Abstract
Nanoparticles have been used in many areas of biotechnology. In this study, an alternative surface sterilisation method was established for plant tissue cultures. Silver nanoparticles synthesised via green synthesis were used for the surface sterilisation of Lamiaceae seeds (Salvia farinecae, Ocimum basilicum – Large Leaf Italian, Thymus vulgaris, Ocimum basilicum var. purpurascens). Water extracts of dried Alkanna tinctorum rhizomes and Syzygium aromaticum flowers were utilised in the bioreduction of silver ions. The seeds were exposed to 0, 1, 7, 14 and 28 day‐old colloidal solutions of silver nanoparticles and their effects on germination and surface sterilisation were determined. Fresh (0 and 1 day‐old) colloidal solutions of silver nanoparticles were found very effective on surface sterilisation (100%). Moreover, they showed no negative effect on both germination and morphology of plantlets. It was shown that silver nanoparticles can be used as a surface sterilisation agent and they have no adverse effects on seed germination and in vitro plantlet growth.
Inspec keywords: nanoparticles, microorganisms, sterilisation (microbiological), silver, nanofabrication, antibacterial activity, colloids, nanobiotechnology, agriculture
Other keywords: green synthesised silver nanoparticles, Lamiaceae seeds, silver ions, surface sterilisation agent, seed germination, colloidal solutions, surface sterilisation method, Ag
1 Introduction
Nanotechnology is the science of objects smaller than 100 nm. Nanoparticles are the main elements of this rapidly expanding technology. Silver nanoparticles are one of the most popular ones among them. They have strong antiseptic, antibacterial, antifungal and antiviral effects depending on their sizes and shapes. Moreover, they are eco‐friendly elements which have relatively low toxicity towards to environment and human [1, 2, 3].
Mainly, there are three types of silver nanoparticle synthesis: physical, chemical and bio‐based (green synthesis) methods. Many parameters such as temperature, pH alteration, the concentration of solutions, type of chemicals used in the synthesis and so on have been studied and characteristics of nanoparticles have been identified under certain circumstances [4].
Silver ions are highly reactive and can form silver nanoparticles in different shapes and sizes. This feature gives a great opportunity to synthesise selectively antimicrobial or selectively toxic silver nanoparticles [5]. For example, silver nanoparticles smaller than 100 nm were found less toxic to Drosophila eggs than silver particles of conventional (>100 nm) size [6]. There is another interesting evidence about silver nanoparticles; Kittler et al. [7] reported that the toxicity of stored silver nanoparticles increases because the particles slowly dissolve into silver ions on a time scale of several days and this causes an increased presence of silver ions in the dispersion. Similar toxicity results of increased presence of silver ions were also reported by Asghari et al. [8].
Lamiaceae is one of the most useful medicinal plant family in the Mediterranean region. It is a cosmopolitan botanical family that comprises 186 genera and 7200 species. Lamiaceae plants are generally used for food seasoning and their medicinal properties in many countries all over the world [9]. The essential oils of these plants are of special interest for pharmacology; they have antibacterial, bactericidal, spasmolytic, sedative, expectorant, antipyretic, antifungal and antiviral activities because of their phenolic contents. They are also used in the cosmetic and perfume industry due to their aromatic potential [10, 11].
Surface sterilisation is the very first step of plant biotechnology. It is also the first bottleneck of biotechnological procedures. Without a successful surface sterilisation of plant materials, procedures cannot proceed further. Therefore, use of surface sterilants and their application durations and concentrations should be defined. Sodium chlorite, ethanol, calcium hypochlorite, mercuric chlorite, surfactants and antibiotics are the most frequently used agents for surface sterilisation. However, they are toxic to plant cells and tissues and have side effects such as necrosis, browning and growth inhibition on plant cells and tissues in culture conditions. They are also toxic to all living organisms and not eco‐friendly [12, 13].
Silver nanoparticles have been used in preventing contamination of medical products [14]. Recently, they have also been used in plant biotechnology as a surface sterilisation agent and an elicitor [15, 16, 17, 18, 19, 20, 21, 22, 23].
In this study, silver nanoparticles synthesised via biological procedure (green synthesis) were used as surface sterilisation agents (NP samples: Alkanna‐NP and Syzygium‐NP) on the seeds of four Lamiaceae plants. Surface sterilisation and germination percentages of seeds were recorded in order to determine the effects of sterilisation agents at different times (0, 1, 7, 14 and 28 day‐old NP samples).
2 Materials and methods
2.1 Materials
Commercial seeds (Sveryverts) of Salvia farinecae (SF), Ocimum basilicum – Large Leaf Italian (OB‐L), Thymus vulgaris (TV), Ocimum basilicum var. purpurascens (OB‐P) were used for the surface sterilisation applications. Dried Alkanna tinctorum rhizomes and Syzygium aromaticum flowers, whose water extracts were used as bioreductors, were obtained from a local market.
2.2 Methods
2.2.1 Preparation of water extracts of A. tinctorum rhizoms and S. aromaticum flowers
Dried A. tinctorum rhizomes and S. aromaticum flowers were surface cleaned with running tap water and rinsed in distilled water twice. After they were dried, they were ground with mortar. About 5 g of each were mixed with 100 ml distilled water and kept in water bath at 80°C for 1 h. The mixtures were filtered through filter paper and kept in glass bottles for one day at room temperature (22 ± 2°C).
2.2.2 Silver nanoparticle synthesis
An aqueous solution of 1 mM silver nitrate was prepared in 250 ml glass bottle and used for the synthesis of silver nanoparticles. Five millilitres of plant extracts were added swiftly into 95 ml silver nitrate solution with the help of a pipette in a few seconds and incubated at room temperature (22 ± 2°C). At the end of bioreduction, these colloidal solutions were used for surface sterilisation of Lamiaceae seeds at day 0, 1, 7, 14 and 28.
2.2.3 Characterisation of silver nanoparticle samples
The formation of silver nanoparticles was confirmed by colour and pH changes, UV–visible (UV–Vis) spectrophotometry and scanning electron microscopy (SEM) analysis. The alterations of pH in NP samples were tracked during 28 days. The pH of water extracts and the aqueous solution of 1 mM silver nitrate were also recorded. The UV–Vis spectra of NP samples were monitored at 320–500 nm by using UV–Vis spectrophotometer (Shimadzu UV‐1201V). Distilled water was used as blank to adjust the baseline. Scanning electron microscope FEI‐QUANTA FEG 250 equipped with energy‐dispersive spectroscopy (EDS, FEI) was used to determine the shapes and sizes of silver nanoparticles. Samples were prepared by depositing a drop of colloidal solution on an aluminium grid sample holder and drying at room temperature.
2.2.4 Surface sterilisation of Lamiaceae seeds
Alkanna‐NP and Syzygium‐NP were used for the surface sterilisation of SF, OB‐L, TV, OB‐P seeds. Prior to NP samples applications, 70% ethanol was applied for 3 min to seeds and they were rinsed with sterile distilled water twice. After that, the seeds were exposed to 0, 1, 7, 14 and 28 day‐old Alkanna‐NP and Syzygium‐NP for 15 min. In control groups, instead of silver nanoparticle samples, the seeds were exposed to 10% NaOCl for 15 min for surface sterilisation. They were rinsed in sterile distilled water three times and placed in semi‐solid woody plant medium (WPM) [24] medium supplemented with 3% sucrose and 0.7% agar (pH = 5.8). Each experiment was conducted with 15 seeds with three replicates and they were incubated at 22 ± 2°C with 16 h light/8 h dark photoperiods. At the fourth week, sterile and germinated seed percentages were calculated by observations of seeds in culture conditions.
2.2.5 Statistical analysis
Germination and surface sterilisation experiments were repeated three times. Experiments were implemented in a factorial randomised plots design with three factors: seed type: SF, OB‐L, TV, OB‐P; NP sample type: Alkanna‐NP, Syzygium‐NP; days of NP samples: 0, 1, 7, 14, 28. Data were analysed with one‐way analysis of variance test and a posthoc least significant difference (LSD) tests were also performed (p ˂0.05).
3 Results and discussion
Bioreduction of silver ions to colloidal silver nanoparticles was visually observed by the colour change; Alkanna‐NP changed from light‐yellow to yellow, whereas Syzygium‐NP changed from red‐brown to grey‐brown. The colouration was caused by the excitation of surface plasmon resonance of conducting electrons in the silver nanoparticles. The colour changes of the samples stopped after 2 h, indicating that the silver nanoparticle biosynthesis was completed (Fig. 1). Silver nanoparticle biosynthesis also confirmed by UV–Vis spectrophotometric analysis. Alkanna‐NP and Syzygium‐NP showed characteristic bands in UV–Vis region (Fig. 2): 369 and 430 nm, respectively. Similar results and observations about the colour changes and UV–Vis chromatograms were reported in green synthesis of silver nanoparticle studies [15, 16, 25, 26].
Fig. 1.

Colours of A. tinctorum rhizoms water extract, Alkanna‐NP, S. aromaticum water extract and Syzygium‐NP
Fig. 2.
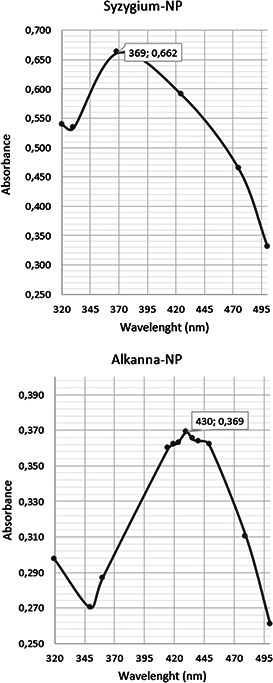
UV–Vis chromatograms of 2 h‐old Syzygium‐NP and Alkanna‐NP
The alterations in pH were also recorded prior to surface sterilisation application for 0, 1, 7, 14 and 28 day‐old Alkanna‐NP and Syzygium‐NP (Fig. 3). In Alkanna‐NP, pH was descended rapidly from 4.17 to 3.5 at the end of the first day and reached to 3.28 at the 28th day. Similarly, in Alkanna‐NP, pH was decreased from 6.3 to 6.11 at the end of the first day and gradually decreased to 3.72 at 28th day.
Fig. 3.
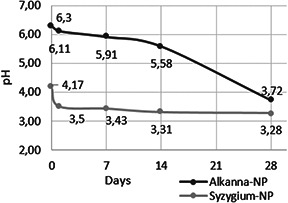
pH alterations in Alkanna‐NP and Syzygium‐NP
OB‐L, OB‐P and TV seeds, including control groups, started to germinate at the third day of culture. However, no germination was observed at SF seeds before two weeks of cultivation. Therefore, the germination and surface sterilisation percentages were recorded at the fourth week of cultivation (Table 1).
Table 1.
Percentage of seed germination and surface sterilisation of Lamiaceae seeds (Alkanna‐NP; A0: day‐0, A1: 1st day, A7: 7th day, A14: 14th day, A28: 28th day) (Syzygium‐NP; K0: day‐0, K1: 1st day, K7: 7th day, K14: 14th day, K28: 28th day)
| Plant seed | Sterilisation methods | Seed germination, %* | Surface sterilisation, %** |
|---|---|---|---|
| Salvia farinecae (SF) | control | 51.11 ± 4.45 | 100.00 ± 0.00 |
| A0 | 46.66 ± 0.00 a | 100.00 ± 0.00 a | |
| A1 | 33.33 ± 0.00 a | 100.00 ± 0.00 a | |
| A7 | 51.11 ± 5.89 a | 100.00 ± 0.00 a | |
| A15 | 51.11 ± 2.22 a | 97.78 ± 2.22 a | |
| A30 | 37.78 ± 8.02 a | 100.00 ± 0.00 a | |
| K0 | 55.56 ± 9.70 a | 100.00 ± 0.00 a | |
| K1 | 48.89 ± 2.22 ab | 100.00 ± 0.00 a | |
| K7 | 51.11 ± 4.45 ab | 84.44 ± 12.39 a | |
| K15 | 53.33 ± 6.67 ab | 88.89 ± 11.12 a | |
| K30 | 31.11 ± 13.53 b | 57.78 ± 19.40 b | |
| Ocimum basilicum ‐ Large Leaf Italian (OB‐L) | control | 88.89 ± 8.02 | 100.00 ± 0.00 |
| A0 | 88.89 ± 2.22 a | 100.00 ± 0.00 a | |
| A1 | 86.67 ± 5.89 a | 100.00 ± 0.00 a | |
| A7 | 95.55 ± 2.22 a | 93.17 ± 3.86 a | |
| A15 | 46.67 ± 3.85 b | 100.00 ± 0.00 a | |
| A30 | 37.78 ± 5.89 b | 97.78 ± 2.22 a | |
| K0 | 80.00 ± 3.85 a | 100.00 ± 0.00 a | |
| K1 | 66.67 ± 7.71 a | 97,78 ± 2,22 a | |
| K7 | 84.45 ± 2.22 a | 93.33 ± 0.00 a | |
| K15 | 75.56 ± 12.39 a | 97.78 ± 2.22 a | |
| K30 | 66.67 ± 3.85 a | 100.00 ± 0.00 a | |
| Thymus vulgaris (TV) | control | 75.56 ± 2.22 | 100.00 ± 0.00 |
| A0 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | |
| A1 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | |
| A7 | 80.00 ± 3.85 ab | 100.00 ± 0.00 a | |
| A15 | 71.11 ± 5.89 b | 100.00 ± 0.00 a | |
| A30 | 68.89 ± 9.70 b | 91.11 ± 8.90 a | |
| K0 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | |
| K1 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | |
| K7 | 71.11 ± 4.45 b | 95.56 ± 4.45 a | |
| K15 | 53.33 ± 13.89 b | 8.89 ± 4.45 c | |
| K30 | 66.67 ± 10.20 b | 60.00 ± 10.20 b | |
| Ocimum basilicum var. Purpurascens (OB‐P) | control | 82,22 ± 8,02 | 100,00 ± 0,00 |
| A0 | 97.78 ± 2.22 a | 100.00 ± 0.00 a | |
| A1 | 66.67 ± 6.67 b | 100.00 ± 0.00 a | |
| A7 | 55.56 ± 2.22 b | 100.00 ± 0.00 a | |
| A15 | 51.11 ± 2.22 b | 100.00 ± 0.00 a | |
| A30 | 53.33 ± 6.67 b | 100.00 ± 0.00 a | |
| K0 | 95.56 ± 2.22 a | 100.00 ± 0.00 a | |
| K1 | 91.11 ± 5.89 a | 100.00 ± 0.00 a | |
| K7 | 75.56 ± 2.22 ab | 64.44 ± 4.45 b | |
| K15 | 55.56 ± 14.59 ab | 95.56 ± 4.45 a | |
| K30 | 75.56 ± 4.45 b | 97.78 ± 2.22 a |
*F = 2.643; LSD = 23.817; p <0.01.
**F = 11.836; LSD = 16.804; p <0.01.
Values within column followed by different letters are significantly different at the 0.01 level by LSD's test.
According to the seed germination percentages, interactions between the three factors (seed type × NP type × days of NP samples) were found statistically significant at p ˂0.01 (F = 2.643; LSD = 23.817). In NP sterilisation, the average germination percentage was 69.89% in Alkanna‐NP, whereas Syzygium‐NP was 65.55%. In control group, the average germination percentage was found 74.45%, which was very close to NP sterilisation rates. The highest average germination rate was recorded in TV seeds at 88.11%, which was 16% higher than its control group (75.56%). The effect of NP samples showed a timewise variation on germination.
As the NP sample got older, lower germination percentages were obtained. The highest average germination percentage (83.06%) was recorded at 0‐day and this rate gradually decreased; 73.06% at 1st day, 70.56% at 7th day; 57.22% at 14th day and 54.72% at 28th day. As mentioned before, stored silver nanoparticles toxicity ascend as the presence of silver ion concentration increase [7]. This may be the consequence of enhanced silver ions in the older NP samples. However, there was no visible morphological deformation was observed in the in vitro grown plantlets (Fig. 4).
Fig. 4.
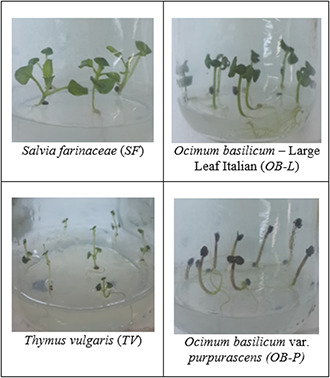
In vitro grown Lamiaceae plantlets after surface sterilisation with NP samples
In surface sterilisation, interactions between the three factors (seed type × NP type × days of NP samples) were also found statistically significant at p ˂0.01 (F = 11.836; LSD = 16.804). The average surface sterilisation percentages were found very satisfactory in NP samples: 97.98% in OB‐L; 95.78% in OB‐P; 93.70% in SF; 88.51% in TV. The lowest percentage (88.51%) was found 11.49% lower than its control group (100%). Alkanna‐NP showed higher average sterilisation activity (98.99%) than Syzygium‐NP (89.00%). Their effects were gradually increased due to their freshness: 100% at 0‐day; 99.72% at 1st day; 92.39% at 7th day; 89.86% at 14th day; 88.06% at 28th day. Zero‐day and first day results showed that NP sterilisation can be as effective as NaOCl sterilisation. Although NP samples lost some of their sterilisation activity, they still can be used as sterilisation agent at the end of 28th day.
Water extracts of A. tinctorum rhizomes and S. aromaticum flowers were also applied to the seeds for 15 min, after exposing them to 70% ethanol for 3 min in order to see their surface sterilisation performance. However, all the seeds were contaminated at the third day of culture.
The antibacterial effects of silver nanoparticles were strongly affected by their sizes and shapes. Smaller and spherical nanoparticles enhance the extent of bacterial elimination and are more effective than bigger ones, due to their larger contact surface [3]. Therefore, in this study, shapes of Alkanna‐NP and Syzygium‐NP were visualised via SEM. According to Figs. 5 and 6, Alkanna‐NP were more spherical and smaller than Syzygium‐NP, which clarify the higher sterilisation percentages of Alkanna‐NP. At the first day, NP samples were relatively sparse (Figs. 5 a and c). However, at the 28th day, they were more intense (Figs. 5 b and d). Moreover, in Syzygium‐NP, precipitation can be observed at the bottom of glass bottle after the 14th day. These results showed that silver nanoparticles engaged to each other and narrow their surface area as their colloidal solutions get older. Therefore, their sterilisation activity was found higher in fresh NP samples. EDS results of NP samples were also given in Fig. 6.
Fig. 5.
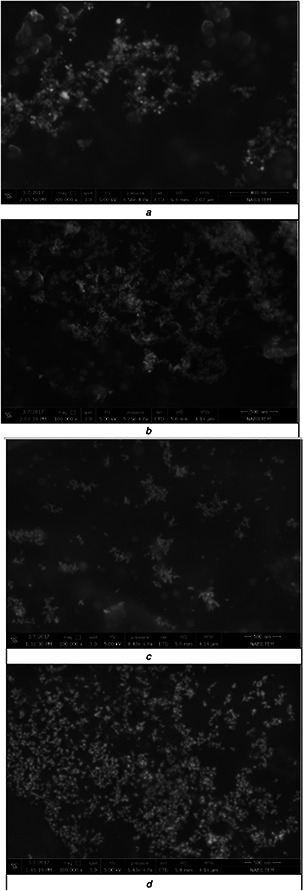
SEM images of NP samples at 1st and 28th days
(a) Alkanna‐NP at 1st day, (b) Alkanna‐NP at 28th day, (c) Syzygium‐NP at 1st day, (d) Syzygium‐NP at 28th day
Fig. 6.
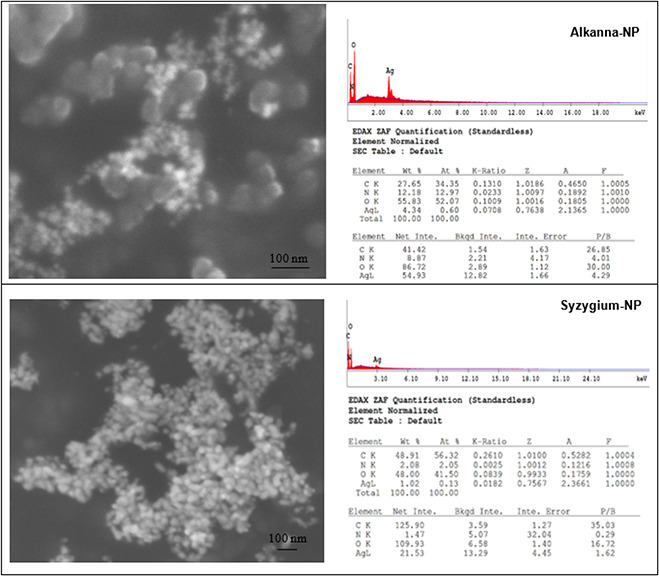
SEM images and EDS results of Alkanna‐NP and Syzygium‐NP
Silver nanoparticles were previously used for the surface sterilisation of plant materials. Pyracantha coccinea stem explants were exposed to green sterilisation with Alkanna‐NP. The percentages of sterile explants were 62.96 and 53.70%, when Alkanna‐NP was implemented for 20 and 40 min, respectively [16]. Rubia tinctorum cell culture extract was also used for the bioreduction of silver ions and its colloidal solution was utilised for the surface sterilisation of Rosmarinus officinali s stem explants. The surface sterilisation percentages of stem explants were varied between 40 and 96.67% [15]. In both studies, 1‐day‐old NP samples were used. In this study, surface sterilisation percentages of all seeds were 100% at day‐0 and first day.
4 Conclusion
Nanoparticles have a wide range of applications, especially in biotechnology, and have created a new and rapidly growing multidisciplinary area called nanobiotechnology. Among other metals, such as gold, zinc and copper, silver is the source of most popular nanoparticles. Silver nanoparticles are mostly used in medical applications because of their low toxicity towards human and eco‐friendly characteristics. In this study, silver nanoparticle colloidal solutions were used successfully in plant biotechnology as surface sterilisation agent for Lamiaceae plants’ seeds. Moreover, the seeds germinated at high percentages and formed healthy Lamiaceae plantlets. It was shown that silver nanoparticles can be used as surface sterilisation agents. These studies about silver nanoparticles associate plant biotechnology and nanotechnology for better, more practical and beneficial applications.
5 References
- 1. Rudramurthy G.R. Swamy M.K. Sinniah U.R. et al.: ‘Nanoparticles: alternatives against drug‐resistant pathogenic microbes’, Molecules, 2016, 21, p. 836, doi:10.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yah C.S. Simate G.S.: ‘Nanoparticles as potential new generation broad spectrum antimicrobial agents’, DARU J. Pharm. Sci., 2015, 23, p. 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pal S. Tak Y.K. Song J.M.: ‘Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram‐negative bacterium Escherichia coli ’, Appl. Environ. Microbiol., 2007, 73, (6), pp. 1712 –1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iravani S. Korbekandi H. Mirmohammadi S.V. et al.: ‘Synthesis of silver nanoparticles: chemical, physical and biological methods’, Res. Pharm. Sci., 2014, 9, (6), pp. 385 –406 [PMC free article] [PubMed] [Google Scholar]
- 5. Cao H.: ‘Toward selectively toxic silver nanoparticles’, in Cao H. (Ed.): ‘Silver nanoparticles for antibacterial devices: biocompatibility and toxicity’, Chapter 12 (RCR Press, Taylor & Francis Group, Boca Raton, 2017), ISBN 9781498725323 [Google Scholar]
- 6. Gorth D.J. Rand D.M. Webster T.J.: ‘Silver nanoparticle toxicity in drosophila: size does matter’, Int. J. Nanomed., 2011, 6, pp. 343 –350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kittler S. Greulich C. Diendorf J. et al.: ‘Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions’, Chem. Mater., 2010, 22, (16), pp. 4548 –4554 [Google Scholar]
- 8. Asghari S. Johari S.A. Lee J.H. et al.: ‘Toxicity of various silver nanoparticles compared to silver ions in daphnia magna ’, J. Nanobiotechnol., 2012, 10, (14), doi: 10.1186/1477‐3155‐10‐14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El‐Gharbaouia A. Beníteza G. González‐Tejeroa M.R. et al.: ‘Comparison of Lamiaceae medicinal uses in eastern Morocco and eastern Andalusia and in Ibn al‐Baytar's compendium of simple medicaments (13th century CE)’, J. Ethnopharm., 2017, 202, pp. 208 –224 [DOI] [PubMed] [Google Scholar]
- 10. Milevskaya V.V. Temerdashev Z.A. Butyl'skaya T.S. et al.: ‘Determination of phenolic compounds in medicinal plants from the Lamiaceae family’, J. Anal. Chem., 2017, 72, (3), pp. 342 –348 [Google Scholar]
- 11. Waller S.B. Cleff M.B. Serra E.F. et al.: ‘Plants from Lamiaceae family as source of antifungal molecules in humane and veterinary medicine’, Microb. Pathog., 2017, 104, pp. 232 –237 [DOI] [PubMed] [Google Scholar]
- 12. Çölgeçen H. Koca U. Toker G.: ‘Influence of different sterilization methods on callus initiation and production of pigmented callus in Arnebia densiflora Ledeb.’, Turk. J. Biol., 2011, 35, pp. 513 –520 [Google Scholar]
- 13. Mbah E.I. Wakil S.M.: ‘Elimination of bacteria from in vitro yam tissue cultures using antibiotics’, J. Plant Pathol., 2012, 94, (1), pp. 53 –58 [Google Scholar]
- 14. Tran Q.H. Nguyen V.Q. Le A.‐T.: ‘Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives’, Adv. Nat. Sci., Nanosci. Nanotechnol., 2013, 4, p. 033001 (20 pp) [Google Scholar]
- 15. Nartop P.: ‘Green sterilization of Rosmarinus officinalis L. stem surfaces with silver nanoparticles synthesized using Rubia tinctorum L. cell culture cxtracts’, Iran. J. Sci. Tech. Trans. A, Sci., 2016a, pp. 1 –4 (in press) [Google Scholar]
- 16. Nartop P.: ‘Use of biosynthetic silver nanoparticles in surface sterilization of Pyracantha coccinea stem explants’, Pamukkale Univ. Muh. Bilim. Derg., PAJES–04809, 2016, 23, (6), pp. 759 –761 [Google Scholar]
- 17. Spinoso‐Castillo J.L. Chavez‐Santoscoy R.A. Bogdanchikova N. et al.: ‘Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system’, Plant Cell Tiss. Organ Cult., 2017, 129, (2), pp. 195 –207 [Google Scholar]
- 18. Fazal H. Abbasi B.H. Ahmad N. et al.: ‘Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L.’, App. Biochem. Biotechn., 2016, 180, (6), pp. 1076 –1092 [DOI] [PubMed] [Google Scholar]
- 19. Bhat P. Bhat A: ‘Silver nanoparticles for the enhancement of accumulation of capsaicin in suspension culture of Capsicum sp.’, J. Exp. Sci., 2016, 7, (2), pp. 1 –6 [Google Scholar]
- 20. Ghanati F. Bakhtiarian S.: ‘Changes of natural compounds of Artemisia annua L. by methyl jasmonate and silver nanoparticles’, Adv. Environ. Biol., 2016, 7, (9), pp. 2251 –2258 [Google Scholar]
- 21. Zaka M. Abbasi B.H.: ‘Effects of bimetallic nanoparticles on seed germination frequency and biochemical characterisation of Eruca sativa ’, IET Nanobiotechn., 2016, 11, (3), pp. 255 –260, doi: 10.1049/iet‐nbt.2016.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deepthi S. Satheeshkumar K.: ‘Enhanced camptothecin production induced by elicitors in the cell suspension cultures of Ophiorrhiza mungos Linn.’, Plant Cell Tiss. Organ Cult., 2016, 124, (3), pp. 483 –493 [Google Scholar]
- 23. Meier P. Hotti H. Rischer H.: ‘Elicitation of furanocoumarins in poison hemlock (Conium maculatum L.) cell culture’, Plant Cell Tiss. Organ Cult., 2015, 123, (3), pp. 443 –443 [Google Scholar]
- 24. Lloyd G.B. McCown B.H.: ‘Commercial‐feasible micropropagation of mountain laurel‐Kalmia latifolia by use of shoot‐ tip culture’, Proc. Int. Plant Prop. Soc., 1980, 30, pp. 421 –427 [Google Scholar]
- 25. Kumar V. Singh D.K. Mohan S. et al.: ‘Green synthesis of silver nanoparticle for the selective and sensitive colorimetric detection of mercury (II) ion’, J. Photochem. Photobiol. B, Biol., 2017, 168, pp. 67 –77 [DOI] [PubMed] [Google Scholar]
- 26. Sinha S.N. Paul D.: ‘Eco‐friendly green synthesis and spectrophotometric characterization of silver nanoparticles synthesized using some common Indian spices’, Int. J. Green Herb. Chem., 2014, 3, (2), pp. 401 –408 [Google Scholar]


