Reaction mixtures were made with 4 ml using distilled water and incubated at room temperature for 20 min under dark conditions. Absorbance was read at 517 nm (Shimadzu UV 1800) and gallic acid was used as a standard. The percentage of antioxidant activity was determined using the formula
2.4 Estimation of total polyphenols and protein content
The total phenolic content of root extracts and SNPs were determined by Folin–Ciocalteau (FC) reagent method [22]. Aliquots of root extracts and SNPs (0.05 and 0.1 ml) were made up to 3 ml with distilled water and mixed with 0.15 ml FC reagent. The mixture was neutralised with 0.3 ml of saturated sodium carbonate solution and incubated at room temperature for 30 min. The absorbance was read at 660 nm (Shimadzu UV 1800) and the total polyphenol content of the samples was estimated with reference to the standard gallic acid concentrations. Amount of protein content estimated using Lowry's method using bovine serum albumin as standard.
2.5 Antibacterial activity
2.5.1 Preparation of bacterial inoculum
Five bacterial strains, Bacillus cereus (MTCC 430), Bacillus licheniformis (MTCC 2465), Escherichia coli (E. coli) (MTCC 10312) Staphylococcus aureus (MTCC 7443) and Pseudomonas aeruginosa (ATCC 27853) were used for the antibacterial assy. These organisms were sub cultured on a nutrient agar and maintained at 4°C. Inoculum was prepared using 10 ml autoclaved nutrient broth was inoculated with a loop full of bacterial culture and incubated at 37°C, for 18 h in an incubator shaker at 220 rpm. The optical density of the inoculum was adjusted to 0.4–0.5, at 660 nm (Shimadzu UV 1800 Spectrophotometry).
2.5.2 Antibacterial activity test
A total of 25 ml of sterilised Muller–Hinton agar medium was transferred into sterile petri plates and allowed to solidify and 100 µl of bacterial cultures from the inoculum were spread on Muller–Hinton agar plates using sterile bent glass rod. Wells of 10 mm diameter were made on each plate using sterile cork borer. A stock solution of SNPs of concentration 10 mg/ml was prepared in distilled water and was used for antibacterial assy. Aliquots of SNPs from the stock (25, 35 and 45 µl) were added into the wells and sterile distilled water was used as a negative control. Antibiotic discs of Ampicillin (25 mcg/disc), Noroflocin (10 mcg/disc) and Vancomycin (5 mcg/disc) were used as a positive control. The plates were incubated at 37°C for 24 h. The zone of inhibition was measured in terms of millimetres. All experiments were carried out in triplicates and mean value was calculated.
3 Results and discussion
3.1 Synthesis of SNPs
The colour change from pale yellow to dark brown due to the rapid reduction of AgNO3 to SNPs was observed immediately after the addition of 1 mM aqueous AgNO3 solution to DH root extract. The colour intensity increased along with the incubation time due to the surface plasmon resonance effect and reducing property of AgNO3 [21]. However, there was no colour change observed in control, i.e. AgNO3 solution (Fig. 1 A).
Fig. 1.

Change in colour due to SNP synthesis
(a) Control (left) reaction mixture (right), (b) UV–vis absorbance spectra of SNPs, (c) Fluorescence emission spectra of SNPs
3.2 Characterisation of SNPs
3.2.1 UV–vis analysis and fluorescence spectroscopic measurements
The UV–vis spectrum recorded showed an increase in colour intensity from initiation to completion of the reaction (Fig. 1 B). Absorption spectra of SNPs had absorption maxima range from 386 to 448 nm and at 432 nm the maximum absorbance was recorded [22].
The maximum fluorescence intensity, 82.45 was recorded when the SNPs were exited at 340 nm corresponding to the emission wavelength of 495 nm (Fig. 1 C). At different excitation wavelengths from 300 to 380 nm, broad emission peaks were noticed from 450 to 483 nm. It was noticed that as the excitation wavelength increases, there was a decrease in fluorescence intensity and emission was observed at wavelength region 469 nm [23].
3.2.2 SEM and EDX spectroscopy analysis of SNPs
Figs. 2 A–C show the scanning electron micrographs of DH mediated synthesis of SNPs. SEM images reveal the appearance of uniform spherical NPs in well dispersed aqueous extracts which conform to the shape of an SPR band in the UV–vis spectrum [24]. The particle size of SNPs 49.3, 55.1 and 105 nm agrees with DLS histogram with an average diameter of around 34 nm. In case of powder SNPs aggregates were seen. EDX spectrum showed three moderate to weak absorbance peaks of Ag with 30, 30 and 40 scale counts corresponding to 2.5, 3 and 3.5 keV, respectively (Fig. 2 D). The concentrations of Ag were observed as 1.19 at% and 7.11 wt%. The presence of other elements such as carbon, oxygen, magnesium and chlorine is due to the elements present in root extracts which may help in capping and stabilising the SNPs [25]. The absorption peaks at 3 keV confirm that the NPs formed are exclusively silver. The strong signals of carbon are due to the grid used for EDX spectra analysis and also the strong peaks of oxygen observed are mainly due to the oxidation of samples [9].
Fig. 2.

SEM and EDX analysis of biosynthesised SNPs
(a) SEM images of Powder SNPs, (b) and (c) SEM images of aqueous SNPs, (d) EDX spectrum of SNPs
3.2.3 DLS and ZP analysis of SNPs
DLS studies indicated the particle size distribution of SNPs between 49.23 and 24.86 nm with the average hydrodynamic size of 32.5 nm which can be well correlated with the powder XRD and AFM studies (Fig. 3 and Table 1). ZP indicates the surface charges present on the NPs and mainly depends on shape, size and methodology of synthesis of NPs. Hence, these external factors influence the colloidal stability of NPs [26].
Fig. 3.

DLS particle size distribution pattern of SNPs
Table 1.
ZP analysis of water and acetone dispersed SNPs
| Sample | ZP, mV | Polarity | Charge, fC | Conductivity, µS/cm | Field strength, kV/m |
|---|---|---|---|---|---|
| aqueous | 8.3 | positive | 0.003 | 671 | 5.0 |
| acetone | 0.5 | positive | 0.003 | 59 | 5.0 |
3.2.4 Powder XRD measurements
The XRD studies showed strong peaks at 2θ angles 27.78°, 32.23°, 46.23°, 57.45° and 76.63° corresponding to the lattice planes 111, 200, 220, 222 and 420, respectively. The crystallite size of SNPs was observed at 33.23 nm at the 2θ angle 27.78° (Fig. 4). By combining the EDX and XRD results, it was confirmed the face‐centred‐cubic (FCC) structure of SNPs and the characteristic pattern corresponds to silver suggests that the main composition of NPs is silver [27].
Fig. 4.

XRD pattern of SNPs
3.2.5 FTIR spectroscopy analysis of SNPs
The FTIR spectrum of DH root extract revealed the characteristics absorbance bands at 3621.66 and 921.807 cm−1 (O–H stretch); 3282.25 and 858.168 and 1565.92 cm−1 (N–H stretch due to primary amine); 2937.06 and 2890.77 cm−1 (C–H stretch); 1741.41 cm−1 (C=O stretch); 1646.91 (aromatic ring); 1450.21 cm−1 (aliphatic alkane CH3); 1218.79 cm−1 (C–N stretch due to amines); 605.539 cm−1 (S–S linkage mainly found in protein structures); and 605.539 cm−1 (S–S linkage) (Fig. 5 A). Interestingly, C–O–C groups at 1149.37 cm−1 due to polysaccharides or chlorophyll were recorded in the case of DH root extracts (control) which was not found in SNPs. In case of DH root mediated SNPs the characteristic absorbance bands at 3936 and 3781.72 cm−1 (O–H stretch); 3583.09 cm−1 (N–H stretch); 2979 and 997.017 cm−1 (C–H stretch); 2441.44 cm−1 (C≡N due to nitriles); 1764.55 cm−1 (C=O stretch); sharp intense peak at 1594 cm−1 (N–H stretch); 1427.07 cm−1 (aliphatic alkane CH3); 1201.43 cm−1 (C–N stretch due to amines); and 601.682 cm−1 (S–S linkage mainly found in protein structures) were observed (Fig. 5 B). There was a shift in frequencies after synthesis of SNPs from 3621.66 to 3781.72 cm−1; 3282.25 to 3583.09 cm−1; 2937.06 to 2979 cm−1; 1741.41 to 1764.55 cm−1; 1450.21 to 1427.07 cm−1; 1218.79 to 1201.43 cm−1 corresponding to O–H, N–H, C–H, C=O, aliphatic alkane CH3 and C–N stretches. This may be due to the reduction and stabilisation of SNPs by phytochemicals present in the root extract such as triterpene and their derivatives, glycosides, flavonoids and polyphenols [20].
Fig. 5.

FTIR spectrum analysis
(a) DH root extract powder, (b) Biosynthesised SNPs
3.2.6 AFM analysis of SNPs
The average size and surface area of SNPs were observed as 70.20 nm and 25.94 µm2, respectively, from AFM image (Fig. 6). Furthermore, the height distribution profile of SNPs is said to be asymmetrical due to the positive skewness value (0.145), the flat surface of NPs was mainly because the kurtosis is lesser than 3. Hence asymmetrical height distribution profile was observed from AFM studies [28].
Fig. 6.

AMF image of SNPs: 2D image (left); 3D image (right)
3.3 Anti‐oxidant activity study of SNPs by DPPH method
The health promoting potential of DH was studied by different researchers. It is known that SNPs possess antioxidant activity [29]. This particularly true in treatment of various diseases such as cancer. The SNPs synthesised through biological route were found to have higher antioxidant activity and therapeutic action. The antioxidant activity of DH root extracts and SNPs was comparatively studied by DPPH method. The radical scavenging effect of 50 and 100 μl of DH root extracts on the DPPH free radical was compared with the standard antioxidant, gallic acid. The results were expressed as %RSA are shown in Table 2. The dose dependant scavenging effects were observed in both DH root extracts and SNP [30].
Table 2.
Estimation of antioxidant activity, total polyphenol and protein concentration in root extract and SNPs
| Extract | Volume, µL | Antioxidant activity, %RSA | Polyphenol content, mg/ml | Protein concentration, µg/ml |
|---|---|---|---|---|
| root (aqueous) | 50 | 11.11 | 5.341 | 183.47 |
| 100 | 23.148 | 5.329 | 364.34 | |
| SNPs | 50 | 11.15 | 1.102 | 33.043 |
| 100 | 22.44 | 1.119 | 66.52 |
3.4 Estimation of total polyphenols and protein concentration in root extract and SNPs
The total polyphenol content of aqueous root extracts was expressed in terms of gallic acid equivalents. The concentration of total polyphenols present in the DH root extract was 534.08 GAE mg/100 g of plant powder. The protein content of root extracts and SNPs was expressed in terms of BSA equivalents. There was four fold reduction of protein and total polyphenols content between plant extract and synthesised aqueous SNPs (Table 2). This is mainly because the plant mediated SNPs involves the sequential steps in the reduction and stabilisation of NPs by capping and thus substantial amount of proteins are utilised as a reducing and capping agents [31, 32].
3.5 Antibacterial activity of SNPs and standard antibiotics
The diligence of antibiotic‐resistant bacteria has exploited the anti‐microbial properties of silver and silver‐based compounds, including SNPs [5]. The antibacterial efficacy of SNPs mainly depends on their size, shape and concentration. Mohanty et al. (2012) evaluated the anti‐bacterial activity of starch‐stabilised AgNPs against human pathogens – Staphylococcus aureus, Salmonella typhi and Pseudomonas aeruginosa. Greater antibacterial effect on E. coli was found when Amoxicillin and SNPs were combined. It is widely accepted that SNPs can anchor to and successively penetrate the bacterial cell wall, thus causing a structural change of the cell membrane and increasing cell permeability, leading to cell death. The formation of free radicals and subsequent free radical‐induced membrane damage is another potential mechanism. SNPs can release silver ions and interact with phosphorus‐containing bases and the thiol groups of many enzymes, this leads to inhibition of certain functions inside the cell, such as affecting DNA replication and cell division. In addition, SNPs may modulate signal transduction through changing the phosphotyrosine profile of bacterial peptides for the potential antibacterial mechanism [4, 33].
SNPs showed reasonable antibacterial activity against all five tested bacterial species (Figs. 7 and 8). The diameter of inhibition zone was observed in the range of 13.5 ± 0.5–18.66 ± 0.942 mm, which is significantly comparable to standards (11.66 ± 0.57–27.25 ± 0.35 mm). There was no inhibition zone observed in case of sterile distilled water used as a negative control. Therefore, SNPs exhibited higher bactericidal activity against Pseudomonas aeruginosa compared to other bacterial species. Overall the DH root mediated SNPs revealed appreciable antibacterial and antioxidant properties which can be further utilised for its various applications [24, 34].
Fig. 7.
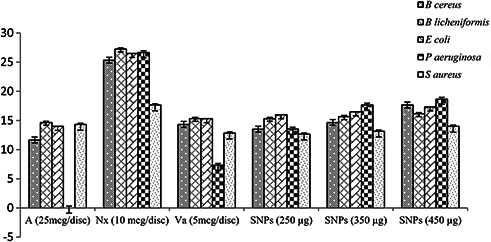
Antibacterial activity of SNPs and standard antibiotics
Fig. 8.
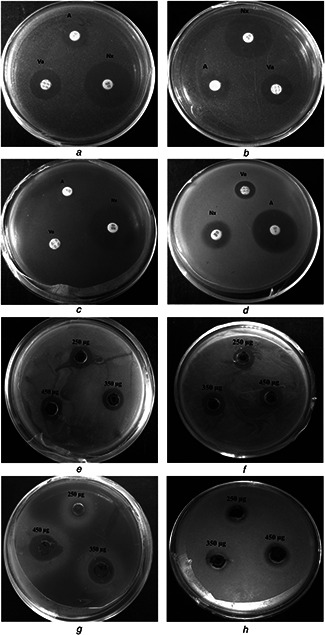
Zone of inhibition of standard antibiotics and SNPs and
(a) and (e) Bacillus cereus, (b) and (f) E. coli, (c) and (g) Pseudomonas aeruginosa, (d) and (h) Staphylococcus aureus
4 Conclusion
This work emphasises the greener synthesis of SNPs utilising medicinal value root extract following cost effective and ecologically pleasant process with broad spectrum applications like electronics, optics, medicine, cosmetics and so on, and also aids in other noble metal NP synthesis.
5 Acknowledgments
The authors are thankful to Principal and Department of Biotechnology, Sri Jayachamarajendra College of Engineering, Mysuru and Postgraduate Department of Biochemistry, JSS College of Arts, Commerce and Science, Mysuru for providing all facility to conduct research.
6 References
- 1. Tran Q.H. Nguyen V.Q. Le A. T.: ‘Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives’, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2013, 4, pp. 1 –20, doi: 10.1088/2043‐6262/4/3/033001 [Google Scholar]
- 2. Mody V.V. Siwale R. Singh A. et al.: ‘Introduction to metallic nanoparticles’, J. Pharm. Bioallied. Sci., 2010, 4, pp. 282 –289, doi: 10.4103/0975‐7406.72127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gericke M. Pinches A.: ‘Biological synthesis of metal nanoparticles’, Hydrometallurgy, 2006, 83, pp. 132 –140, doi: 10.1016/j.hydromet.2006.03.019 [Google Scholar]
- 4. Prabhu S. Poulose E.K.: ‘Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects’, Int. Nano Lett., 2012, 2, (32), pp. 1 –10, doi: 10.1186/2228‐5326‐2‐32 [Google Scholar]
- 5. Mohanty S. Mishra S. Jena P. et al.: ‘An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch‐stabilized silver nanoparticles’, Nanomedicine: NBM, 2012, 8, (6), pp. 916 –924, doi: 10.1016/j.nano.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 6. Mirza A.Z. Siddiqui F.A.: ‘Nanomedicine and drug delivery: a mini review’, Int. Nano Lett., 2014, 4, (4), pp. 1 –7, doi: 10.1007/s40089‐014‐0094‐7 [Google Scholar]
- 7. Shameli K. Bin Ahmad M. Jazayeri S. D. et al.: ‘Investigation of antibacterial properties silver nanoparticles prepared via green method’, Chem. Cent. J., 2012, 6, (1), p. 73, doi: 10.1186/1752‐153X‐6‐73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajan R. Chandran K. Harper S. L. et al.: ‘Plant extract synthesized silver nanoparticles: an ongoing source of novel biocompatible materials’, Ind. Crop. Prod., 2015, 70, pp. 356 –373, doi: 10.1016/j.indcrop.2015.03.015 [Google Scholar]
- 9. Ahmad N. Sharma S. Alam M. K. et al.: ‘Rapid synthesis of silver nanoparticles using dried medicinal plant of basil’, Colloids Surf B Biointerfaces, 2010, 81, pp. 81 –86, doi: 10.1016/j.colsurfb.2010.06.029 [DOI] [PubMed] [Google Scholar]
- 10. Kannan N. Mukunthan K.S. Balaji S.: ‘A comparative study of morphology, reactivity and stability of synthesized silver nanoparticles using Bacillus subtilis and Catharanthus roseus (L.) G. Don’, Colloids Surf B Biointerface, 2011, 86, pp. 378 –383, doi: 10.1016/j.colsurfb.2011.04.024 [DOI] [PubMed] [Google Scholar]
- 11. Banerjee P. Satapathy M. Mukhopahayay A. et al.: ‘Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis’, Bioresour. Bioprocess., 2014, 1, (3), pp. 1 –10, doi: 10.1186/s40643‐014‐0003‐y [Google Scholar]
- 12. Zhang Y. Cheng X. Zhang Y. et al.: ‘Biosynthesis of silver nanoparticles at room temperature using aqueous aloe leaf extract and antibacterial properties’, Colloids Surf A Physicochem Eng. Asp., 2013, 423, pp. 63 –68, doi: 10.1016/j.colsurfa.2013.01.059 [Google Scholar]
- 13. Loo Y.Y. Chieng B.W. Nishibuchi M. et al.: ‘Synthesis of silver nanoparticles by using tea leaf extract from Camellia sinensis ’, Int. J. Nanomed., 2012, 7, pp. 4263 –4267, doi: org/10.2147/IJN.S33344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sankar R. Karthik A. Prabu A. et al.: ‘Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity’, Colloids Surf B Biointerfaces, 2013, 108, pp. 80 –84, doi: 10.1016/j.colsurfb.2013.02.033 [DOI] [PubMed] [Google Scholar]
- 15. Prasad T.N.V.K.V. Elumalai E.K.: ‘Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity’, Asian Pac. J. Trop. Biomed., 2011, 1, (6), pp. 439 –442, doi: 10.1016/S2221‐1691(11)60096‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paulkumar K. Gnanajobitha G. Vanaja M. et al.: ‘ Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens’, Sci. World J., 2014, 1, pp. 1 –9, doi: 10.1155/2014/829894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nazeruddin F.M. Prasad N.R. Prasad S. R. et al.: ‘ Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti‐microbial activity’, Ind. Crop. Prod., 2014, 60, pp. 212 –216, doi: 10.1016/j.indcrop.2014.05.040 [Google Scholar]
- 18. Sharma S. Shahzad A.: ‘An overview on decalepis: a genus of woody medicinal’, Climbers J. Plant Sci. Res., 2014, 1, pp. 1 –13 [Google Scholar]
- 19. Srivastava A. Harish S.R. Shivanandappa T.: ‘Antioxidant activity of the roots of Decalepis hamiltonii ’, LWT, 2006, 39, pp. 1059 –1065, doi: 10.1016/j.lwt.2005.07.005 [Google Scholar]
- 20. Prakash P. Thiyagarajan G.: ‘Phytochemical screening and antibacterial activity of root extracts of Decalepis hamiltonii Wight & Arn’, Int. J. Pharm. Res. Rev., 2014, 3, (11), pp. 33 –38 [Google Scholar]
- 21. Muthukrishnan S. Bhakya S. Kumar T. S. et al.: ‘Biosynthesis, characterization and antibacterial effect of plant‐mediated silver nanoparticles using Ceropegia thwaitesii – an endemic species’, Ind. Crop. Prod., 2015, 63, pp. 119 –124, doi: 10.1016/j.indcrop.2014.10.022 [Google Scholar]
- 22. Alhakmani F. Kumar S.F. Khan S. A.: ‘Estimation of total phenolic content, in‐vitro antioxidant and anti‐inflammatory activity of flowers of Moringa oleifera ‘, Asian Pac. J. Trop. Biomed., 2013, 3, pp. 623 –627, doi: 10.1016/S2221‐1691(13)60126‐4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parang Z. Keshavarz A.: ‘University of technology fluorescence emission spectra of silver and silver/cobalt nanoparticles’, Sci. Iran, 2012, 19, pp. 943 –947, doi: 10.1016/j.scient.2012.02.026 [Google Scholar]
- 24. Ahmed S. Ahmad S.M. Swami B. L. et al.: ‘Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract’, J. Radiat. Res. Appl. Sci., 2016, 9, (1), pp. 1 –7, doi: 10.1016/j.jrras.2015.06.006 [Google Scholar]
- 25. Mehmood A. Murtaza G.T. Bhatti T. M. et al.: ‘Mahmood, phyto‐mediated synthesis of silver nanoparticles from Melia azedarach L. leaf extract: characterization and antibacterial activity’, Arab. J. Chem., 2013, doi: 10.1016/j.arabjc.2013.11.046 [Google Scholar]
- 26. Chowdhury I.H. Ghosh S. Roy M. et al.: ‘Green synthesis of water‐dispersible silver nanoparticles at room temperature using green carambola (star fruit) extract’, Sol‐Gel Sci. Technol., 2015, 73, pp. 199 –207, doi: 10.1007/s10971‐014‐3515‐1 [Google Scholar]
- 27. Krishnaraj C. Jagan G.E. Mohan N. et al.: ‘Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens’, Colloids Surf B Biointerfaces, 2010, 76, pp. 50 –56, doi: 10.1016/j.colsurfb.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 28. Raposo M. Ferreira Q. Ribeiro P. A.: ‘A guide for atomic force microscopy analysis of soft‐ condensed matter’, Mod. Res. Educ. Top. Microsc., 2007, 1, pp. 758 –769 [Google Scholar]
- 29. Kalaiyarasu T. Karthi N. Manju V.: ‘ In vitro assessment of antioxidant and antibacterial activity of green synthesized silver nanoparticles from Digitaria radicosa leaves’, Asian J. Pharm. Clin. Res., 2016, 9, (1), pp. 297 –302 [Google Scholar]
- 30. Chung I. Park I. Seung‐Hyun K. et al.: ‘Plant‐mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications’, Nanoscale Res. Lett., 2016, 11, (1), p. 1, doi: 10.1186/s11671‐016‐1257‐4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ajitha B. Reddy Y. A. K. Reddy P. S.: ‘Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract’, Mater. Sci. Eng. C, 2015, 49, pp. 373 –381, doi: 10.1016/j.msec.2015.01.035 [DOI] [PubMed] [Google Scholar]
- 32. Suman T.Y. Radhika Rajasree S.R. Jayaseelan C. et al.: ‘GC‐MS analysis of bioactive components and biosynthesis of silver nanoparticles using Hybanthus enneaspermus at room temperature evaluation of their stability and its larvicidal activity’, Environ. Sci. Pollut. Res., 2016, 23, pp. 2705 –2714, doi: 10.1007/s12668‐016‐0199‐8 [DOI] [PubMed] [Google Scholar]
- 33. Ge L. Li Q. Ouyang J. et al.: ‘Nanosilver particles in medical applications: synthesis, performance, and toxicity’, Int. J. Nanomed., 2014, 9, pp. 2399 –2407, doi: http://doi.org/10.2147/IJN.S55015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poojary M.M. Passamonti P. Adhikari A. V.: ‘Green synthesis of silver and gold nanoparticles using root bark extract of Mammea suriga: characterization, process optimization, and their antibacterial activity’, BioNanoSci, 2016, 1, pp. 1 –11, doi: 10.1007/s12668‐016‐0199‐8 [Google Scholar]


