Abstract
In this study, the synthesis of ultra‐fine grade antimony trioxide (Sb2 O3) using plant extract for the first time is reported. Antimony chloride was used as a starting material and Dioscorea alata tuber extract was used as a reducing and capping agent. The synthesised nanoparticles were characterised by X‐ray diffraction (XRD), field emission scanning electron microscopy (FE‐SEM), dynamic light scattering (DLS), and Fourier transform infrared spectroscopy. XRD analysis indicates the formation of pure Sb2 O3 nanoparticles. The result from FE‐SEM and DLS showed that the particles have a cube‐like morphology and have an average size of 346.4 nm which falls within the range of ultra‐fine grade Sb2 O3.
Inspec keywords: field emission electron microscopy, scanning electron microscopy, X‐ray diffraction, particle size, nanofabrication, light scattering, transmission electron microscopy, ultraviolet spectra, nanoparticles, antimony compounds, Fourier transform infrared spectra
Other keywords: field emission scanning electron microscopy, FE‐SEM, dynamic light scattering, DLS, XRD analysis, antimony chloride, starting material, reducing agent, ultrafine grade antimony trioxide, plant extract, dioscorea alata tuber extract, capping agent, X‐ray diffraction, pure antimony trioxide nanoparticles, cube‐like morphology, Sb2 O3
1 Introduction
Antimony trioxide (Sb2 O3) is the most important commercial compound of antimony [1]. Its global production was 130,000 tonnes in 2012 which is increasing every year. The safety of the Sb2 O3 was confirmed by World Health Organization (WHO) (2003) [2] and the European Food safety authority (2004) [3]. It has wide applications in industries and used as an excellent catalyst to accelerate the polymerisation in polyester manufacturing. In ceramic industries, it is used as an improver while glass manufacturer uses it as a degassing agent. It is also used as an opacifier in porcelain and enamelling products. In titanium dioxide production, it is incorporated as a flocculent [4]. In lubricants, it is added to increase stability. In fluorescent light bulbs, it is used as a phosphorescent agent [5]. Sodium stibogluconate has been used as medicine since 1940 which is synthesised using Sb2 O3. It is on WHO list of essential medicine [6], the most effective and safe medicine used for the treatment of leishmaniasis [7, 8].
Since ancient times, Sb2 O3 is used as a white pigment. The pigmentation of antimony in plastics can be controlled and adjusted by selecting a proper grade of Sb2 O3 having a specific particle size. The product with smaller particle size will impart the whitest colour and highest opacity to plastic. Translucent plastic can be prepared using large particles. Particle size during manufacturing is controlled by adjusting temperature and rate at which antimony vapour are precipitated as these vapours exist furnace. The grades of commercially available Sb2 O3 are ultra‐fine (particle size 250–450 nm), high tint (800–1800 nm), and low tint (1900–3200 nm) [9].
Sb2 O3 is used as a flame retardant synergist with halogenated hydrocarbon compounds in plastic industries to provide maximum flame retardancy in synthetic plastic and resin such as high impact polystyrene, polypropylene, polyethylene, and synthetic textile fibres such as modacrylic. Addition of any type of additive to plastic usually alters the physical properties of the original plastic. Hence, it is always desired to improve flame retardancy while minimising the effect on physical properties of the plastic substrate. It is been proposed that when Sb2 O3 is finely divided and distributed in plastic, the physical properties are less affected. Blizzard and Martin have demonstrated a method and apparatus for making Sb2 O3 powder having a specific sub‐micron size. Sb2 O3 powder was prepared using a plasma arc process using vaporisation and condensation of crude Sb2 O3. This complex system requires sophisticated instrumentation and very high operating temperature. Particles of 320 nm were obtained by this process [10].
Conventionally, Sb2 O3 is produced by high‐temperature smelting using antimony trisulphide as a starting material. The main drawback of this process is the release of sulfur dioxide which leads to acid production which has a detrimental effect on the environment. In a typical conventional technology for manufacturing of Sb2 O3, the capital cost of scrubbing SO2 emission will be double than the capital cost of production of Sb2 O3. Other methods for synthesis of Sb2 O3 nanoparticles are hydrothermal [11], gamma radiation‐oxidation [12], micro‐emulsion [13], solution phase reduction [14], thermal oxidation [15], vacuum evaporation [16], ultra‐sound [17], and biosynthesis [18]. In spite of the success of all these methods, there are several drawbacks such as the requirement of high temperature and pressure, sophisticated instrumentation, extensive use of solvents, expensive raw materials, tedious procedures, long incubation times etc. All these factors add cost to the final product.
Dean Thibault et al have also demonstrated a two‐step method for synthesis of Sb2 O3. The first step comprises production of antimony trichloride by reacting antimony trisulphide and iron (III) chloride. The second step comprises hydrolysis of antimony trichloride for production of Sb2 O3 [19].
Green synthesis of nanoparticles is an eco‐friendly, cost‐effective approach in which plant extract can be effectively used as reducing and stabilising agents for the synthesis of metal and metal oxide nanoparticles. This way we can adapt the benign synthesis approach that uses non‐toxic reactions and maintains mild reaction conditions [20]. Plants are capable of generating a wide variety of nanostructures matching the elegance of current engineered materials. Water‐soluble plant metabolites (such as alkaloids, phenolics, flavonoids, terpenoids, and catechins) are responsible for reduction [21]. Green route eliminates or minimise the use of the harmful polluting substance in the synthesis of nanomaterials.
Dioscorea is a genus of flowering plants with over 600 species in the family Dioscoreaceae. Dioscorea alata is one such edible species which has the highest yield among all Dioscorea species and can be stored for a relatively longer period [22]. It is a rich source of phytochemical such as flavonoids, phenolics, alkaloids, saponin etc. and is also rich in vitamin and mineral contents [23]. As Dioscorea species are rich in polyphenolic content and thus can be used for bio‐reduction of metal to nanoparticles [24]. We have successfully synthesised bismuth trioxide nanoparticles using D. alata tuber extract [25].
Particle size is an important criterion for industrial application of Sb2 O3. In this paper, we have proposed a rapid, cost‐effective, green‐route for the synthesis of ultra‐fine Sb2 O3 nanoparticles.
2 Materials and method
2.1 Chemicals
Antimony trichloride (SbCl3) and ethanol were procured from S.D. Fine‐Chem India. All reagents used in the experiment were of analytical grade and used without further purification.
2.2 Collection of plant material
D. alata tubers were collected from the field and identified in Botany Department, Shivaji University, Kolhapur, India.
2.3 Preparation of plant extract
Initially, D. alata tubers were thoroughly washed with water, then peeled off and diced into small pieces, and dried in a hot air oven. Dried tubers were pulverised and the powder was stored at room temperature. For the preparation of plant extract, the dried powder (5 g) was weighed and transferred to 500 mL of Erlenmeyer flask containing 100 mL of deionised water, mixed using magnetic stirrer for 10 min and then transferred to preheated water bath at 80°C and incubated for 15 min. The extract was stained using a sieve, and then centrifuged at 8000 rpm for 15 min and the supernatant was filtered through Whatman no. 1 filter paper. The clear filtrate was collected and used for synthesis.
2.4 Synthesis of Sb2 O3 nanocubes
For the synthesis of Sb2 O3, 0.456 g (0.1M) of antimony trichloride was dissolved in 20 mL of 70% ethanol. Then 50 mL of tuber extract was added dropwise under constant stirring. After addition of tuber extract, the mixture turns turbid. After mixing for 20 min, culture bottles were autoclaved at 121°C at 15 lbs for 21 min. The obtained solution was cooled at room temperature and the precipitate was isolated by centrifugation. The obtained pellet was washed using ethanol and water thrice. Then dried in a vacuum desiccator for 6 h. The resultant white colour powder was used as it is for X‐ray diffraction (XRD) and scanning electron microscopy (SEM) analysis. KBr pellet method was used for Fourier transform infrared (FTIR) analysis.
2.5 Characterisation of Sb2 O3 nanocubes
XRD was measured on Bruker AXS D2 phaser diffractometer using Cu kα radiation (k = 1.5406 Å). Field emission scanning electron microscopy (FE‐SEM) by Mira‐3 Tescan and dynamic light scattering (DLS) by Malvern instrument Zetasizer Nano ZS‐90 was used to investigate the morphology and particle size distribution of synthesised nanoparticles. FTIR spectroscopy was done using the Shimadzu FTIR spectrophotometer.
3 Result and discussion
White colour is a characteristic property of Sb2 O3 nanoparticles which was obtained after completion of the reaction. The obtained sample was marginally soluble in water and ethanol, this is also a characteristic property of Sb2 O3 nanoparticles [26].
3.1 XRD analysis
To evaluate the crystalline size and phase of Sb2 O3, the XRD pattern (Fig. 1) was recorded. The observed pattern was identified with the α phase of antimony oxide using JCPDS data card (card no. 00‐003‐0530) with reveals high purity of Sb2 O3. From intense peak (1 2 1), the grain size was estimated using the Scherrer formula [27]. The average grain size was found to be 305.81 nm.
Fig. 1.
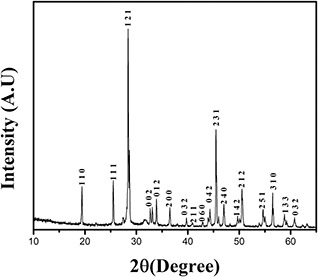
XRD pattern of synthesised Sb2 O3 nanocubes
3.2 Field emission scanning electron microscope
FE‐SEM reveals the formation of morphology and particle size. Cube‐like structures are shown in Fig. 2. The FE‐SEM was analysed on ImageJ 1.50i software. These measurement states that the nanocubes have a wide distribution range from 200 to 800 nm. The same trend is seen in the DLS histogram. The morphology of synthesised material matches with morphology reported in the literature [28, 29, 30, 31, 32].
Fig. 2.
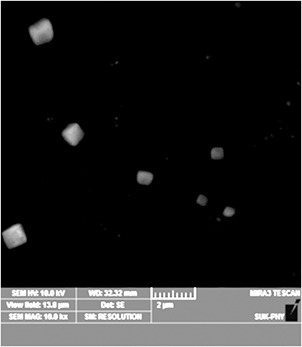
FE‐SEM of Sb2 O3 nanocubes
3.3 DLS
The histogram in Fig. 3. shows the distribution of particle size which indicates that the particles have a wide size distribution from 10 to 1000 nm. The mean diameter of nanoparticles obtained from the Gaussian fit of the histogram is 346.4 nm. The size of commercial ultra‐fine Sb2 O3 powder ranges from 250 to 450 nm. The mean size obtained from DLS falls within the range of ultra‐fine grade of Sb2 O3.
Fig. 3.
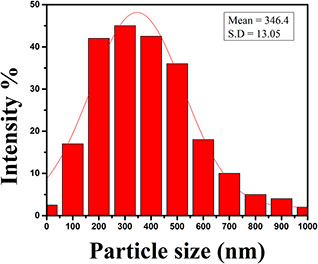
DLS histogram of the size distribution of Sb2 O3 nanocubes
3.4 FTIR analysis
FTIR spectrum in Fig. 4. reveals the chemical groups and characteristic bands of Sb2 O3 nanoparticles. The peaks obtained at 750 and 912 cm−1 correspond to symmetric stretching (V s) and overtone (V s + V as) vibrations. The sharp peak at 675 and 862 cm−1 and peak at 630 cm−1 correspond to symmetric and asymmetric bending, respectively [33, 34, 35].
Fig. 4.
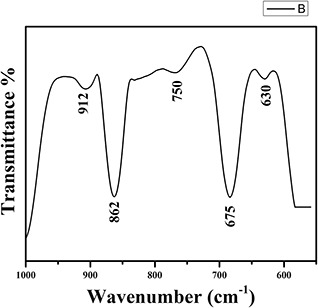
FTIR spectra of Sb2 O3 nanocubes
Dean Thibault et al in their patent WO1998011021A1 demonstrated a two‐step process to eliminate impurities present in the starting material SbCl3 [19]. The same starting material was used in our process. The XRD pattern as well as the FTIR spectra did not show any impurity. This confirms that pure Sb2 O3 nanoparticles are synthesised by a green route.
Flame retardancy decreases when particle size is <250 nm [36]. The nanoparticles synthesised by gamma radiation‐oxidation [12], solution phase reduction [14], thermal oxidation [15], vacuum evaporation [16], ultra‐sound [17], and biosynthesis [18] have size distribution from 2 to 100 nm.
Blizzard and Martin in their patent US4347060A [10] have synthesised Sb2 O3 powder of size 320 nm. This process requires very high operating temperature, which increases the cost of the final product. In this paper, we have demonstrated eco‐friendly prices operating at ambient temperature and pressure. The final product was obtained within 6 hours. Due to these parameters, the cost of the final product will decrease, which will benefit the industry.
4 Conclusion
For the first time, ultra‐fine Sb2 O3 nanoparticles were successfully synthesised using plant extract. D. alata tuber extract is a promising reducing and capping agent in the synthesis of metal oxide nanoparticles. This green approach overcomes the drawbacks of conventional methods such as incubation time, high temperatures and pressure conditions, complex and expensive equipment, and use of toxic precursors.
5 Acknowledgment
The authors would like to thank Dr. D.K. Gaikwad, Head of Botany Department Shivaji University, Mr. M.V. Patgaonkar, Head of Biotechnology Department, KIT College of Engineering, Kolhapur, and Dr. P.S. Patil and Mr. P.P. Waifalkar, Shivaji University, Kolhapur for their immense support and useful inputs.
6 References
- 1. National Center for Biotechnology Information. : Pubchem compound database, CID = 27652, Available at: https://pubchem.ncbi.nlm.nih.gov/compound/27652, (accessed on Aug 2, 2018)
- 2.Antimony in Drinking water [World Health Organisation], 2003, Available at: http://www.who.int/water_sanitation_health/dwq/chemicals/antimony,pdf, (accessed on Aug 2, 2018)
- 3. European Food Safety Authority. : ‘Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to a 2nd list of substances for foods contact materials’, EFSA J., 2004, 2, (2), pp. 1 –13 [Google Scholar]
- 4. Antimony Trioxide Chemical [Chemistry Learner] , Available at: http://www.chemistrylearner.com/antimony‐trioxide.html, (accessed on March 9, 2019)
- 5.Uses and Formulation of Antimony [United States Antimony Corporation], Available at: http://usantimony.com/uses_formulations.htm, (accessed on March 9, 2019)
- 6.Model List of Essential Medicines [World Health Organisation], 19th edition, 2015, Available at: http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf, (accessed on Aug 2, 2018)
- 7. Mookerjee‐Basu J. Mookerjee A. Sen P., et.al.: ‘Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3‐kinase and mitogen‐activated protein kinase activation in Leishmania donovani‐infected macrophages’, Antimicrob. Agents Chemother., 2016, 50, (5), pp. 1788 –1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirk R. Sati M.H.: ‘Observations on the use of sodium antimony gluconate (sodium stibogluconate) in the treatment of kala‐azar’, Ann. Tropical Med. Parasitology, 1947, 41, (1), pp. 14 –21 [DOI] [PubMed] [Google Scholar]
- 9.Antimony Compounds [Flame Retardants], Available at: http://fr.polymerinsights.com/fr‐types/synergists/antimony, (accessed on March 9, 2019)
- 10. Blizzard R.L. Martin T.O.: ‘Making of antimony powder’, US4347060A
- 11. Chen X.Y. Huh H.S. Lee S.W. et al.: ‘Hydrothermal synthesis of antimony oxychloride and oxide nanocrystals: Sb4O5Cl2, Sb8O11Cl2, and Sb2 O3 ’, J. Solid State Chem., 2008, 181, pp. 2127 –2132 [Google Scholar]
- 12. Liu Y. Huh H.S. Lee S.W. et al.: ‘Preparation of nanocrystalline antimony oxide powders by use of γ‐ray radiation — oxidization route’, Mater. Sci. Eng., 1997, B49, pp. 42 –45 [Google Scholar]
- 13. Zhang Z. Guo L. Wang W.: ‘Synthesis and characterization of antimony oxide nanoparticles’, J. Mater. Res., 2001, 16, pp. 803 –805 [Google Scholar]
- 14. Chin H.S. Cheong K.Y.: ‘Review on oxides of antimony nanoparticles: synthesis, properties, and applications’, J. Mater. Sci., 2010, 45, pp. 5993 –6008 [Google Scholar]
- 15. Xu C.H. Shi S.Q. Surya C. et al.: ‘Synthesis of antimony oxide nano‐particles by vapor transport and condensation’, J. Mater. Sci., 2007, 42, pp. 9855 –9858 [Google Scholar]
- 16. Keqiang Q. Rongliang Z.: ‘Research on preparation of nanometer antimony trioxide from slag containing antimony by vacuum evaporation method’, Vacuum, 2006, 80, pp. 1016 –1020 [Google Scholar]
- 17. Han C. Chen D. Li X. et al.: ‘Synthesis and catalytic performance of antimony trioxide nanoparticles by ultrasonic‐assisted solid‐liquid reaction ball milling’, Adv. Powder Technol., 2017, 28, pp. 1136 –1140 [Google Scholar]
- 18. Jha A.K. Prasad K. Prasad K.: ‘Biosynthesis Sb2 O3 nanoparticles: a low‐cost green approach’, Biotechnol. J., 2009, 4, pp. 1582 –1585 [DOI] [PubMed] [Google Scholar]
- 19. Dean Thibault J. Macdonald M.D. Stevens D.A.: ‘Process for producing antimony trioxide’, WO1998011021A1
- 20. Rai M. Posten C.: ‘Green biosynthesis of nanoparticles’ (Center for Agriculture and Biosciences International, India, 2013) [Google Scholar]
- 21. Mohammadinejad R. Karimi S. Iravani S. et al.: ‘Plant‐derived nanostructures: types and applications’, Green Chem., 2016, 18, pp. 20 –52 [Google Scholar]
- 22. Faustina Dufie W.M. Oduro I. Ellis W.O. et al.: ‘Potential health benefits of water yam (Dioscorea alata)’, Food Funct., 2013, 4, pp. 1496 –1501 [DOI] [PubMed] [Google Scholar]
- 23. Okwu D.E. Ndu C.U.: ‘Evaluation of the phytonutrients, mineral and vitamin contents of some varieties of yam (Dioscorea sp.)’, Int. J. Mol. Med. Adv. Sci., 2006, 2, (2), pp. 199 –203 [Google Scholar]
- 24. Mittal A.K. Chisti V.K. Banerjee U.C.: ‘Synthesis of metallic nanoparticles using plant extracts’, J. Biotech Adv., 2013, 31, (2), pp. 346 –356 [DOI] [PubMed] [Google Scholar]
- 25. Indurkar A. Sangoi V. Patil P. et al.: ‘Rapid synthesis of Bi2 O3 nano‐needles via ‘green route’ and evaluation of its anti‐fungal activity’, IET Nanobiotechnol., 2018, 12, (4), pp. 496 –499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antimony Trioxide Chemical [TODINI chemicals], Available at: https://www.todini.com/chemicals/antimony/antimony‐trioxide, (accessed on March 9, 2019)
- 27. Cullity B.D.: ‘Elements of X‐ray diffraction’ (Addison‐Wesley Publishing, Phillippines, 1978, 2nd Edn.) [Google Scholar]
- 28. Liu L. Hu Z. Cui Y. et al.: ‘A facile route to the fabrication of morphology‐controlled Sb2 O3 nanostructures’, Solid State Sci., 2010, 12, (5), pp. 882 –886 [Google Scholar]
- 29. Wang D. Zhou Y. Song C. et al.: ‘Phase and morphology controllable synthesis of Sb2 O3 microcrystals’, J. Crystal Growth, 2009, 311, (15), pp. 3948 –3953 [Google Scholar]
- 30.Nano research element: Antimony Oxide Micro‐Powder (Sb2O3), 2018, Available at: http://www.nanorh.com/product/antimony‐oxide‐sb2o3‐micropowder‐sb2o3/, (accessed on Aug 2, 2018)
- 31. Wang S.S. Xing Z.H. Chen G.Y. et al.: ‘Cuboctahedral Sb2 O3 mesocrystals organized from octahedral building blocks: more than self‐similarity’, Cryst. Growth Des., 2016, 16, (7), pp. 3613 –3617 [Google Scholar]
- 32. Bozorth R.: ‘The crystal structures of the cubic forms of arsenious and antimonous oxides’, J. Am. Chem. Soc., 1923, 45, (7), pp. 1621 –1627 [Google Scholar]
- 33. Deng Z. Chen D. Tang F. et al.: ‘Synthesis and purple‐blue emission of antimony trioxide single‐crystalline nanobelts with elliptical cross section’, Nano Res., 2009, 2, (2), pp. 151 –160 [Google Scholar]
- 34. Kaviyarasu K. Sajan D. Devrajan P.A.: ‘A rapid and versatile method for solvothermal synthesis of Sb2 O3 nanocrystals under mild conditions’, Appl. Nanosci., 2013, 3, (6), pp. 529 –533 [Google Scholar]
- 35. Cody C.A. DiCarlo L. Darlingtom R.K.: ‘Vibrational and thermal study of antimony oxides’, Inorg. Chem., 1979, 18, (6), pp. 1572 –1576 [Google Scholar]
- 36. Jian‐Lin X.U. Sheng‐Gang Z. Lei N. et al.: ‘ effect of modified by various surface active agents on flame retardant properties of PVC composite’, J. Mater. Sci., 2016, 44, (8), pp. 64 –69 [Google Scholar]


