Abstract
Breast cancer accounts for the first highest mortality rate in India and second in world. Though current treatment strategies are effectively killing cancer cells, they also end in causing severe side effects and drug resistance. Curcumin is a nutraceutical with multipotent activity but its insolubility in water limits its therapeutic potential as an anti‐cancer drug. The hydrophilicity of curcumin could be increased by nanoformulation or changing its functional groups. In this study, curcumin is loaded on mesoporous silica nanoparticle and its anti‐cancer activity is elucidated with MCF‐7 cell death. Structural characteristics of Mobil Composition of Matter ‐ 41(MCM‐41) as determined by high‐resolution transmission electron microscopy (HR‐TEM) shows that MCM‐41 size ranges from 100 to 200 nm diameters with pore size 2–10 nm for drug adsorption. The authors found 80–90% of curcumin is loaded on MCM‐41 and curcumin is released efficiently at pH 3.0. The 50 µM curcumin‐loaded MCM‐41 induced 50% mortality of MCF‐7 cells. Altogether, their results suggested that increased curcumin loading and sustained release from MCM‐41 effectively decreased cell survival of MCF‐7 cells in vitro.
Inspec keywords: cancer, cellular biophysics, nanoparticles, nanomedicine, biomedical materials, polymers, mesoporous materials, transmission electron microscopy, drugs, adsorption
Other keywords: polyethylenimine‐modified curcumin‐loaded mesoporus silica nanoparticle, MCF‐7 cell line, breast cancer, cancer cells, drug resistance, multipotent activity, therapeutic potential, anticancer drug, mesoporous silica nanoparticle, MCF‐7 cell death, high‐resolution transmission electron microscopy, drug adsorption, curcumin‐loaded MCM‐41, nutraceutical curcumin, size 2 nm to 10 nm, size 100 nm to 200 nm
1 Introduction
Breast cancer incidence in India is predicted to be 90,723 in year 2015 which is less when compared with world breast cancer occurrence. However, mortality of breast cancer patients in India is around 50% which is much higher compared with other countries [1]. Till now drugs developed for breast cancer are being targeted for its receptors on cell surface, angiogenesis blockade, histone deacetylase inhibitors and inhibitors for survival pathway. Of these, drugs are being targeted for human epidermal growth factor receptor (EGFR), and human epidermal growth factor receptor (HER2) such as Trastuzumab, Pertuzumab, Lapatinib, Neratinib, and Afatinib are in clinical trials. These drugs are potential anti‐cancer effect and they lead to severe side effects such as nausea, diarrhoea, fatigue, neutropenia, elevate liver enzymes, epistaxis, and conjuctivital disorder [2]. Therefore, there is a need for developing drug which overcomes above side effects.
Curcumin is an anti‐oxidant and polyphenolic compound found in turmeric (curcuma longa) is known for its non‐toxic nature. Curcumin pleotropic effects include anti‐oxidant, anti‐inflammatory, antibiotic, antidiabetic, anti‐cancer, anti‐depressive, and inhibit of reactive oxygen species formation. Curcumin hampers the proliferation of various cell lines including MDA‐MB‐435 and human colateral cancer ‐ 116 (HCT‐116) HCT‐116 and arrest these cells at G1 phase [3]. Curcumin inhibits the cell growth and induced apoptosis in vitro by increasing Bcl‐2‐associated X protein / B‐ cell lymphoma 2 (Bax/Bcl2) ratio [4] and blocks the nuclear factor kappa BNF‐kb [5].
In spite of all these effects, curcumin's low bioavailability, absorption, and digestion decline its eligibility in therapy [6]. In phase I clinical trial, oral consumption of curcumin is found only in the gastrointestinal track but not in other parts of the body. Oral uptake of 8 g of curcumin showed peak plasma curcumin concentration of 2 nm after 1 h. The number of clinical trials is proved that curcumin as potential cancer preventive molecules. However, it is limited by its poor availability in different parts of the human body and inhibits therapeutic potential [7]. The encapsulation of curcumin with drug delivery vehicles such as liposomes, nanoparticles, polymers, emulsion, colloidal suspension, and adjuvant were proven to increase curcumin retention in animal system and also increase its bioavailability [8, 9].
The present paper focuses on mesoporous silica nanoparticles (MCM‐41) as a carrier for delivering curcumin. MCM‐41 has cylindrical channels, tunable pore size of 2–12 nm, high surface area, non‐toxicity, and inertness which make them a better carrier for delivering the curcumin molecules. The shape of these nanoparticles determines its biological activity and distribution in different organelles of the cells [9, 10, 11, 12]. The present paper investigates the characterisation of curcumin loaded with polyethylenimine (PEI)‐coated MCM‐41 (CUR‐MCM‐41P), efficiency of drug loading and release, drug delivery inside the cell, and apoptosis induction in MCF‐7 cell. Improving the therapeutic potential of CUR‐MCM‐41P could be a better curation of breast cancer.
2 Experimental Methodology
2.1 Materials
PEI, curcumin, tetraethyl orthosilicate (TEOS) were purchased from Alfa Aesar (Ward Hill, MA, USA). Iscove's Modified Dulbecco's Media (IMDM) FBS‐ Fetal bovine serum (FBS), and trypsin Ethylenediaminetetraacetic acid (EDTA) were purchased from Gibco BRL (Waltham, MA USA). All tissue wares are purchased from Greiner (Bahlingen, Germany). The chemicals such as cetyl trimethyl ammonium bromide (CTAB), tetra sodium (Na) salt WST‐1, paraformaldehyde (PFA), and 4',6‐diamidino‐2‐phenylindole (DAPI) were purchased from Sigma (Bengaluru, India).
2.2 MCM‐41 synthesis, curcumin loading, and release
For MCM‐41 synthesis, 48 ml of water containing 100 mg of CTAB, 350 µl of 2M NaOH was heated and stirred at 80°C. About 500 µl of 21.6 mM TEOS was added into the mixture and stirred for 2 h. The white precipitate obtained refluxed for CTAB was removed by adding HCl and methanol for 18 h [13]. The precipitate was washed with ethanol, dried, and coated with 0.3% of 10 kDa PEI [14]. The 10 mg of MCM‐41 coated with PEI (MCM‐41P) was kept in a shaker along with 50 µM curcumin in ethanol for 24 h. The unbounded curcumin was washed with ethanol and dried with CUR‐MCM‐41P.
The drug release studies were performed with 4 mg/ml concentration of curcumin loaded with MCM‐41P kept in three different pH ranges of 3, 6, and 7.4 in phosphate buffer saline (PBS). About 200 µl of PBS was collected at every 6 h for up to 48 h and curcumin concentration was measured in fluorescent microplate reader excited at 420 nm and emission and excitation at 495/40 nm [Biotek, Model FLx800, Vermont, USA].
2.3 Characterisation of MCM‐41 and CUR‐MCM‐41P
The synthesised silica nanoparticles were dried and characterised by HR‐TEM, X‐ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR). To determine the structure of MCM‐41 and CUR‐MCM‐41P by using HR‐TEM and these nanoparticles were dissolved in ethanol and loaded on copper grid. A Carl Zeiss microscope operated with HT650 ES1000 W at 100 kV was used for TEM analysis and pore size of MSN measured by using ‘measurement’ and image J software. XRD analysis was done in X‐ray tube 3 kW with copper target, real‐time multiple strip solid‐state detectors was used, K alpha was maintained at 0.001°, and it was scanned fast by D8 Advance ECO XRD System (Bruker, Madison, WI, USA). FTIR measurements of MCM‐41, MCM‐41P, and CUR‐MCM‐41P were analysed by Shimadzu spectrometer (Nishinokyo, Japan) in the ranges of 400–4000 cm−1 in transmission mode.
2.4 Culture condition
Breast adenocarcinoma cell line MCF‐7 were grown in IMDM medium containing 10% FBS with 5% CO2 at 37°C. MCM‐41 and CUR‐MCM‐41P were treated separately with MCF‐7 cells containing serum free media for all the experiments.
2.5 Toxicity analysis of MCM‐41 and curcumin
The MCM‐41 and curcumin toxicity were analysed with WST‐1 [15] MTT [16] assay for MCF‐7 cells in 96 well plate separately. Briefly, 10,000 cells were grown in 96 well plate for 36 h, followed by 500 ng/ml to 1 mg/ml of MCM‐41P and 10–100 µM of curcumin added separately in each well. After 24 h 5 µl WST‐1 and 5 µl MTT reagent was added to each well and incubated for 30 min. followed by read at 450 nm for WST‐1 and 595 nm for MTT assay in a microplate reader.
2.6 Accumulation of nanoparticles in MCF‐7 cells
The accumulation of MCM‐41P in MCF‐7 cell was analysed in confocal laser scanning microscopy (CLSM). MCF‐7 cells were grown in Petri dishes (MetTek, Ashland, MA, USA) and treated with doxorubicin‐coated MCM‐41P (DOX‐MCM‐41P) at different time intervals 20 min, 40 min, 1 h, 2 h, 3 h, and 4 h. Then cells were fixed with 4% PFA followed by DAPI stain and analysed in CLSM (Zeiss LSM 500, Heidelberg, Germany) exited with 405 nm and emission from 580 to 620 nm.
2.7 Curcumin release and MCF‐7 cell viability
Approximately 10,000 MCF‐7 cells were seeded on 96 well plate and incubated at 37°C with 5% CO2 for 36 h. CUR‐MCM‐41P was added to MCF‐7 cells and incubated for 4 h and the used media was replaced with fresh media. This was further incubated for different time intervals such as 3, 7, 15, 24, and 48 h. Followed by 5 µl WST‐1 reagent added to each well and incubated for 30 min and the plate was read at 450 nm in a microplate reader.
2.8 Apoptosis assay
MCF‐7 cells were treated with curcumin, MCM‐41P, and 50 µM CUR‐MCM‐41P for 24 h and percentage of cell death by fluorescence‐activated cell sorting (FACS) analysis. The cells were trypsinised and incubated in 10 µg/ml propidium iodide (PI) for 15 min and apoptotic cells were analysed for PI positive staining.
2.9 Statistical analysis
All results were analysed as the mean±standard error of the mean values. Statistical analysis was approved by using Graph pad prism 5 (Graph pad software, San Diego, CA, USA). A significance level of P value is <0.0001, 95% confidence interval was used and data with control and sample.
3 Results and discussion
3.1 Characterisation of MCM‐41 and CUR‐MCM‐41P
MCM‐41P was synthesised and different concentration of curcumin was loaded in these nanoparticles. These nanoparticles were characterised by HR‐TEM, FTIR, and XRD. The MCM‐41 and CUR‐MCM‐41P were analysed by HR‐TEM (Fig. 1 a) revealed that carrier size ranges from 100 to 200 nm and measured pore size from the instrument software ranges from 4 to 12 nm. The MCM‐41 pores are wide open however; CUR‐MCM‐41P pore closure because of significantly curcumin loaded in MCM‐41. Amorphous nature of MCM‐41 was analysed with XRD, (Fig. 1 b) addition of curcumin and PEI reduces the amorphous nature of MCM‐41 at 20–30 2θ °. FTIR analysis Fig. 1 c revealed that peaks around 500 and 1055 cm−1 represented the presence of silica functional group Si–O–Si and Si–OH. C=O and phenolic –OH group peaks were found in the ranges of 1750 and 3780 cm−1 which indicated that curcumin loaded on MCM‐41. Therefore, significant differences were found in CUR‐MCM‐41P when compared with MCM‐41. The peak of 3050 cm−1 indicated the presence of imine group and –C–H group at peak of 2660 cm−1 which are functional groups of PEI.
Fig. 1.
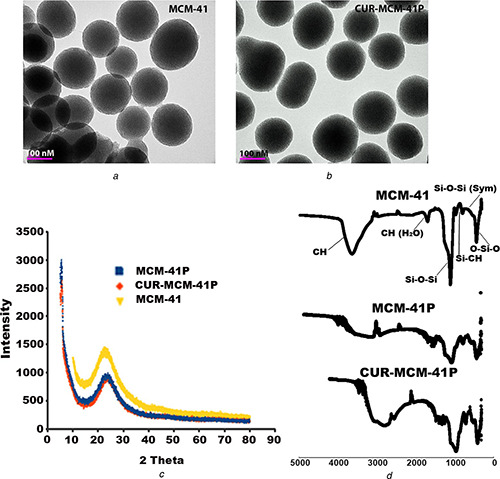
Characterisation of MCM‐41
(a) HR‐TEM images of MCM‐41 and curcumin loaded and PEI coated with MCM‐41, (b) FTIR analysis of MCM‐41 alone and in combination of curcumin and PEI loaded, (c) Functional groups of curcumin, silica, and PEI peaks were observed in XRD
3.2 Curcumin loading and release in MCM‐41
The MCM‐41 and MCM‐41P containing flasks were kept in a shaker separately and added 50 µM curcumin dissolved in 100% ethanol. The amount of curcumin loaded in MCM‐41 and MCM‐41P was calculated from unbound curcumin in a flask at 0 and 24 h after incubation (Fig. 2 a). Curcumin loaded on MCM‐41P was found to be 90%, whereas MCM‐41 showed significantly reduced 10% of curcumin. Therefore, curcumin loading on MCM‐41 has significantly enhanced 80% through PEI coating. The release of curcumin from MCM‐41 in PBS with three different pH ranges 3.0, 6.0, and 7.4 as shown in Fig. 2 b. We found that curcumin is released effectively at pH 3.0 when compared with pH 6 and 7.4. The 65 nM curcumin released in pH 3.0 and 58 M in pH 7.4 at 48 h. Amount of curcumin released depends on degradability of PEI coating with MCM‐41. The secondary amines of PEI are easily protonated with acid, resulting in releasing curcumin effectively at acidic pH [17].
Fig. 2.
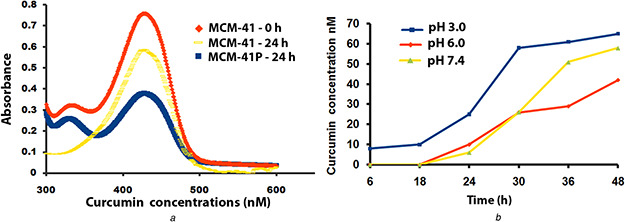
Curcumin loading and released from MCM‐41
(a) Curcumin was loaded in PEI‐coated MCM‐41 nanoparticles at 0 and 24 h after incubation at room temperature, (b) Curcumin released from PEI‐coated MCM‐41 at different pH 3, 6, and 7.4
3.3 In vitro accumulation of DOX‐MCM‐41P in MCF‐7 cells
The DOX‐MCM‐41P was treated with MCF‐7 cells in Petri dishes for different time intervals. The cells were fixed, stained, and documented in CLSM. Fig. 3 showed that DOX‐MCM‐41P started accumulating in MCF‐7 cells from 1 h incubation and after 3 h there is no significant accumulation of DOX compared with 1 h after incubation. Our result was consistent with the previous report [18] that MSN accumulating DOX inside the cell from 30 min to 1 h. Therefore, the accumulation of MCM‐41 starts accumulating from 1 h and gets saturated at 3 h.
Fig. 3.
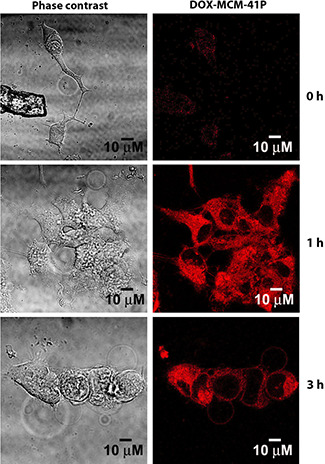
Intracellular accumulation DOX‐loaded MCM‐41
DOX released from MCM‐41 in MCF‐7 cells was observed in fluorescence microscope at consecutive intervals such as 0, 1, and 3 h
3.4 Toxicity analysis of MCM‐41 and curcumin
The different concentrations of curcumin are treated with MCF‐7 cells and analysing the IC50 by MTT assay (Fig. 4 a). About 50 µM concentration of curcumin loaded could induce 50% cell death in MCF‐7 cells. Therefore, these studies suggested that threshold levels of toxicity were depending on the concentration of curcumin in MCF‐7 cells.
Fig. 4.
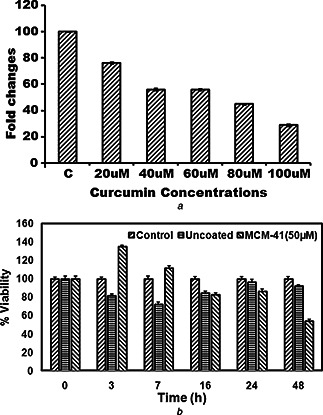
Cell survival of MCF‐7 with curcumin and MCM‐41‐loaded curcumin
(a) IC50 of curcumin against MCF‐7 cells, (b) Different time intervals of curcumin released from MCM‐41 and its effect on MCF‐7 survival
3.5 Curcumin released from MCM‐41 and induction of cell death in MCF‐7 cells
The accumulation of curcumin released in MCF‐7 cells from 50 µM CUR‐MCM‐41P particles is observed in different time intervals (Fig. 4 a). We observed intracellular MCM‐41 concentration increased from 1 h and saturated within 3 h. Therefore, curcumin released from MCM‐41 could effectively increase MCF‐7 cell death. Curcumin released from MCM‐41 inside MCF‐7 cell reduced the viability of MCF‐7 cells (Fig. 4 a). The 50 µM CUR‐MCM‐41P reduced cell viability around 50% at 48 h. The similar results are previously reported [19] that IC50 of curcumin released from curcEmulsome.
3.6 Cell death induced by CUR‐MCM‐41P
PI is used to stain the DNA of apoptotic cells which have damaged cell membrane. PI positive cells analysed with FACS suggest that curcumin (Fig. 5 b) induced 41.6% of cell death, whereas CUR‐MCM‐41P (Fig. 5 d) induced 50.47% of cell death. The sustained release of curcumin from CUR‐MCM‐41P was able to cause 50% cell death at 48 h. The amount of curcumin released from MCM‐41P (58 nm) curcumin dissolved in ethanol. Low solubility, short half‐life, large volume distribution, and low absorption [6] could be the reason for high concentration of free curcumin required to cause 50% of cell death, whereas the same is acquired from 58 nm curcumin released from MCM‐41 inside the cell.
Fig. 5.
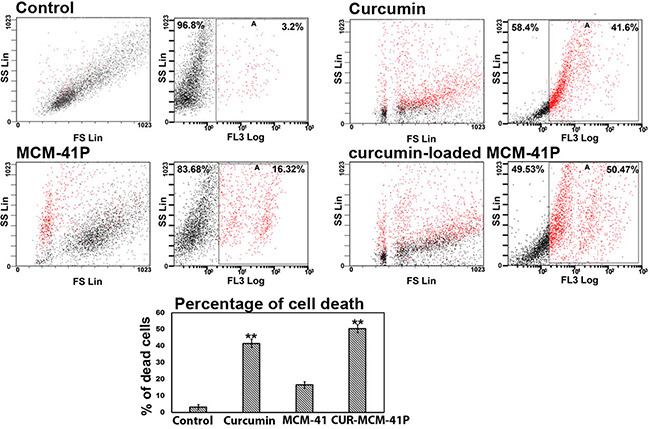
Curcumin‐loaded MCM‐41 altered nuclear morphology and cell death
Altered nuclear morphology is indicated by change in DNA intactness. Curcumin‐induced DNA fragment is shown in arrows
4 Conclusion
Conjugating curcumin with MCM‐41 increased its hydrophilicity and dispersity. Our results suggested that MCM‐41 could enter the cell and release curcumin which results in reduced cell survival and induced apoptosis.
5 Acknowledgment
This study was supported in part by a Grant‐in‐Aid from the Department of Science and Technology – Science and Engineering Research Board (SB/FT/LS‐204/2012) of India.
6 References
- 1. Singh N. Handa T.S. Kumar D. et al.: ‘Mapping of breast cancer research in India: a bibliometric analysis’, Curr. Sci., 2016, 11, (117), pp. 1178 –1183 [Google Scholar]
- 2. Bourdeanu L. ThahanLuu T.: ‘Targeted therapies in breast cancer: implications for advanced oncology practice’, J. Adv. Pract. Oncol., 2014, 5, (4), pp. 246 –260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bandyopadhyay D.: ‘Farmer to pharmacist: curcumin as an anti‐invasive and anti‐metastatic agent for the treatment of cancer’, Front. Chem., 2014, 113, (2), pp. 1 –13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lv Z.D. Liu X.P. Wei‐Jun Zhao W.J.: ‘Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo’, Int. J. Clin. Exp. Pathol., 2014, 7, (6), pp. 2818 –2824 [PMC free article] [PubMed] [Google Scholar]
- 5. Shakibaei M. John T. Tanzil S.G. et al.: ‘Suppression of NF‐kappa B activation by curcumin leads to inhibition of expression of cyclo‐oxygenase‐2 and matrix metalloproteinase‐9 in human articular chondrocytes: Implications for the treatment of osteoarthritis’, Biochem. Pharmacol., 2007, 73, (9), pp. 1434 –1445 [DOI] [PubMed] [Google Scholar]
- 6. Prasad S. Tyagi A.K. Aggarwal B.B.: ‘Recent developments in delivery bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice’, Cancer Res Treat., 2014, 46, (1), pp. 1434 –1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng A.L. Hsu C.H. Lin J.K. et al.: ‘Phase I clinical trial of curcumin, a chemo preventive agent, in patients with high‐risk or pre‐malignant lesions’, Anticancer Res.., 2001, 21, (4B), pp. 2895 –2900 [PubMed] [Google Scholar]
- 8. Anand P.: ‘Bioavailability of curcumin: problems and promises’, Mol. Pharma., 2007, 4, (6), pp. 807 –818 [DOI] [PubMed] [Google Scholar]
- 9. Tsai Y.M. Chien C.F. Lin L.C. et al.: ‘Curcumin and its nano‐formulation: the kinetics of tissue distribution and blood–brain barrier penetration’, Int. J. Pharm., 2011, 416, (1), pp. 331 –338 [DOI] [PubMed] [Google Scholar]
- 10. Meng H. Yang S. Li Z. et al.: ‘Aspect ratio determines the quantity of mesoporous silica nanoparticle uptake by a small GTPase‐dependant macro pinocytosis mechanism’, ACS Nano, 2011, 5, (6), pp. 4434 –4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang X. Li L. Liu T. et al.: ‘The shape effect of mesoporous silica nanoparticles on biodistribution’, Clearance Biocompat. In Vivo, ACS Nano, 2011, 5, (7), pp. 5390 –5399 [DOI] [PubMed] [Google Scholar]
- 12. Tang F. Li L. Chen D.: ‘Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery’, Adv. Mater., 2012, 24, (12), pp. 1504 –1534 [DOI] [PubMed] [Google Scholar]
- 13. Cho Y. Shi R. Borgens R.B. et al.: ‘Functionalized mesoporous silica nanoparticle‐based drug delivery system to rescue acrolein‐mediated cell death’, Nanomedicine, 2008, 3, (4), pp. 507 –518 [DOI] [PubMed] [Google Scholar]
- 14. Balakrishnan V. Wab H.A. Razak K.A. et al.: ‘In vitro evaluation of cytotoxicity of colloidal amorphous silica nanoparticles designed for drug delivery on human cell lines’, J. Nanomater., 2013, (2013), pp. 1 –8 [Google Scholar]
- 15. Reeves A. Vinogradov S.V. Morrissey P. et al.: ‘Curcumin‐encapsulating nanogels as an effective anticancer formulation for intracellular uptake’, Mol. Cell. Pharmacol., 2015, 7, (3), pp. 25 –40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xia T. Kovochich M. Liong M. et al.: ‘Polyethylenimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs’, ACS Nano, 2009, 3, (10), pp. 3273 –3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun C. Tang T. Uludag H. et al.: ‘Molecular dynamics simulations of DNA/PEI complexes: effect of PEI branching and protonation state’, Biophys. J., 2011, 100, (11), pp. 2754 –2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ekkapongpisit M. Giovia A. Follo C. et al.: ‘Biocompatibility, endocytosis, and intracellular trafficking of mesoporous silica and polystyrene nanoparticles in ovarian cancer cells: effects of size and surface charge groups’, Int. J. Nanomed., 2012, 7, pp. 4147 –4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ucisik M.H. Küpcü S. Schuster B. et al.: ‘Characterization of CurcuEmulsomes: nanoformulation for enhanced solubility and delivery of curcumin’, J. Nanobiotechnol., 2013, 11, (37), pp. 1 –13 [DOI] [PMC free article] [PubMed] [Google Scholar]


