Abstract
The present investigation was done to explore the potential of Lantana camara (L. camara) flower in the fabrication of gold nanoparticles (AuNPs). The shape and size of AuNPs have been successfully controlled by introducing small amounts of L. camara flower extract. It produced spherical nanogold of average size 10.6 ± 2.9 nm without any aggregation and showed significant photocatalytic degradation activity of the methylene blue (>62%, 10 mg/L) in the presence of solar light. In addition, the experimental approach is inexpensive, rapid and eco‐friendly for industrial scale production of nanoparticles.
Inspec keywords: nanoparticles, gold, nanofabrication, botany, catalysis, photochemistry, organic compounds
Other keywords: biofabrication, Lantana camara flower extract, gold nanoparticles fabrication, spherical nanogold, photocatalytic degradation activity, methylene blue, solar light, size 10.6 nm to 2.9 nm, Au
1 Introduction
During the last few decades, the research interest in gold nanoparticles (AuNPs) is largely due to ease of their synthesis, oxidation resistance, stability and their good biocompatibility. In general, as the size of materials is reduced to nanoscale, the resulting properties are unique when compared with their bulk equivalents. AuNPs have novel applications in environmental sensing [1], drug and gene delivery [2], microbiology and medicine [3], biosensors [4], photothermal therapy [5], tissue/tumour imaging [6], catalysis [7], electrochemical immunoassay [8] and antimicrobial applications [9]. These applications derive from the unusual chemical, optical, thermal and physical properties of AuNPs. Several complicated and expensive methods have been employed for their preparation, which involve either toxic chemicals or high temperature and pressure, and also generate enormous toxic wastes [10]. In addition, chemical methods are not compatible in medical applications due to the attachment of toxic chemical moieties on the surface of nanoparticles.
As nanotechnologists and environment‐conscious researchers, our effort is towards the development of an environmental friendly and cost‐effective method for the fabrication of bulk amount of nanoparticles of desired size and shape. Recently, several novel biofabrication methods have been reported for the production of a large amount of nanoparticles by using mixed‐valence polyoxometallates [11], solar light irradiation [12], polysaccharides [13], tollens [14], biological methods [15, 16] and so on. In biological methods, exposure of the biomaterials – both intracellular and extracellular – to metal ions over a lapse of time produces metal nanoparticles of different morphology [17, 18]. For AuNPs, a few reports concerned shape‐controlled synthesis with sizes of about 50 nm or less – including sodium citrate and D2 O [19], alkanethiolate [20], amine‐terminated ionic liquid [21], glucose [22], Ralstonia eutropha bacteria [23], plant extracts [24], Morinda citrifolia roots [25], Punica granatum fruit [26], Mirabilis jalapa flowers [27], geranium leaves and its endophytic fungus [28].
Lantana camara L. (L. camara) is an ornamental weed growing generally in tropical, sub‐tropical and temperate regions. Its major phytoconstituents are terpenes (mono, sesqui, tri), iridoid glycosides, flavanoids and so on. L. camara has various uses in folk medicine owing to its various pharmacological effects that include asthma, vermifuge, chicken pox, measles, rheumatism, leprosy, scabies as well as fever, influenza, stomach ache and high blood pressure [29]. However, there is no scientific report on the fabrication of AuNPs by using the L. camara flower. Therefore, a rapid and eco‐friendly biofabrication method was developed to synthesise stable AuNPs by using an extract of L. camara flower. The produced AuNPs are small and showed photocatalytic degradation activity of methylene blue (MB) in the presence of solar light. The synthesised AuNPs were visually characterised by using UV–vis absorption spectroscopy, dynamic light scattering (DLS), transmission electron microscopy (TEM) and X‐ray diffraction analysis (XRD). MB is a thiazine‐based cationic dye, commonly used as dyeing material for cotton, silk, paper and wood. Its acute exposure can cause some harmful effects to humans (vomiting, shock, cyanosis, jaundice etc.) and the aquatic environment [12]. Thus, remediation of MB from wastewater is very important.
2 Experimental
2.1 Biofabrication of AuNPs
Gold (III) chloride hydrate (HAuCl4 · x H2 0, 99.99%) was purchased from Sigma‐Aldrich, USA and MB (99.8%) was purchased from Spectrum, USA. Colourful L. camara flowers were collected from the local garden of Playa Chica 1, near Universidad de las Fuerzas Armadas ESPE, Sangolqui, Ecuador. The collected L. camara flower (2 g) was washed thoroughly with Milli‐Q water and heated (55–60°C) in 20 mL of ethanol (95%) for 10 min. After cooling, the yellow extract was filtered using Whatman No.1 paper. For the extracellular biofabrication of AuNPs, 1 ml of extract was mixed with 9 ml of 0.5 mM AuCl4 − solution at room temperature (22–25°C). Reduction of Au3+ to Au occurs rapidly as indicated by the appearance of a purple‐pink colour after 15 min; the formation of the AuNPs was studied at different time intervals. To determine the concentration of AuNPs, the resulting AuNPs colloid was separated out by centrifugation at 5000 rpm for 10 × 3 min, washed with deionised water/acetone (9/1) mixture (three times) and then freeze dried overnight. Finally, the dried AuNPs (1.35 mg) were resuspended in 10 ml deionised water for further characterisation.
2.2 Photocatalytic activity of AuNPs
Photocatalytic activity of AuNPs was evaluated by degradation of MB in the presence of direct solar light source (900–1000 cd/m2), a method adapted from Kumar et al. [12]. MB 2, 500 μl of AuNPs (135 μg/ml) and 500 μl of H2 O were added to 5 ml of 10 mg/l MB solution. A control setup was also maintained without AuNPs (MB 1). The reaction mixture was put under the direct solar light and the absorbance spectrum of the reaction mixture was subsequently measured using UV–vis spectrophotometer (λ max = 664 nm) at different time intervals. The average degradation (%) of MB in solution was calculated using the following equation
| (1) |
where η is the rate of degradation of MB in terms of percentage, A o is the initial absorbance of the dye solution and At is the absorbance of the MB at time t [11].
2.3 Instrumental characterisation of AuNPs
The L. camara flower mediated AuNPs were confirmed by UV–vis spectrophotometer (Thermo Spectronic, GENESYS™ 8, England). The particle size distributions and polydispersity of nanoparticles were determined by using DLS instrument (HORIBA LB‐550). Size, shape and selective area electron diffraction (SAED) pattern of nanoparticles were studied on TEM (FEI, TECNAI, G2 spirit twin, Holland). XRD studies on thin films of the nanoparticle were carried out using a diffractometer (EMPYREAN, PANalytical) and a θ–2θ configuration (generator–detector), wherein a copper X‐ray tube emitted a wavelength of λ = 1.54 A°.
3 Results and discussion
Fig. 1 (inset) presents the addition of L. camara flower extract to 0.5 mM AuCl4 − solution; this led to the appearance of a purple‐pink and then red colour in the solution over the reaction time. This colour evaluation of gold solution indicates the formation of small AuNPs [19]. The UV–vis absorption spectra for green synthesised AuNPs showed an absorption maximum, λ max, at 530 nm (Fig. 1) corresponding to the transverse surface plasmon vibration [19, 24]. Initially, λ max appeared at 540 nm, but it shifted towards 530 nm with the progress of reaction time. This clearly indicates the blue shift and formation of small spherical AuNPs. The additional peak observed at 340–350 nm can be assigned to phytochemicals associated gold clusters that by way of intermediates build up metallic AuNPs. To monitor the long‐term stability of the gold colloid (AuNPs), we have measured the absorption spectra of the colloid after 3 months. It was observed that there is no more change in their λ max = 530 nm in UV–vis spectrum; this remained so for 3 months and showed stability without any precipitation.
Fig. 1.
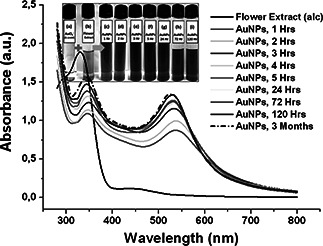
Visible and UV–vis absorbance spectra of AuNPs at different time points
The DLS measures the hydrodynamic particle size distribution of nanoparticles and the histogram of AuNPs were obtained after 72 h and 3 months of reaction time (Fig. 2). The average particle size of AuNPs for 72 h and 3 months are 10.6 ± 2.9 and 9.5 ± 1.9 nm, respectively. The polydispersity index (PDI) is a measure of the width of the particle size distribution and PDI <0.1 typically refers to ‘monodisperse’ [30]. The formula for polydispersity is: PDI = (SD/mean)2. The PDI for AuNPs at 72 h and 3 months are 0.07 and 0.04, respectively, and this clearly shows that synthesised AuNPs are monodispersed and stable for 3 months, without any aggregation.
Fig. 2.
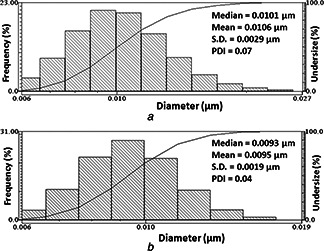
DLS pattern of AuNPs for
a 72 h
b 3 months
TEM characterisation was performed to further investigate the effect of L. camara flower extract on the morphologies of AuNPs. Figs. 3 a –c show that AuNPs were monodispersed, spherical in shape and around 4–12 nm in size. Interestingly, the particles are well dispersed; this may be due to phytochemicals of L. camara flower acting as a strong stabilising agent for the AuNPs [31]. The bright circular spot in the SAED pattern (Fig. 3 d) revealed that the biosynthesised AuNPs are spherical and polycrystalline in nature [23]. The size obtained from the TEM was confirmed with the DLS results. It was also observed that the AuNPs solution was stable for more than 3 months, with a little sign of aggregation and did not show any significant changes in λ max.
Fig. 3.

Images of AuNPs by
a –c TEM
d SAED
Fig. 4 shows the XRD image of the green synthesised AuNPs and this was confirmed by the observed characteristic peaks. Bragg reflection peaks that were observed at the 2θ angles of 38.27 (111), 44.54° (200), 64.89° (220) and 78.03° (311) are in the agreement with the ICSD no. 98‐005‐3763 of gold and may be indexed on the basis of the face‐centred cubic structure of gold. The corresponding four Bragg reflection peaks of AuNPs were in agreement with the results reported by Srivastava and Mukhopadhyay [23], and Rajeshkumar et al. [32].
Fig. 4.

XRD spectrum of AuNPs
Thus, the XRD pattern confirms that the AuNPs were essentially crystalline in nature. The broad reflection hump at 20°–30° is due to the low crystallinity of the organic phytochemicals and the other unidentified peaks at 28.29° and 31.60° may be due to the association of organic material of flower extracts with the synthesised AuNPs.
Besides the optical properties, AuNPs demonstrate excellent catalytic activity. The role of metal nanoparticle as an electron transfer catalyst is expected to vary with size. Fig. 5 shows the main MB absorption peak (λ max) at 664 nm that decreased gradually with the exposure of the reaction mixture (AuNPs 135 μg/ml) to solar light using glass tubes (glass is opaque to UV light), indicating the photocatalytic degradation, 62.29%, 10 mg/l dye of MB in 120 min. The decrease of absorbance with lapse of time is indicative of the degradation of MB and also highlights the ability of unreacted phytochemicals of AuNPs to degrade MB through electron relay [12]. This confirms that AuNPs act as a redox catalyst by transferring electron from phytochemicals to MB by way of electron relay.
Fig. 5.

Photocatalytic patterns of AuNPs for remediation of MB
4 Conclusion
The L. camara flowers are highly compatible for the biofabrication of spherical nanogold of average size 10.6 ± 2.9 nm. This eliminates the use of toxic reducing and stabilising agents as it can be used as an alternative agent for remediation of MB from waste water. AuNPs did not show any significant changes in λ max for more than 3 months. Thus, the surface modified AuNPs can be safely used for various engineering and biotechnological applications.
5 Acknowledgments
This scientific work was funded by the Prometeo Project of the National Secretariat of Higher Education, Science, Technology and Innovation (SENESCYT), Ecuador. The authors thank Dr. Alexis Debut, ESPE for providing technical assistance for the TEM and XRD measurements.
6 References
- 1. Saha K. Agasti S.S. Kim C. et al.: ‘Gold nanoparticles in chemical and biological sensing’, Chem. Rev., 2012, 112, pp. 2739 –2779 (doi: 10.1021/cr2001178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghosh P. Han G. De M. et al.: ‘Gold nanoparticles in delivery applications’, Adv. Drug Deliv. Rev., 2008, 60, pp. 1307 –1315 (doi: 10.1016/j.addr.2008.03.016) [DOI] [PubMed] [Google Scholar]
- 3. Pissuwan D. Cortie C.H. Valenuela S.M. et al.: ‘Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends’, Biotechnol., 2010, 28, pp. 207 –213 [DOI] [PubMed] [Google Scholar]
- 4. Storhoff J.J. Marla S.S. Bao P. et al.: ‘Gold nanoparticle‐based detection of genomic DNA targets on microarrays using a novel optical detection system’, Biosens. Bioelectron., 2004, 19, pp. 875 –883 (doi: 10.1016/j.bios.2003.08.014) [DOI] [PubMed] [Google Scholar]
- 5. Sperling R.A. Rivera Gil P. Zhang F. et al.: ‘Biological applications of gold nanoparticles’, Chem. Soc. Rev., 2008, 37, pp. 1896 –1908 (doi: 10.1039/b712170a) [DOI] [PubMed] [Google Scholar]
- 6. Narayanan K.B. Sakthivel N.: ‘Synthesis and characterization of nano‐gold composite using Cylindrocladium floridanum and its heterogeneous catalysis in the degradation of 4‐nitrophenol’, J. Hazard. Mater., 2011, 189, pp. 519 –525 (doi: 10.1016/j.jhazmat.2011.02.069) [DOI] [PubMed] [Google Scholar]
- 7. Boote E. Fent G. Kattumuri V. et al.: ‘Gold nanoparticle contrast in a phantom and juvenile swine: models for molecular imaging of human organs using X‐ray computed tomography’, Acad. Radiol., 2010, 17, pp. 410 –417 (doi: 10.1016/j.acra.2010.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang H. Chen J. Nie L. et al.: ‘A label‐free electrochemical immunoassay for carcinoembryonic antigen (CEA) based on gold nanoparticles (AuNPs) and nonconductive polymer film’, Biosens. Bioelectron., 2007, 22, pp. 1061 –1067 (doi: 10.1016/j.bios.2006.04.027) [DOI] [PubMed] [Google Scholar]
- 9. Wani I.A. Ahmad T.: ‘Size and shape dependant antifungal activity of gold nanoparticles: a case study of Candida ’, Colloids Surf. B, Biointerfaces, 2013, 101, pp. 162 –170 (doi: 10.1016/j.colsurfb.2012.06.005) [DOI] [PubMed] [Google Scholar]
- 10. Boisselier E. Astruc D.: ‘Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity’, Chem. Soc. Rev., 2009, 38, pp. 1759 –1782 (doi: 10.1039/b806051g) [DOI] [PubMed] [Google Scholar]
- 11. Zhang G. Keita B. Biboum R.N. et al.: ‘Synthesis of various crystalline gold nanostructures in water: the polyoxometalate β‐[H4 PMo12 O40]3− as the reducing and stabilizing agent’, J. Mater. Chem., 2009, 19, pp. 8639 –8644 (doi: 10.1039/b903599k) [DOI] [Google Scholar]
- 12. Kumar B. Smita K. Cumbal L. et al.: ‘Sacha inchi (Plukenetia volubilis L.) oil for one pot synthesis of silver nanocatalyst: an ecofriendly approach’, Ind. Crops Prod., 2014, 58, pp. 238 –243 (doi: 10.1016/j.indcrop.2014.04.021) [DOI] [Google Scholar]
- 13. Kumar B. Smita K. Cumbal L. et al.: ‘Sonochemical synthesis of silver nanoparticles using starch: A comparison’, Bioinorganic Chem. Appl., 2014, 2014, p. 8, Article ID 784268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panaceka A. Kolarb M. Vecerovab R. et al.: ‘Antifungal activity of silver nanoparticles against Candida spp.’, Biomaterials, 2009, 30, pp. 6333 –6340 (doi: 10.1016/j.biomaterials.2009.07.065) [DOI] [PubMed] [Google Scholar]
- 15. Kumar B. Smita K. Cumbal L. et al.: ‘Synthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extracts’, Saudi J. Bio.l Sci., 2014, 21, pp. 605 –609 (doi: 10.1016/j.sjbs.2014.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vankar P.S. Shukla D.: ‘Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric’, Appl. Nanosci., 2012, 2, pp. 163 –168 (doi: 10.1007/s13204-011-0051-y) [DOI] [Google Scholar]
- 17. Kaushik N.T. Snehit S.M. Rasesh Y.P.: ‘Biological synthesis of metallic nanoparticles’, Nanomed. Nanotechnol. Biol. Med., 2010, 6, pp. 257 –262 (doi: 10.1016/j.nano.2009.07.002) [DOI] [PubMed] [Google Scholar]
- 18. Shukla D. Vankar P.S.: ‘Synthesis of plant parts mediated gold nano particles’, Int. J. Green Nanotechnol., 2012, 4, pp. 277 –288 (doi: 10.1080/19430892.2012.706175) [DOI] [Google Scholar]
- 19. Ojea‐Jimenez I. Romero F.M. Bastus N.G. et al.: ‘Small gold nanoparticles synthesized with sodium citrate and heavy water: insights into the reaction mechanism’, J. Phys. Chem. C, 2010, 114, pp. 1800 –1804 (doi: 10.1021/jp9091305) [DOI] [Google Scholar]
- 20. Petroski J. Chou M. Creutz C.J.: ‘The coordination chemistry of gold surfaces: formation and far‐infrared spectra of alkanethiolate‐capped gold nanoparticles’, Organometallic Chem., 2009, 694, pp. 1138 –1143 (doi: 10.1016/j.jorganchem.2008.11.057) [DOI] [Google Scholar]
- 21. Wang Z. Zhang Q. Kuehner D. et al.: ‘Green synthesis of 1–2 nm gold nanoparticles stabilized by amine‐terminated ionic liquid and their electrocatalytic activity in oxygen reduction’, Green Chem., 2008, 10, pp. 907 –909 (doi: 10.1039/b806453a) [DOI] [Google Scholar]
- 22. Raveendran P. Fu J. Wallen S.L.: ‘A simple and ‘green’ method for the synthesis of Au, Ag, and Au‐Ag alloy nanoparticles’, Green Chem., 2006, 8, (1), pp. 34 –38 (doi: 10.1039/B512540E) [DOI] [Google Scholar]
- 23. Srivastava N. Mukhopadhyay M.: ‘Ralstonia eutropha (Cupriavidus metallidurans) mediated biosynthesis of gold nanoparticles and catalytic treatment of 2, 4 dichlorophenol’, Synth. React. Inorg., Metal‐Organic, Nano‐Metal Chem., 2015, 45, pp. 238 –247 (doi: 10.1080/15533174.2013.831879) [DOI] [Google Scholar]
- 24. Elia P. Zach R. Hazan S. et al.: ‘Green synthesis of gold nanoparticles using plant extracts as reducing agents’, Int. J. Nanomed., 2014, 9, pp. 4007 –4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suman T.Y. Radhika Rajasree S.R. Ramkumar R. et al.: ‘The green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L.’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2014, 118, pp. 11 –16 (doi: 10.1016/j.saa.2013.08.066) [DOI] [PubMed] [Google Scholar]
- 26. Basavegowda N. Sobczak‐Kupiec A. Fenn R.I. et al.: ‘Bioreduction of chloroaurate ions using fruit extract Punica granatum (pomegranate) for synthesis of highly stable gold nanoparticles and assessment of its antibacterial activity’, Micro Nano Lett., 2013, 8, (8), pp. 400 –404 (doi: 10.1049/mnl.2013.0137) [DOI] [Google Scholar]
- 27. Vankar P.S. Bajpai D.: ‘Preparation of gold nanoparticles from Mirabilis jalapa flowers’, Indian J. Biochem. Biophys., 2010, 47, (3), pp. 157 –160 [PubMed] [Google Scholar]
- 28. Shankar S.S. Ahmad A. Pasricha R. et al.: ‘Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes’, J. Mater. Chem., 2003, 13, (7), pp. 1822 –1826 (doi: 10.1039/b303808b) [DOI] [Google Scholar]
- 29. Ghisalberti E.L.: ‘ Lantana camara L. (Verbenaceae)’, Fitoterapia, 2000, 71, pp. 467 –486 (doi: 10.1016/S0367-326X(00)00202-1) [DOI] [PubMed] [Google Scholar]
- 30. Lim J.K. Yeap S.P. Che H.X. et al.: ‘Characterization of magnetic nanoparticle by dynamic light scattering’, Nanoscale Res. Lett., 2013, 8, p. 381 (doi: 10.1186/1556-276X-8-381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumar B. Smita K. Cumbal L. et al.: ‘ Lantana camara berry for the synthesis of silver nanoparticles’, Asian Pac. J. Trop. Biomed., 2015, 5, (3), pp. 930 –933 (doi: 10.1016/S2221-1691(15)30005-8) [DOI] [Google Scholar]
- 32. Rajeshkumar S. Malarkodi C. Gnanajobitha G. et al.: ‘Seaweed‐mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization’, J. Nanostruct. Chem., 2013, 3, p. 44 (doi: 10.1186/2193-8865-3-44) [DOI] [Google Scholar]


