Abstract
This study reports the unprecedented, novel and eco‐friendly method for the synthesis of three‐dimensional (3D) copper nanostructure having flower like morphology using leaf extract of Ficus benghalensis. The catalytic activity of copper nanoflowers (CuNFs) was investigated against methylene blue (MB) used as a modal dye pollutant. Scanning electron micrograph evidently designated 3D appearance of nanoflowers within a size range from 250 nm to 2.5 μm. Energy‐dispersive X‐ray spectra showed the presence of copper elements in the nanoflowers. Fourier‐transform infrared spectra clearly demonstrated the presence of biomolecules which is responsible for the synthesis of CuNFs. The catalytic activity of the synthesised CuNFs was monitored by ultraviolet–visible spectroscopy. The MB was degraded by 72% in 85 min on addition of CuNFs and the rate constant (k) was found to be 0.77 × 10−3 s−1. This method adapted for synthesis of CuNFs offers a valuable contribution in the area of nanomaterial synthesis and in water research by suggesting a sustainable and an alternative route for removal of toxic solvents and waste materials.
Inspec keywords: catalysis, dyes, nanostructured materials, nanofabrication, scanning electron microscopy, X‐ray chemical analysis, copper, Fourier transform infrared spectra, visible spectra, ultraviolet spectra, molecular biophysics
Other keywords: catalytic degradation, methylene blue, biosynthesised copper nanoflowers, F. benghalensis leaf extract, three‐dimensional copper nanostructure synthesis, 3D copper nanostructure synthesis, flower like morphology, Ficus benghalensis leaf extract, modal dye pollutant, electron micrograph, 3D appearance, energy‐dispersive X‐ray spectra, copper elements, Fourier‐transform infrared spectra, biomolecules, ultraviolet‐visible spectroscopy, toxic solvent removal, waste materials, size 250 nm to 2.5 mum, Cu
1 Introduction
Nanotechnology is amongst one of the most interesting areas which have been applied for the description of the formation and utilisation of materials with structural features between those of atoms and bulk materials with at least one dimension in nanorange [1]. Nanomaterials illustrate new and enhanced properties on the basis of their size, morphology and distribution [2, 3]. Synthesis of metal nanomaterials is an enormous and expanding area due to their potential applicability in various fields such as healthcare, agriculture, environment and energy. To fulfil the growing demand of nanomaterials in various fields, researchers are using environmental friendly methods using bio‐templates such as microbes [4, 5, 6] and plant extract [7, 8] for the synthesis of nanomaterials. This synthesis process offers a promising route to existing synthetic method due to its eco‐friendliness. Moreover, this process also eradicates the toxicity‐related concerns making these materials to be used for a wide variety of biological applications. Further, plant‐mediated synthesis of nanomaterial is the more advantageous method as it eliminates various cumbrous process involved in the microbial‐mediated synthesis. Copper and copper‐based compounds have been of great importance due to its co‐effectiveness and electrical conductivity which plays a significant role in the modern electronic circuits [9]. Copper nanoparticles have also gained crucial technological interest due to their catalytic and optoelectronic properties [10, 11]. Metallic copper has potential applications in the field of nanodevices [12] and non‐linear optical devices [13]. However, in the recent time, use of copper‐based nanomaterials in catalysis gained significant interest. A number of reports in the literature show copper as an effective catalyst [14].
The waste effluents of textile industry, in particular dye‐containing waste water has been proclaimed as one of the considerable sources of water pollution worldwide [15, 16, 17]. The coloured waste water leads to reduced sunlight penetration and oxygen dissolution which poses a great threat to marine life [18]. The application of various metal nanoparticles for the degradation of organic dyes has proved to be an effective and competent scheme for the degradation of end products, such as aromatic amines [19, 20, 21]. A number of studies for catalytic dye degradation using noble metal nanoparticles such as silver, gold and platinum have been performed [22, 23, 24]. However, use of these nanoparticles is very expensive on an industrial scale. Hence, our research in this group suggests an alternative and cost‐effective approach for removal of these contaminants, which has enormous potential to be used for industrial processes.
Herein, we report a facile, cost‐effective method for synthesis of copper nanoflowers (CuNFs) using leaf extract of Ficus benghalensis which acts as reducing and stabilising agents in the synthesis process. The synthesised nanoflowers were used as a catalyst for the degradation of methylene blue (MB) and the catalytic activity of these nanoflowers was analysed by means of the reaction kinetics.
2 Experimental
2.1 Materials
Leaves of F. benghalensis were acquired from Amity University Uttar Pradesh (AUUP), Noida, India. Copper sulphate pentahydrate (CuSO4. 5H2 O), MB and sodium borohydride (NaBH4) were obtained from Qualigens Fine Chemicals, Mumbai, India.
2.2 Preparation of leaf extract
The leaf extract was prepared by taking 50 g of leaf, thoroughly washed, dried and chopped into fine pieces, mixed with 100 ml of deionised water in a 250 ml Erlenmeyer flask. The mixture was boiled at 80°C for 60 min before decanting. The solution was then cooled and filtered using Whatman paper No. 1. The filtrate obtained was collected and stored at 4°C for further use.
2.3 Biosynthesis of CuNFs
Our group has already developed an unprecedented method for the biosynthesis of zinc oxide nanoflowers [25]. Now, we have synthesised CuNFs using following biological method. Aqueous solution of (10−3 M) copper sulphate pentahydrate (CuSO4. 5H2 O) was prepared and used for the synthesis of CuNFs. The bio‐reduction of cuprous ions was achieved by adding 5 ml leaf extract of F. benghalensis into 50 ml solution of copper sulphate penta‐hydrate (CuSO4. 5H2 O) in a 200 ml Erlenmeyer flask. The solution was allowed to stir for 15 min at 60°C and then 10 ml of F. benghalensis leaf extract was added to the reaction mixture. It was observed that the colour of solution changed from blue to green and then solution was stirred continuously for 60 min. Again 6 ml of leaf extract was added dropwise for 10 min and the colour of solution was finally turned into reddish black due to excitation of surface plasmon resonance (SPR) which indicates the formation of CuNFs. The pH of the reaction solution was maintained at 9 ± 1 using 0.1 M NaOH solution.
2.4 Material characterisation
Ultraviolet–visible (UV–Vis) spectral analysis was performed using UV‐1601 PC Shimadzu Spectrometer, Japan working at a resolution of 1 nm between 400 and 800 nm. Size range and morphology of synthesised CuNFs were determined using field‐emission scanning electron microscopy (Σigma™, Carl Zeiss NTS Ltd, Cambridge, UK) working at an accelerating voltage of 5 kV, coupled with energy‐dispersive X‐ray (EDX) for elemental analysis. FTIR spectra of the biosynthesised CuNFs were recorded in transmission mode using Perkin Elmer 1750 IR Spectrometer, Norwalk, CT. All characterisations were performed using standard operating procedures.
2.5 Catalytic activity of CuNFs
The catalytic activity of the synthesised CuNFs was investigated using MB. The standard quartz cuvette having 1 cm path length was used to perform the reaction of NaBH4 (15 mM) with 1.5 ml MB (100 μM) and absorbance was taken at different time intervals. Another reaction mixture was prepared like above solution with the addition of biosynthesised CuNFs to evaluate the degradation of MB. The reaction mixture was scanned in the range of 400–700 nm by UV–Vis spectroscopy.
3 Results and discussions
3.1 UV–Vis spectroscopy
The formation of CuNFs by using the F. benghalensis leaf extract was determined with the help of UV–Vis spectrometer. Addition of F. benghalensis leaf extract to the cuprous ion complex leads to the reduction of Cu2+ to Cu0 which was monitored by UV–Vis spectrum of the reaction media. The UV spectrum reveals the formation of copper, as shown by the SPR peak occurring at 542 nm (Fig. 1). Deionised water was used as a blank solution. The reaction mixture was scanned in the wavelength range from 400 to 800 nm with a resolution of 1 nm.
Fig. 1.
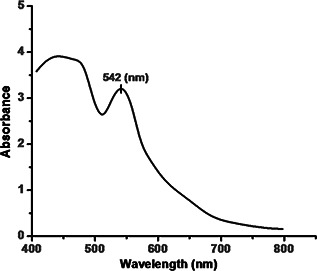
UV–Vis spectra of CuNFs
3.2 Scanning electron microscopy (SEM)
The size and morphology of the CuNFs were analysed using SEM working at an accelerating voltage of 5 kV. SEM micrograph revealed that the synthesised nanostructures have flower like morphology. SEM micrograph evidently designated the three‐dimensional (3D) appearance of nanoflowers with a range from 250 nm to 2.5 μm in size (Figs. 2 a –c). Nanoflowers have nanopetals base (sepals) with about 25 nm in diameter (Fig. 2 e). The average mean size of nanoflowers is found to be 500 nm (Fig. 2 a). The micrographs show three to four nanopetals, but all are combined together and make single nanopetals with 500 nm in diameter (Fig. 2 d) and length of nanopetals is about 150 nm (Fig. 2 e).
Fig. 2.
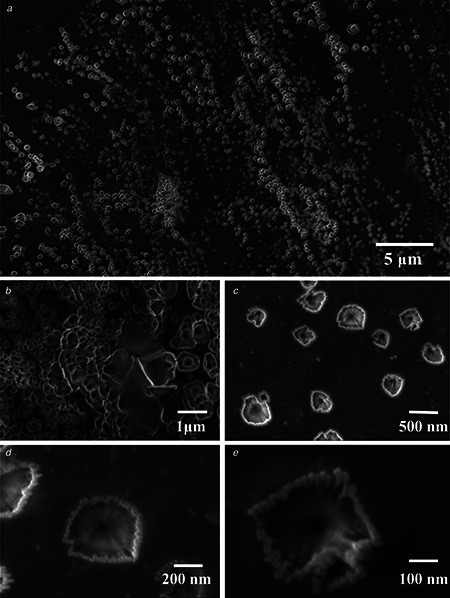
SEM micrograph of CuNFs
a, b CuNFs showing a size range from 250 nm to 2.5 μm
c SEM micrograph reveals no agglomeration
d SEM micrograph showing length of nanopetals
e Micrograph reveals the diameter of the nanopetals base (sepal)
3.3 EDX spectroscopy
Elemental analysis was performed using EDX spectroscopy; CuNFs were coated on to the grid for EDX analysis. The number of X‐ray counts is displayed on the vertical axis while the energy in keV is displayed on the horizontal axis. EDX spectra corroborates the elemental signal of copper (Cu) in the nanoflowers, thus certifying the formation of CuNFs. The additional peaks observed in the spectrum are present due to the sample holder (Fig. 3).
Fig. 3.
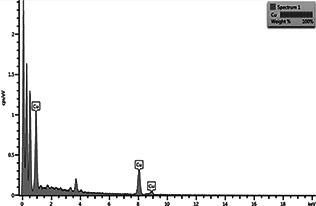
EDX spectra of biosynthesised CuNFs
3.4 Fourier‐transform infrared (FTIR) spectroscopy
FTIR spectra of liquid samples were taken in the frequency range 4000–500 cm−1 to identify the biomolecules responsible for the formation of CuNFs by F. benghalensis leaf extract (Fig. 4). The absorbance bands illustrated from 3645.46 to 3196.05 cm−1 correspond to the stretching vibrations of the –OH group with a presence of N–H (amide) group showing the presence of protein in the sample [26]. An intense peak located at 1633.71 cm−1 and peak occurring at 1566.55 cm−1 correspond to amide I and amide II adsorptions of protein molecule, respectively [26, 27]. An intense peak at 604.32 cm−1 corresponds to stretching vibration of the C–Cl bond. A peak at 574.79 cm−1 corresponds to stretching of the C–Br bond. Previous reports showed that amine group binds protein molecules to the nanoparticles surface [28]. Therefore, the FTIR spectra clearly indicate that the biological molecules which were present in the F. benghalensis, participated in the synthesis of CuNFs.
Fig. 4.
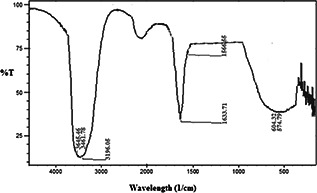
FTIR spectra of the biosynthesised CuNFs using F. benghalensis leaf extract
3.5 Catalytic reduction of MB
The catalytic activity of the biologically synthesised CuNFs was investigated by the reduction of MB through NaBH4 as a model reaction (Fig. 5 a). The characteristic absorbance of MB was found at λ = 664 nm which was used as reference for the analysis of catalytic degradation. UV–Vis spectra of MB and NaBH4 mixture in the absence of CuNFs showed negligible degradation as shown by the control analysis. However, after addition of CuNFs to MB and NaBH4 mixture, the reductive degradation was observed by UV–Vis spectra at different time intervals as shown in Fig. 5 a. UV–Vis spectra decreases at 664 nm after addition of CuNFs with increase in the reaction time and after 85 min, 72% reduction of MB was observed (Fig. 5 b). In this reduction reaction, NaBH4 concentration is higher than the MB, which causes the complete reduction of MB and the concentration of BH4 − remains stable throughout the reaction [29, 30]. Thus, the catalytic activity of the biosynthesised CuNFs can be determined by the pseudo‐first‐order kinetics with respect to MB [31, 32, 33]. The rate constant (k) was determined from the linear plot of ln(At /A 0) against reduction time in seconds and was estimated to be 0.77 × 10−3 s−1 (Fig. 5 c).
Fig. 5.
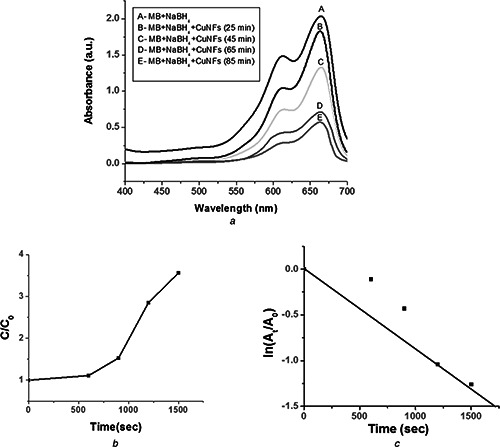
Catalytic activity of the biologically synthesised CuNFs
a UV–Visible spectral changes of MB and NaBH4 mixture in presence of CuNFs
b Catalytic degradation of MB in the presence of CuNFs
c Selected fitting results using pseudo‐first‐order reaction
4 Conclusions
We have developed a novel and biogenic method for the synthesis of CuNFs using leaf extract of F. benghalensis in ambient laboratory conditions. The biosynthesised nanoflowers were characterised using SEM, EDX and FTIR. SEM analysis revealed that CuNFs are 3D in appearance with a size range from 250 nm to 2.5 μm. EDX spectra corroborates the presence of the elemental signal of copper in the synthesised nanoflowers. A strong characteristic peak was observed in UV–Vis measurement at 524 nm depicting the formation of CuNFs. Further, the FTIR analysis of the sample showed that the bio‐macromolecules are responsible for the formation of CuNFs. Synthesised nanoflowers showed excellent catalytic activity towards reduction of MB by NaBH4 in aqueous reaction media. MB was degraded by 72% in 85 min by the catalytic activity of CuNFs and the rate constant (k) was found to be 0.77 × 10−3 s−1. Thus, taking in consideration the remarkable performance of CuNFs, our research suggests that these CuNFs offer an excellent alternative route for removal of pollutants from our environment.
5 Acknowledgments
The authors are grateful to the management of Amity University Uttar Pradesh, Noida, India, for providing financial support for the above work.
6 References
- 1. Saxena A. Tripathi R.M. Zafar F. et al.: ‘Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity’, Mater. Lett., 2012, 67, pp. 91 –94 (doi: 10.1016/j.matlet.2011.09.038) [DOI] [Google Scholar]
- 2. Song J.Y. Kiml B.S.: ‘Rapid biological synthesis of silver nanoparticles using plant leaf extracts’, Bioprocess. Biosyst. Eng., 2009, 32, pp. 79 –84 (doi: 10.1007/s00449-008-0224-6) [DOI] [PubMed] [Google Scholar]
- 3. Nalwa H.S.: ‘Handbook of nanostructured materials and nanotechnology’ (Synthesis and Processing, Academic Press, New York, 2000), vol. l [Google Scholar]
- 4. Zhao G. Stevens S.E. Jr.: ‘Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion’, Biometals, 1998, 11, pp. 27 –32 (doi: 10.1023/A:1009253223055) [DOI] [PubMed] [Google Scholar]
- 5. Tripathi R.M. Gupta R.K. Singh P. et al.: ‘Ultra‐sensitive detection of mercury(II) ions in water sample using gold nanoparticles synthesized by Trichoderma harzianum and their mechanistic approach’, Sens. Actuators B, Chem., 2014, 204, pp. 637 –646 (doi: 10.1016/j.snb.2014.08.015) [DOI] [Google Scholar]
- 6. Tripathi R.M. Gupta R.K. Shrivastav A. et al.: ‘ Trichoderma koningii assisted biogenic synthesis of silver nanoparticles and evaluation of their antibacterial activity’, Adv. Nat. Sci., Nanosci. Nanotechnol., 2013, 4, (3), pp. 035005 –035010 (doi: 10.1088/2043-6262/4/3/035005) [DOI] [Google Scholar]
- 7. Saxena A. Tripathi R.M. Singh R.P.: ‘Biological synthesis of silver nano particles by using onion (Allium cepa) extract and their antibacterial activities’, Dig. J. Nanomater. Bios., 2010, 5, pp. 427 –432 [Google Scholar]
- 8. Prathna T.C. Chandrasekaran N. Raichur A.M. et al.: ‘Biomimetic synthesis of silver nanoparticles by citrus limon (lemon) aqueous extract and theoretical prediction of particle size’, Colloids Surf. B, Biointerfaces, 2011, 82, pp. 152 –159 (doi: 10.1016/j.colsurfb.2010.08.036) [DOI] [PubMed] [Google Scholar]
- 9. Schaper A.K. Hou H. Greiner A. et al.: ‘Copper nanoparticles encapsulated in multi‐shell carbon cages’, Appl. Phys. A, Mater. Sci. Process., 2004, 78, pp. 73 –77 (doi: 10.1007/s00339-003-2199-0) [DOI] [Google Scholar]
- 10. Dhas N.A. Raj C.P. Gedanken A.: ‘Synthesis, characterization and properties of metallic copper nanoparticles’, Chem. Mater., 1998, 10, pp. 1446 –1452 (doi: 10.1021/cm9708269) [DOI] [Google Scholar]
- 11. Huang H.H. Yan F.Q. Kek Y.M. et al.: ‘Synthesis, characterization and nonlinear optical properties of copper nanoparticles’, Langmuir, 1997, 13, pp. 172 –175 (doi: 10.1021/la9605495) [DOI] [Google Scholar]
- 12. Pergolese B. Muniz M.M. Bigotto A.: ‘Surface enhanced raman scattering investigation of the adsorption of 2‐mercaptobenzoxazole on smooth copper surfaces doped with silver colloidal nanoparticles’, J. Phys. Chem., 2006, 110, pp. 9241 –9245 (doi: 10.1021/jp0605698) [DOI] [PubMed] [Google Scholar]
- 13. Flytzanis C.: ‘Nonlinear optics in mesoscopic composite materials’, J. Phys. B, At. Mol. Opt. Phys., 2005, 38, pp. S661 –S679 (doi: 10.1088/0953-4075/38/9/015) [DOI] [Google Scholar]
- 14. Decan M.R. Impellizzeri S. Marin M.L. et al.: ‘Copper nanoparticle heterogeneous catalytic ‘click’ cycloaddition confirmed by single‐molecule spectroscopy’, Nat. Commun., 2014, 5, p. 4612 (doi: 10.1038/ncomms5612) [DOI] [PubMed] [Google Scholar]
- 15. Sau T.K. Pal A. Pal T.: ‘Size regime dependent catalysis by gold nanoparticles for the reduction of eosin’, J. Phys. Chem. B, 2001, 105, pp. 9266 –9272 (doi: 10.1021/jp011420t) [DOI] [Google Scholar]
- 16. Huanga J.H. Zhou C.F. Zenga G.M. et al.: ‘Micellar‐enhanced ultrafiltration of methylene blue from dye wastewater via a polysulfone hollow membrane’, J. Membr. Sci., 2010, 365, pp. 138 –144 (doi: 10.1016/j.memsci.2010.08.052) [DOI] [Google Scholar]
- 17. Martín J.S. Velasco M.G. Heredia J.B. et al.: ‘Novel tannin‐based adsorbent in removing cationic dye (methylene blue) from aqueous solution. Kinetics and equilibrium studies’, J. Hazard. Mater., 2010, 174, pp. 9 –16 (doi: 10.1016/j.jhazmat.2009.09.008) [DOI] [PubMed] [Google Scholar]
- 18. Rauf M.A. Meetani M.A. Hisaindee S.: ‘An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals’, Desalination, 2011, 276, pp. 13 –27 (doi: 10.1016/j.desal.2011.03.071) [DOI] [Google Scholar]
- 19. Janus M. Morawski M.W.: ‘New method of improving photocatalytic activity of commercial Degussa P25 for azo dyes decomposition’, Appl. Catal. B, 2007, 75, pp. 118 –123 (doi: 10.1016/j.apcatb.2007.04.003) [DOI] [Google Scholar]
- 20. Wawrzyniak B. Morawski A.W.: ‘Solar‐light‐induced photocatalytic decomposition of two azo dyes on new TiO2 photocatalyst containing nitrogen’, Appl. Catal. B, 2006, 62, pp. 150 –158 (doi: 10.1016/j.apcatb.2005.07.008) [DOI] [Google Scholar]
- 21. Appleton E.L.: ‘A nickel–iron wall against contaminated ground water’, Environ. Sci. Technol., 1996, 30, (12), pp. 536A –539A (doi: 10.1021/es962526i) [DOI] [PubMed] [Google Scholar]
- 22. Tripathi R.M. Kumar N. Shrivastav A. et al.: ‘Catalytic activity of silver nanoparticles synthesized by Ficus panda leaf extract’, J. Mol. Catal. B, Enzym., 2013, 96, pp. 75 –80 (doi: 10.1016/j.molcatb.2013.06.018) [DOI] [Google Scholar]
- 23. Rajesh R. Kumar S.S. Venkatesan R.: ‘Efficient degradation of azo dyes using Ag and Au nanoparticles stabilized on graphene oxide functionalized with PAMAM dendrimers’, New J. Chem., 2014, 38, pp. 1551 –1558 (doi: 10.1039/c3nj01050c) [DOI] [Google Scholar]
- 24. Pal J. Deb M.K. Deshmukh D.K. et al.: ‘Microwave‐assisted synthesis of platinum nanoparticles and their catalytic degradation of methyl violet in aqueous solution’, Appl. Nanosci., 2014, 4, pp. 61 –65 (doi: 10.1007/s13204-012-0170-0) [DOI] [Google Scholar]
- 25. Tripathi R.M. Bhadwal A.S. Gupta R.K. et al.: ‘ZnO nanoflowers: novel biogenic synthesis and enhanced photocatalytic activity’, J. Photochem. Photobiol. B, 2014, 141, pp. 288 –295 (doi: 10.1016/j.jphotobiol.2014.10.001) [DOI] [PubMed] [Google Scholar]
- 26. Tripathi R.M. Bhadwal A.S. Singh P. et al.: ‘Mechanistic aspects of biogenic synthesis of CdS nanoparticles using Bacillus licheniformis ’, Adv. Nat. Sci., Nanosci. Nanotechnol., 2014, 5, pp. 025006 –025010 (doi: 10.1088/2043-6262/5/2/025006) [DOI] [Google Scholar]
- 27. Bao H. Hao N. Yang Y. et al.: ‘Biosynthesis of biocompatible cadmium telluride quantum dots using yeast cells’, Nano Res., 2010, 3, pp. 481 –489 (doi: 10.1007/s12274-010-0008-6) [DOI] [Google Scholar]
- 28. Sanghi R. Verma P.: ‘A facile green extracellular biosynthesis of CdS nanoparticles by immobilized fungus’, Chem. Eng. J., 2009, 155, pp. 886 –891 (doi: 10.1016/j.cej.2009.08.006) [DOI] [Google Scholar]
- 29. Li J. Zhu J. Liu X.: ‘Ultrafine silver nanoparticles obtained from ethylene glycol at room temperature: catalyzed by tungstate ions’, Dalton Trans., 2014, 43, pp. 132 –137 (doi: 10.1039/C3DT52242C) [DOI] [PubMed] [Google Scholar]
- 30. Tripathi R.M. Gupta R.K. Bhadwal A.S. et al.: ‘Fungal biomolecules assisted biosynthesis of Au–Ag alloy nanoparticles and evaluation of their catalytic property’, IET Nanobiotechnol., 2015, 9, (4), pp. 178 –183 (doi: 10.1049/iet-nbt.2014.0043) [DOI] [PubMed] [Google Scholar]
- 31. Yao Y. Li G. Ciston S. et al.: ‘Photoreactive TiO2/carbon nanotube composites: synthesis and reactivity’, Environ. Sci. Technol., 2008, 42, (13), pp. 4952 –4957 (doi: 10.1021/es800191n) [DOI] [PubMed] [Google Scholar]
- 32. Tripathi R.M. Kumar N. Bhadwal A.S. et al.: ‘Facile and rapid biomimetic approach for synthesis of Hap nanofibers and evaluation of their photocatalytic activity’, Mater. Lett., 2014, 140, pp. 64 –67 (doi: 10.1016/j.matlet.2014.10.149) [DOI] [Google Scholar]
- 33. Bhadwal A.S. Tripathi R.M. Gupta R.K. et al.: ‘Biogenic synthesis and photocatalytic activity of CdS nanoparticles’, RSC Adv., 2014, 4, pp. 9484 –9490 (doi: 10.1039/c3ra46221h) [DOI] [Google Scholar]


