Abstract
Retinoblastoma is the most common intraocular malignancy basically occurs among children below five. Certain ocular treatments such as surgery, radiation therapy and chemotherapy are more likely to cause side effects. Here, a rapid method of synthesising silver nanoparticles (AgNPs) from the brown seaweed Turbinaria ornata and its cytotoxic efficacy against the retinoblastoma Y79 cell lines was studied. The AgNPs synthesis was determined by Ultraviolet–visible spectroscopy and was further characterised by X‐ray diffraction, High‐resolution transmission electron microscopy, zeta potential, Energy‐dispersive X‐ray spectroscopy, thermogravimetric analysis, Fourier transform infrared spectrum and inductively coupled plasma‐mass spectroscopy techniques. The synthesised AgNPs were found to be very stable and finely dispersed. The total phenolic content of the synthesised AgNPs was estimated at 43±2.52 mg/g gallic acid equivalent and the nanoparticles exhibited good scavenging activity analysed by 2, 2′‐azinobis‐(3‐ethylbenzothiazoline‐6‐sulphonic acid) assay. Moreover, cytotoxicity of synthesised AgNPs against in vitro retinoblastoma Y79 cell lines showed a dose‐dependent response with an inhibitory concentration (IC50) of 10.5 µg/mL. These results suggest that AgNPs could be a promising anticancer agent with enhanced activity in ocular treatment.
Inspec keywords: toxicology, silver, nanoparticles, cellular biophysics, cancer, nanomedicine, nanofabrication, X‐ray diffraction, transmission electron microscopy, electrokinetic effects, X‐ray chemical analysis, thermal analysis, Fourier transform infrared spectra, ultraviolet spectra, visible spectra, biomedical materials, mass spectroscopic chemical analysis
Other keywords: cytotoxic activity; marine seaweed Turbinaria ornata; intraocular malignancy; silver nanoparticles; brown seaweed Turbinaria ornata; X‐ray diffraction; high‐resolution transmission electron microscopy; zeta potential; EDAX; thermogravimetric analysis; Fourier transform infrared spectrum; inductively coupled plasma‐mass spectroscopy; phenolic content; gallic acid; scavenging activity; in vitro retinoblastoma Y79 cell lines; dose‐dependent response; inhibitory concentration; anticancer agent; 2,2′‐azinobis‐(3‐ethylbenzothiazoline‐6‐sulphonic acid) assay; nanotechnology‐based cancer diagnosis; ocular tumour treatment; ultraviolet‐visible spectroscopy; Ag
1 Introduction
Biosynthesis of metal nanoparticles (NPs) is now established as a promising area of nanoscience research and development [1]. Nanotechnology provides a golden platform to modify and develop the properties of pure metals by converting them into their NPs, which has applications in numerous fields such as diagnostics, drug delivery, antimicrobial agents and treatment of various other diseases [2]. Noble metal NPs such as gold, silver (Ag) and platinum are widely used in medical applications. Silver has long been recognised as an effective antimicrobial agent that exhibits low toxicity in human and has diverse in vitro and in vivo applications [3]. The AgNPs have been synthesised by various physical, chemical and biological methods. Among the various known synthesis methods, biosynthesis of AgNPs is preferred as it is environmentally safe, low cost and less toxic [4]. These biologically synthesised AgNPs could have better applications in therapeutics, drug delivery, anticancer and bio‐imaging techniques [5]. The biological materials were used for the synthesis of NPs such as bacteria, fungi, plant, algae etc. Algae are called as ‘bionanofactories’ among the biological materials, because the live and dead biomass was used for the synthesis of metallic NPs. It is considered as environment friendly, cost effective, macroscopic structured material and has distinct advantage due to its high metal uptake capacity [6].
In India retinoblastoma is a widespread eye cancer that develops from the immature cells of a retina, the light‐detecting tissue of the eye, occurring in 1 in 20,000 children. The survival rate in retinoblastoma is almost 90% but by enucleation successful treatment is achieved in majority of cases [7]. Owing to conventional therapies many complications arise such as impaired vision and sometimes may induce secondary cancer [8].
Seaweeds have been reported to possess biological activity of potential medicinal value [9]. It was reported that seaweeds are rich source of bioactive compounds such as terpenoids, phlorotannins, fucoidans, sterols and glycolipids, and the extracts or isolated pure components from seaweeds possess a wide range of pharmacological properties such as anticancer, antibacterial, antifungal, anti‐viral, anti‐inflammatory, anticoagulant, antioxidant, hypoglycaemic, hypolipidemic, antimelanogenic, anti‐bone loss, hepatoprotective and neuroprotective activities [10, 11]. Earlier reports indicated that the extracts of brown seaweeds belonging to Turbinaria spp. were found to have antioxidant and anti‐inflammatory activities [12, 13]. In the present paper, we investigated the green synthesis of AgNPs using Turbinaria ornata (T. ornata) and its cytotoxic potential against Y79 cell lines in vitro.
2 Materials and methods
2.1 Collection and processing of marine algae
The brown algae, T. ornata were collected from Gulf of Mannar, Tamil Nadu. The algal sample was washed in clean sea water to remove epiphytes and salts attached to the surface of the sample. The sample was transported to the laboratory in sterile polythene bags. It was then rinsed with tap water, shade dried for a week, powdered and stored in a polypropylene container at room temperature.
2.2 Biosynthesis of AgNPs
The pure algal aqueous extract solution of 10 ml was mixed with 90 ml of 1 mM Ag nitrate (AgNO3) solution and kept in room temperature with constant stirring at 120 rpm. The appearance of dark brown colour indicated the formation of AgNPs.
2.3 Characterisation of AgNPs
Bioreduction of Ag ions using T. ornata aqueous extract was monitored by ultraviolet–visible (UV–vis) spectrophotometer at different wavelengths from 300 to 700 nm (UV–vis Schimadzu 1800 spectrophotometer). The biosynthesised AgNPs were purified by repeated centrifugation at 12,500 rpm for 15 min and freeze dried. The crystalline nature of the purified AgNPs was coated onto the glass substrate and X‐ray diffraction (XRD) measurements were carried out using Rigaku smart lab instrument with copper (Cu) Kα 1 radiation (40 kV, 30 mA). Transmission electron micrographs were taken using high‐resolution transmission electron microscopy (HRTEM – JEOL 3010) equipped with selected area electron diffraction pattern (SAED) by taking a little volume of AgNPs solution on Cu grid and allowed to dry completely and operated at 300 kV. The elemental composition was analysed using Energy‐dispersive X‐ray spectroscopy. The particle size distributions and the stability of NPs were characterised by dynamic light scattering (DLS) using a Malvern Zetasizer Nano (Malvern).
Fourier transform infrared spectrum (FT‐IR) of the biosynthesised AgNPs was recorded at 4000–400 cm−1 range using the Perkin Elmer system. Thermogravimetric analysis (TGA) was performed using a TGA instrument (Mettler Toledo). The loss of material was determined by heating the sample in alumina crucible at the rate of 10 °C/min from 0 to 1100 °C under continuous nitrogen purging (20 ml/min). Initial and final concentration after reduction of Ag+ ions was determined using inductively coupled plasma‐mass spectroscopy (ICP‐MS – Agilent 7700, USA).
2.4 Antioxidant assays
2.4.1 Determination of total phenolic content
Phenolic contents of the AgNPs were estimated spectrophotometrically according to the Folin–Ciocalteu colorimetric method [14]. The sample of 200 µl was mixed with 1 ml of 7.5% sodium carbonate and incubated for 2 min at room temperature. Then 1 ml of Folin–Ciocalteu phenol reagent was added and the tubes were incubated for 2 h. The absorbance was read at 726 nm using a spectrophotometer (Beckman, USA). The total phenolic content was expressed as gallic acid equivalents (GAEs) in milligrams/grams of the sample.
2.4.2 2, 2′‐Azinobis‐(3‐ethylbenzothiazoline‐6‐sulphonic acid) (ABTS) radical scavenging assays
The free radical scavenging activity of AgNPs was determined using ABTS radical cation (diammonium salt) decolorisation assay [15]. ABTS radical cation was generated by mixing 7 mM ABTS stock solution with 2.45 mM potassium persulphate under darkness at room temperature for 12–16 h. The ABTS radical cation solution was diluted with 50% absolute ethanol and the absorbance was measured at 734 nm. The ABTS radical cation scavenging activity was assessed by mixing 2.0 ml of ABTS solution (absorbance of 0.7 ± 0.02) with 0.2 ml of AgNPs at different concentrations (10, 50, 100, 250, 500 and 1000 µg). The final absorbance was taken at 734 nm with spectrophotometer. The percentage of scavenging was calculated by the following formula:
where A 0 is the absorbance of control and A 1 is the absorbance of sample.
2.5 Cytotoxic activity
To observe the cytotoxicity assay, retinoblastoma cell line Y79 was purchased from National Centre for Cell Science, Pune, India. The cell lines were grown in Dulbecco's modified eagle medium supplemented with 10% Fetal Bovine Serum, 100 µg/ml penicillin, 100 μg/ml streptomycin and grown at 37 °C in a humidified atmosphere of 95% air and 5% CO2. The Y79 cells were cultured and seeded in 96 well plates (∼1 × 106 cells per well) and incubated for 24 h to perform cytotoxic analysis using 3‐(4, 5‐dimethyl‐2‐thiazolyl)‐2, 5‐diphenyl‐tetrazolium bromide (MTT) assay. Various concentrations of the AgNPs in 0.1% Dimethyl sulfoxide were added and incubated for 24 h at 95% air and 5% CO2 incubator. After incubation, 10 µl (5 mg/ml in Phosphate Buffered Saline) of MTT was added to each well and incubated for 4 h at 37°C. The resulting formazan was dissolved in 100 µl of DMSO and the viable cells were determined by measuring the absorbance at 570 nm. The effect of AgNPs on the proliferation of Y79 was expressed as the percentage of cell viability using the following formula:
The treated cells were observed under microscope for cell morphology analysis and images of each concentration were captured and recorded.
3 Results and discussion
The synthesis of the NPs took place on mixing T. ornata extract (1 ml from stock) with 1 mM aqueous AgNO3 solution. The colour change of the aqueous extract was noted visually from yellow colour to dark brown indicating the formation of AgNPs [16]. The following characterisation methods were used to confirm the formation of AgNPs through biosynthesis.
The formation of AgNPs was confirmed using UV–vis spectroscopy. Fig. 1 shows UV–vis spectrum recorded from seaweed extract and the biosynthesised AgNPs after the reduction process. The seaweed extract showed many distinct peaks between 250 and 800 nm that confirmed the presence of phenols, flavonoids, polysaccharides and proteins. The biosynthesised AgNPs showed a plasmon peak of reduced AgNPs at 428 nm in the UV–vis spectra indicates the presence of AgNPs. These results conclude that the above mentioned compounds present in seaweed might be responsible for the reduction process.
Fig. 1.
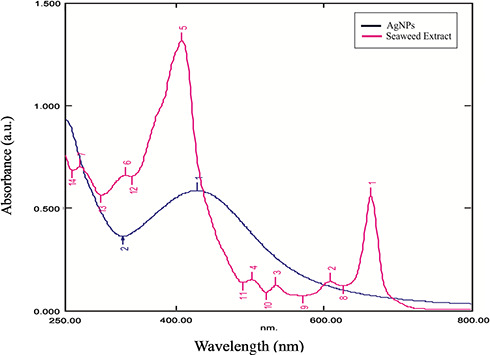
UV spectra of plant extract and reduced AgNPs
The XRD of the synthesised AgNPs is shown in Fig. 2. In XRD analysis, four peaks were identified at 2θ values of 38.07°, 44.28°, 64.41° and 77.29° can be indexed to (111), (200), (220) and (311) crystalline planes of face‐centred‐cubic crystalline structure of metallic NPs, respectively (The Joint Committee on Powder Diffraction Standards File No.: 03‐0921) [17].
Fig. 2.
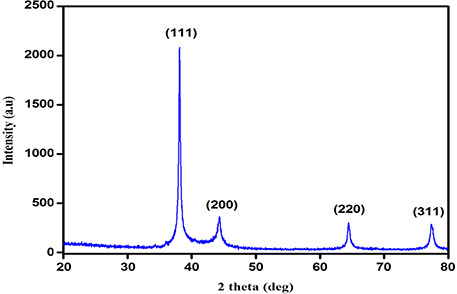
XRD pattern showing sharp peaks of AgNPs
The HRTEM image obtained from AgNPs showed spherical shaped particles in the size range 14–22 nm (Fig. 3 a). The selected area diffraction pattern (SAED) obtained for the AgNPs is shown in Fig. 3 b. This revealed that the bright circular spots are spherical and confirmed the crystalline nature of AgNPs.
Fig. 3.
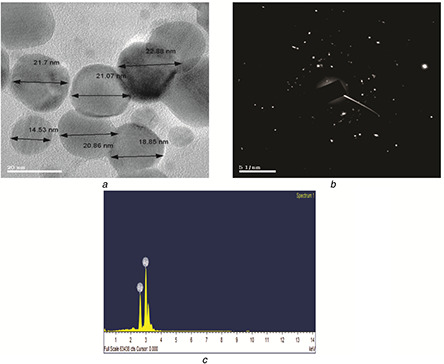
Images of spherical shaped AgNPs, diffraction pattern and elemental composition
(a) HRTEM, (b) SAED (c) EDAX
The elemental compositions can be identified by the EDAX analysis, which confirmed the reduction of AgNO3 to AgNPs. The EDAX spectrum (Fig. 3 c) illustrated the presence of sharp signal peak of Ag.
DLS size distribution pattern of AgNPs are found to be polydispersed with size ranging from 22 to 32 nm. Zeta potential is the net surface charge of NPs and plays an important role in the stability of the solution [18]. The results showed a sharp peak at −28.7 mV that indicated the biogenic NPs are negatively charged on their surface (Figs. 4 a and b).
Fig. 4.
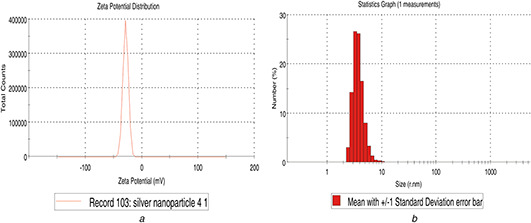
Zeta potential analysis
(a) Particle size measurement of AgNPs, (b) Size distribution analysis of synthesised AgNPs
FT‐IR analysis was carried out to identify the possible biomolecules responsible for the reduction of Ag+ ions and the capping of bioreduced AgNPs synthesised. The FT‐IR studies (Fig. 5 a) found a broad intense peak at 3408 cm−1 that corresponds to O–H and N–H stretch of phenol. The narrow peaks located in 2923 and 2856 cm−1 were identified to be aldehydic C–H stretching [19]. Other three narrow peaks noted in 1629 cm−1 correspond to C=O stretching of a carbonyl group [20], 1397 and 1052 cm−1 regions correspond to amide I band rise due to carbonyl stretch in proteins, C–N stretching vibration of aromatic amine group and C–N stretching vibration of aliphatic amines [21]. This concludes that phenols and proteins present in the seaweed are the active biomolecules for significant reduction of the biogenic AgNPs.
Fig. 5.
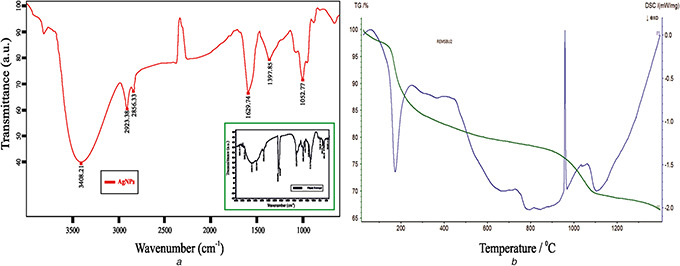
Spectral and thermal analysis
(a) FT‐IR spectra of AgNPs and T. ornata extract, (b) Differential Scanning Calorimetry and TGA of AgNPs
DSC of AgNPs shows two peaks: the endothermic peak was found at 173.4 °C and the other exothermic peak at 959.2 °C due to transition temperature. TGA plot of AgNPs showed weight loss in the temperature range and the initial sample mass was 13 mg and the residual mass was 0.66 mg (42%) (Fig. 5 b).
ICP‐MS analysis showed that the final AgNP solution prepared using T. ornata extract contains 96% of Ag°. It is this percentage of NPs that show good antioxidant property too. The percentage yield (Y) was calculated by the formula mentioned below
The results of total phenol content of the biosynthesised AgNPs from T. ornata was measured by Folin–Ciocalteu method which demonstrated the attachment of certain amount (43 ± 2.52 mg/g GAE) of phenolic compounds. The Folin–Ciocalteu's assay is a frequently used screening method for the measurement of antioxidant properties of food products and dietary supplements [22].
The scavenging capacity of the sample was tested using ABTS radical assay. ABTS radical cation is generated from oxidation of ABTS radical by potassium persulphate, is a good tool for determining the antioxidant activity of hydrogen‐donating and chain breaking antioxidants [23]. The ABTS scavenging activity showed increase in free radical scavenging activity with increase in the concentration of the synthesised AgNPs. The IC50 value for the synthesised AgNPs was 144.21 µg/ml (Fig. 6). There are some evidences that seaweeds contain compounds with a relatively high antioxidant and antiproliferative activity. Seaweeds are low in fat but contain vitamins and bioactive compounds such as terpenoids, sulphated polysaccharides and polyphenolic compounds, the latter being a potential natural antioxidant not found in land plants [13]. The total phenol content and ABTS used in this paper represent convenient methods for the identification of potential sources of antioxidant compounds [24].
Fig. 6.
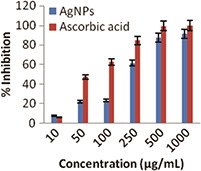
ABTS calibration curve of AgNPs and standard ascorbic acid
The antiproliferative activity of the synthesised AgNPs was evaluated against retinoblastoma cell lines Y79. Here, the size and dose concentration of NPs play an important role in inducing cytotoxicity [25]. The results of the present paper revealed that the cell viability decreased with increase in concentration of synthesised AgNPs (Fig. 7 a). The inhibition concentration (IC50) was recorded at 10.5 μg/ml. The high cytotoxic effect of AgNPs may also be attributed to its size and capping of biomolecules such as protein or phenol on the surface of NPs [26]. It has been reported that the increase in the cytotoxicity of AgNPs was due to active physical and chemical interaction of Ag ions with the functional groups of intracellular proteins [27]. The cells were then observed under inverted light microscope which showed distinct morphological changes after treatment with AgNPs when compared with the control (Fig. 7 b).
Fig. 7.
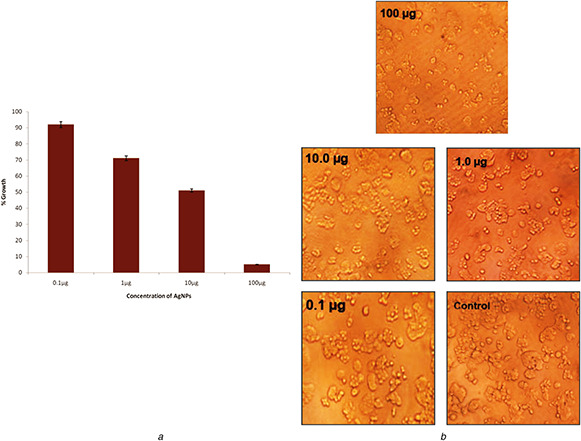
Cytotoxic activity and images of the cells
(a) Cytotoxic effect of AgNPs against Y79 cell lines, (b) Morphological changes of control and the treated cells under inverted microscopy
4 Conclusions
AgNPs were successfully synthesised using marine seaweed T. ornata and the fabricated NPs were also characterised well in this paper. The synthesised NPs were stable and exhibited promising free radical scavenging effect. Furthermore, the seaweed extracts derived NPs exhibited strong cytotoxic effects against the retinoblastoma cell lines Y79. The above results suggest that biologically synthesised AgNPs might be used as a novel anticancer agent and could be used for broad range of medical applications in future.
5 Acknowledgments
The authors are thankful to the management of Sathyabama University for the support in carrying out the research activity. The authors also acknowledge Centre for Ocean Research and Centre for Nanoscience and Nanotechnology of Sathyabama University, Sophisticated Analytical Instrument Facility Indian Institute of Technology Chennai, Sankara Netralyala Chennai for providing necessary instrumental facilities.
6 References
- 1. Shenya D.S. Mathew J. Philip D.: ‘Photosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale ’, Spectrochim. Acta A, 2011, 79, pp. 254 –262 [DOI] [PubMed] [Google Scholar]
- 2. Singh R. Nalwa H.S.: ‘Medical applications of nanoparticles in biological imaging, cell labeling, antimicrobial agents, and anticancer nanodrugs’, J. Biomed. Nanotechnol., 2011, 7, pp. 489 –503 [DOI] [PubMed] [Google Scholar]
- 3. Farooqui M.A. Chauhan P.S. Krishnamoorthy P. et al.: ‘Extraction of silver nanoparticles from the leaf extracts of Clerodendrum inerme ’, Dig. J. Nanomater. Biostruct., 2010, 5, pp. 43 –48 [Google Scholar]
- 4. Buzea C. Pacheco I.I. Robbie K.: ‘Nanomaterials and nanoparticles: sources and toxicity’, Biointerphases, 2007, 2, pp. 17 –71 [DOI] [PubMed] [Google Scholar]
- 5. Indu B. Peeyush R. Suneel K. et al.: ‘Cellular oxido‐reductive proteins of Chlamydomonas reinhardtii control the biosynthesis of silver nanoparticles’, J. Nanobiotechnol., 2011, 56, pp. 1 –12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis T.A. Volesky B. Mucci A.: ‘A review of the biochemistry of heavy metal biosorption by brown algae’, Water Res., 2003, 37, pp. 4311 –4330 [DOI] [PubMed] [Google Scholar]
- 7. De Potter P.: ‘Current treatment of retinoblastoma’, Curr. Opin. Ophthalmol., 2002, 13, pp. 331 –336 [DOI] [PubMed] [Google Scholar]
- 8. Abramson D.H.: ‘Retinoblastoma in the 20th century: past success and future challenges the Weisenfeld lecture’, Invest. Ophthalmol. Vis. Sci., 2005, 46, pp. 2683 –2691 [DOI] [PubMed] [Google Scholar]
- 9. Satoru K. Noboru T. Hiroo N. et al.: ‘Oversulfation of fucoidan enhances its anti‐angiogenic and anti‐tumor activities’, Biochem. Pharmcol.., 2003, 65, pp. 173 –179 [DOI] [PubMed] [Google Scholar]
- 10. Chakraborty K. Paulraj R.: ‘Sesquiterpenoids with free radical scavenging properties from marine macroalga Ulvafasciata delile ’, Food Chem., 2010, 122, pp. 31 –41 [Google Scholar]
- 11. Liu L. Heinrich M. Myers S. et al.: ‘Towards a better understanding of medicinal uses of the brown seaweed Sargassum in traditional Chinese medicine: a phytochemical and pharmacological review’, J. Ethnopharmacol., 2012, 142, pp. 591 –619 [DOI] [PubMed] [Google Scholar]
- 12. Zubia M. Fabre M.S. Kerjean V. et al.: ‘Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts’, Food Chem., 2009, 116, pp. 693 –701 [Google Scholar]
- 13. Vijayabaskar P. Shiyamala: ‘Antioxidant properties of seaweed polyphenol from Turbinaria ornata (Turner) J. Agardh 1848’, Asian Pac. J. Trop. Biomed., 2012, 1, pp. 90 –98 [Google Scholar]
- 14. Singleton V.L. Rossi J.A.: ‘Colorimetry of total phenolics with phosphomolybdic – phosphotungstic acid reagents’, Am. J. Enol. Viticult., 1965, 16, pp. 144 –158 [Google Scholar]
- 15. Re R. Pellegrini N. Proteggente A. et al.: ‘Antioxidant activity applying an improved ABTS radical cation decolorization assay’, Free Radic. Biol. Med., 1999, 26, pp. 1231 –1237 [DOI] [PubMed] [Google Scholar]
- 16. Ahmad A. Mukherjee P. Senapati S. et al.: ‘Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum ’, Colloids Surf. B, 2003, 28, pp. 313 –318 [Google Scholar]
- 17. Narayanan K.B. Sakthivel N.: ‘Facile green synthesis of gold nanostructures by NADPH‐dependent enzyme from the extract of Sclerotium rolfsii ’, Colloids Surf. A, 2011, 380, pp. 156 –161 [Google Scholar]
- 18. Vivek R. Thangam R. Muthuchelian K. et al.: ‘Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF‐7 cells’, Process Biochem., 2011, 47, pp. 2405 –2410 [Google Scholar]
- 19. Ahmad M.B. Shameli K. Tay M.Y. et al.: ‘Antibacterial effect of silver nanoparticles prepared in bipolymers at moderate temperature’, Res. Chem. Intermediates, 2014, 40, pp. 817 –832 [Google Scholar]
- 20. Kumar V. Yadav S.K.: ‘Plant‐mediated synthesis of silver and gold nanoparticles and their applications’, J. Chem. Technol. Biotechnol., 2009, 84, pp. 151 –157 [Google Scholar]
- 21. Prathap M. Alagesan A. Ranjitha Kumari B.D.: ‘Anti‐bacterial activities of silver nanoparticles synthesized from plant leaf extract of Abutilon indicum (L.) sweet’, J. Nanostruct. Chem., 2014, 4, pp. 1 –6 [Google Scholar]
- 22. Chew Y.L. Lim Y.Y. Omar M. et al.: ‘Antioxidant activity of three edible seaweeds from two areas in South East Asia’, Food Sci. Technol., 2008, 41, pp. 1067 –1072 [Google Scholar]
- 23. Leong L.P. Shui G.: ‘An investigation of antioxidant capacity of fruits in Singapore markets’, Food Chem., 2002, 76, pp. 69 –75 [Google Scholar]
- 24. Stratil P. Klejdus B. Kuban V.: ‘Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods’, J. Agric. Food Chem., 2006, 54, pp. 607 –616 [DOI] [PubMed] [Google Scholar]
- 25. Babu G. Arulvasu C. Durai P. et al.: ‘Biosynthesis and characterization of silver nanoparticles from Datura inoxia and its apoptotic effect on human breast cancer cell line MCF7’, Mater. Lett., 2014, 122, pp. 98 –102 [Google Scholar]
- 26. Remya R.R. Radhika Rajasree S.R. Aranganathan L. et al.: ‘An investigation on cytotoxic effect of bioactive AgNPs synthesized using Cassia fistula flower extract on breast cancer cell MCF‐7’, Biotechnol. Rep., 2015, 8, pp. 110 –115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moaddab S. Ahari H. Shahbazzadeh D. et al.: ‘Toxicity study of nanosilver (Nanocid) on osteoblast cancer cell line’, Int. Nano Lett., 2011, 1, pp. 11 –16 [Google Scholar]


