Abstract
Nanobiotechnology is one of the emerging fields and its interventions in agriculture is been attracting the scientific community. Herein, the authors first to report on control of groundnut bruchid (Caryedon serratus O.) using nanoscale zinc oxide (ZnONPs) particles and nanoscale chitosan (CNPs) particles‐based Azadirachtin formulations. ZnONPs and CNPs were prepared using sol–gel and ion tropic gelation techniques, respectively. Neem seed kernel extract (NSKE) 5% and Neem oil (3000 and 1000 ppm) were encapsulated using the prepared nanoscale materials and characterised using the techniques such as dynamic light scattering, high‐resolution transmission electron microscopy. Spherical‐shaped nanoparticles were formed after encapsulation with the required bio‐materials (ZnONPs 33.1 nm; CNPs 78.8 nm; neem oil encapsulated (3000 ppm) ZnONPs 182.9 nm; NSKE encapsulated ZnONPs 84.9 nm) and observed that the particles are stable (52.3 mV for ZnONPs, −36.2 mV for CNPs, −43.0 mV for neem oil encapsulated (3000 ppm) ZnONPs and −39.4 mV for NSKE encapsulated ZnONPs). NSKE encapsulated CNPs were able to contain groundnut bruchid up to 180 days with 54.61% weight loss compared to other formulations tested. Thus biomaterial encapsulated nanoscale material formulations are proved to be effective in controlling stored grain pests to reduce huge economic losses.
Inspec keywords: nanobiotechnology, agricultural products, toxicology, agrochemicals, food safety, sol‐gel processing, food preservation, agriculture, II‐VI semiconductors, storage, nanoparticles, transmission electron microscopy, encapsulation, nanofabrication, zinc compounds, wide band gap semiconductors, food processing industry, light scattering, materials preparation, pest control, nanocomposites
Other keywords: voltage ‐36.2 mV, voltage ‐43.0 mV, voltage ‐39.4 mV, voltage 52.3 mV, size 84.9 nm, size 182.9 nm, size 78.8 nm, size 33.1 nm, NSKE, neem seed kernel extract, caryedon serratus O., CNPs, bio‐materials, nanoscale materials, nanoparticle, encapsulation, spherical‐shaped nanoparticles, high‐resolution transmission electron microscopy, neem oil, ion tropic gelation techniques, sol–gel, nanoscale chitosan particles, nanoscale zinc oxide particles, scientific community, groundnut bruchid, Azadirachtin formulations, biomaterial encapsulated nanoscale material formulations
1 Introduction
Groundnut (Arachishypogaea L.) is a leguminous oilseed crop cultivated in the semi‐arid and subtropical regions of the world, grown in more than 100 countries on six continents spanning an area of nearly 24.6 m ha, with a production of 41.3 m.t. and productivity of 1676 kg ha−1 during 2016. Developing countries in Asia, Africa, and South America account for over 97% of world groundnut area and 95% of total production. However, the productivity of Asia (2217 kg ha−1) and Africa (929 kg ha−1) is very poor as compared to America (3632 kg ha−1). Groundnut is stored as unshelled pods and as kernels for different uses. Both forms are vulnerable to be attacked by a number of insect pests, rodents, atoxigenic spells after harvest. Out of 100 species of insect pests attacking the stored groundnut, pod bruchid Caryedon serratus (Olivier) is a major cosmopolitan pest of economic importance [1]. The groundnut bruchid, C. serratus is the only pest which infests both pods and kernels of groundnut, causing heavy losses in quality and quantity. This pest caused 20% loss in a period of 5 months of storage of groundnut pods in Andhra Pradesh [2], the extent of damage was higher as recorded by Kumari et al. at 77.1% in pods and 67.8% in kernels [3]. However, some of the insect pests do not breed on the pods but their presence in the store becomes harmful as they generate debris, noxious smell and general contamination such as segments, hair debris, excreta and other waste products. The increasing public concern over chemical pesticide safety and possible damage to the environment has resulted in increasing attention being given to natural products majorly, plant origin for the control of storage pests, globally.
Nanotechnology (a set of technologies which deals with the objects, systems with less than 100 nm in at least one dimension) plays a major role in insect pest management in agriculture as it can provide enhanced surface reactivity with target coupled with controlled release of the chemicals. Many researchers have showed potential applications of nanoparticles (NPs) including as bio stimulants [4] and as agents for controlling different microbial populations [5, 6]. The present work focuses on the preparation of ZnO NPs and chitosan NPs then encapsulating the neem oil and neem seed kernel extract (NSKE) to produce nano encapsulated formulations to control the groundnut bruchid in storage condition.
2 Materials and methods
Nanotechnology is been used in an array of field and agriculture has no exception. Agricultural productivity enhancement is one of the important issues to be addressed with the advanced technologies and nanotechnologies have emerged as better options for the same. Nanobiotechnology, one of the nanotechnologies, could have the potential to address some of the important problems in pest management including stored grain pest management. So far nanoscale silver and composite materials of nanosilver were used to develop some nanoscale fungicides and pesticides. However, reports available on antimicrobial activity of nanoscale zinc oxide, nanoscale chitosan and composites prepared thereby. These materials are claimed to exhibit slow release functionality with enhanced reactivity which arises due to their reduced size. Therefore, in this study, we prepared materials of nanoscale zinc oxide, neem oil encapsulated nanoscale zinc oxide, NSKE coated nanoscale zinc oxide and nanoscale chitosan, NSKE loaded chitosan to evaluate their efficacy against bruchids in groundnut.
The experiment was conducted at Institute Frontier Technology (IFT), RARS, Tirupati for the synthesis of nanoscale encapsulated ZnO and chitosan with neem oil and NSKE and rearing of test culture and imposition of treatments during 2015–2016.
2.1 Synthesis of nanoscale zinc oxide particles
Nanoscale zinc oxide particles were prepared using oxalate decomposition method [7]. Equimolar (0.2 M) concentrations of zinc acetate and oxalic acid were mixed thoroughly using magnetic stirrer for 3 h and filtered using Whattman no. 1. Then the collected mixture was dried at 100°C for 24 h and calcined.
2.2 Nanoscale zinc oxide encapsulated neem oil
0.1 gm of nanoscale zinc oxide was thoroughly mixed in 100 ml of distilled water using magnetic stirrer for 30 min. Then add the desired concentration (3000 and 1000 ppm) of neem oil and stirred for 1 h at 60°C and cooled to room temperature and the formulation used for further experimentation.
2.3 Synthesis of chitosan NPs
Chitosan NPs were prepared by ion tropic gelation technique and was first reported in [8] and has been widely examined and developed [9, 10]. The mechanism of chitosan NPs formation is based on electrostatic interaction between amine group of chitosan and negatively charge group of polyanion such as tripolyphosphate (TPP) [11, 12]. This technique offers a simple and mild preparation method in the aqueous environment.
Initially, Chitosan solution 5 mg/mL was prepared by dissolving the polymer in 1% (v/v) acetic acid aqueous solution for 0.5 h under magnetic stirring. The pH of the solution was adjusted to 5.0–6.0 using 1 mol/L NaOH. The chitosan solution was then stirred for 0.5 h at room temperature. Finally, sodium TPP, the counter ion, was dissolved in pure water to prepare a 1 mg/mL solution, and added to the chitosan solution under mild magnetic stirring leads to formation of chitosan NPs due to inter and intra molecular interaction between chitosan and sodium TPP. The NP solution was centrifuged at 18,000 rpm and 4°C for 30 min, after which the NPs at the bottom were collected, extensively washed with water to remove the TPP and the acetic acid. The size and surface charge of particles can be modified by varying the degree of deacetylation and ratio of chitosan and stabiliser [13].
2.4 Dynamic light scattering spectroscopy (DLS for size and zeta potential measurements)
DLS was performed using a particle sizing system (Horiba Nanopartica SZ‐100) at a wavelength of 633 nm from a 4.0 mW, solid‐state He–Ne laser at a scattering angle of 170° [14]. Intensity average, volume average, and number average diameters were calculated from the inbuilt software.
2.5 High‐resolution transmission electron microscopic (HRTEM) measurements
The prepared nanoscale materials were characterised by using the technique, HRTEM (JEOL 3010, Jeol Ltd., Peabody, MA, USA) for surface morphological studies including size and shape of the particles. The HRTEM samples were prepared by drop casting the suspensions on carbon‐coated Cu grids [7].
2.6 Rearing of stock insect culture
The infested pods containing the pupae were collected and transferred to clean plastic jars (18 × 14 × 12 cm). The jars were covered with muslin cloth and kept in the laboratory until the adults emerged. Emerging adults were transferred to another clean plastic jars (12 × 10 × 6 cm) and filled with groundnut pods for oviposition. Before feeding to the adults, the groundnut pods were kept in hot air oven at 70°C for 5 min to get rid of any unwanted insect infestations. After 45 days, emerged adults were used as stock culture in further experiments. The adult beetles were sexed as described by Davey [15]. Male and female adult beetles were identified by observing the last visible segments of the abdomen. These cultures were multiplied continuously for regular supply of insects for experimentation studies. The experiment was laid as completely randomised design with three replications and nine treatments, namely, nanoscale ZnO encapsulated neem oil 1000 ppm; nanoscale ZnO encapsulated neem oil 3000 ppm; nanoscale chitosan encapsulated neem oil 1000 ppm; nanoscale chitosan encapsulated neem oil 3000 ppm; nanoscale ZnO encapsulated NSKE; nanoscale chitosan encapsulated NSKE; nanoscale ZnO particles; nanoscale chitosan particles and control.
During experimentation, test material 10 ml was mixed with 2 kg of groundnut pods and on which ten pairs of freshly emerged adults will be released. The plastic containers were covered with muslin cloth. Destructive sampling was done on 100 g of randomly selected pods from each treatment, at 15 intervals and number of eggs laid, number of adults emerged, per cent pod damage by count and weight, per cent weight loss were recorded as described in the above section and was continued till 180 days.
2.7 Adult emergence
Number of eggs deposited were counted in the plastic containers after 30 days (which coincides with adult emergence) number of adult C. serratus are emerging from different treatments were counted and removed at every 15 days and continued up to 180 days. The adults which were counted and removed after every 15 days could be the adults emerging from the eggs deposited by original population as well as the adults emerging from eggs deposited by subsequent residual population. The experiment was terminated after 180 days and the adults collected from different treatments at every 15 days of interval were pooled and were subjected to statistical analysis to find out total number of adults emerging from different treatments.
2.8 Pod damage
A representative sample of 30 groundnut pods were collected from the same cloth bags used at the onset of oviposition experiment and data was collected on number of damaged pods and healthy pods. Weights of both damaged and healthy pods were also recorded for calculating per cent damage by weight with the help of following formulae [16]
2.9 Weight loss
Weight loss was calculated by deducting the final weight of sample at the period of termination of the experiment, i.e. at 180 days after the initiation of the experiment and from initial weight taken during initiation of the experiment and the data was converted into the percentage
W1 is the initial weight of pods, W2 is the final weight of pods.
2.10 Statistical analysis
The oviposition, adult emergence was subjected to square root transformation and per cent pod damage (by count and by weight) and weight loss were transformed into angular values and then subjected to complete randomised design analysis and were presented in Table 1 [17].
Table 1.
Efficacy of nano encapsulated neemoil and NSKE treatments with respect to oviposition, adult emergence of C. serratus in stored groundnut at fortnight intervals up to 6 months. The data on pods per cent pod damage (count and weight) and per cent weight loss
| Treatments | Mean no. of eggsa /180 days | Mean no adultsa /180 days | % pod damage by countb /180 days | % pod damage by weightb /180 days | Weight loss (%)b /180 days |
|---|---|---|---|---|---|
| T1: nano ZnO encapsulated neem oil 1000 ppm |
35.22h (6.02) |
17.67ab (4.29) |
26.54d (31.00) |
41.23de (39.91) |
59.73a (50.60) |
| T2: nano ZnO encapsulated neem oil 3000 ppm |
29.86g (5.56) |
20.71b (4.66) |
24.41d (29.56) |
37.36d (37.63) |
62.47a (52.20) |
| T3: nano chitosan encapsulated neem oil 1000 ppm |
28.06f (5.39) |
16.67ab (4.17) |
17.40c (24.62) |
31.38c (34.02) |
58.07a (49.63) |
| T4: nano chitosan encapsulated neem oil 3000 ppm |
26.02e (5.20) |
16.46ab (4.17) |
26.46d (30.94) |
38.25d (38.20) |
55.02a (47.86) |
| T5: nano ZnO encapsulated NSKE |
17.06d (4.25) |
17.83ab (4.33) |
5.21ab (13.19) |
18.13b (25.19) |
63.67a (52.92) |
| T6: nano chitosan encapsulated NSKE |
12.31b (3.65) |
12.25a (3.62) |
3.82a (11.25) |
10.93a (18.88) |
54.61a (47.66) |
| T7: nano ZnO particles |
10.11a (3.33) |
18.96ab (4.46) |
4.34ab (12.01) |
16.95b (24.31) |
63.21a (52.65) |
| T8: nano chitosan particles |
14.00c (3.88) |
20.25b (4.61) |
5.67b (13.76) |
18.05b (25.14) |
64.05a (53.14) |
| T9: control |
69.97i (8.42) |
46.96c (6.91) |
49.33e (44.60) |
47.34e (43.43) |
83.70b (66.54) |
| SE(m)± | 0.43 | 0.25 | 0.62 | 1.17 | 2.11 |
| CD (5%) | 1.30 | 0.76 | 1.86 | 3.52 | 6.33 |
Means followed by same letters are not significantly different at p < 0.05 by DMRT.
a Values in parentheses are square root transformed value.
b Values in parentheses are angular transformed value.
3 Results and discussion
Nanoscale materials (measured size 1–99 nm in at least one dimension) with their evidence‐based novel properties, continues to fascinate the researchers who are in search of development of agriculturally beneficial formulations for diverse applications including containing the stored grain pests.
3.1 Dynamic light scattering spectroscopy (DLS for size and zeta potential measurements)
DLS technique is used to measure the hydrodynamic diameter of the particles. It is evident from Fig. 1 a that the mean diameter of formed nanoscale ZnO nano particles is 33.1 nm and found to be uniformly distributed. Further, it has been observed that mean size of chitosan nanoscale particles was 78.8 nm (Fig. 1 b), neem oil encapsulated (at 3000 ppm) nanoscale zinc oxide particles were 182.9 nm (Fig. 1 c) and NSKE encapsulated nanoscale zinc oxide particles was 84.9 nm (Fig. 1 d), respectively.
Fig. 1.
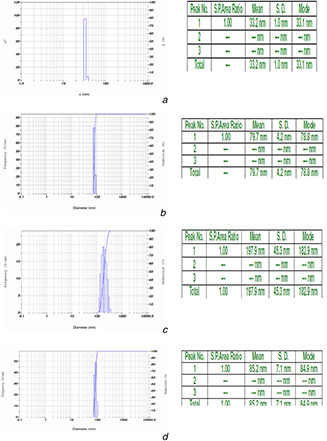
Particle size distribution
(a) Nanoscale zinc oxide particles (mean size 33.1 nm), (b) Chitosan nanoscale particles (mean size 78.8 nm), (c) Neem oil encapsulated (at 3000 ppm) nanoscale zinc oxide particles (mean size 182.9 nm), (d) NSKE encapsulated nanoscale zinc oxide particles (mean size 84.9 nm)
The measured zeta potential was found to be 52.3 mV for zinc oxide particles (Fig. 2 a), −36.2 mV recorded for Chitosan nanoscale particles (Fig. 2 b), the same was found to be −43.0 mV for neem oil encapsulated (at 3000 ppm) nanoscale zinc oxide particles (Fig. 2 c) and −39.4 mV for NSKE encapsulated nanoscale zinc oxide particles (Fig. 2 d).
Fig. 2.
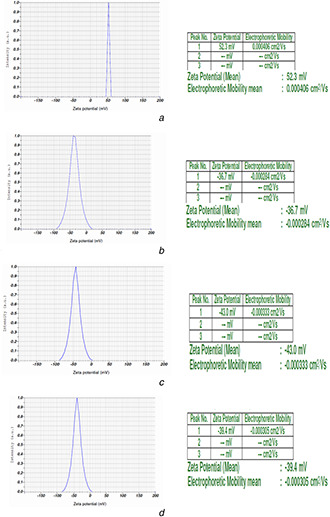
Zeta potential
(a) Nanoscale zinc oxide particles (mean zeta potential 52.3 mV), (b) Chitosan nanoscale particles (mean zeta potential −36.2 mV), (c) Neem oil encapsulated (at 3000 ppm) nanoscale zinc oxide particles (mean zeta potential −43.0 mV), (d) NSKE encapsulated nanoscale zinc oxide particles (mean zeta potential −39.4 mV)
3.2 HRTEM measurements
HRTEM micrographs of prepared chitosan NPs showed relatively spherical shape with bushy nature with a mean size of 30 nm (Fig. 3 a). Further it has been observed that particles are monodispersed. These results are in correlation with the observations made in DLS technique. Well‐defined NPs (with mean size of 25 nm) of ZnO were observed in Fig. 3 b.
Fig. 3.
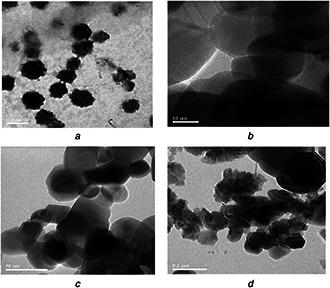
Transmission electronmicroscopic images
(a) Chitosan NPs (mean size 30 nm), (b) ZnO NPs (mean size 25 nm), (c) Neem oil (at 3000 ppm) encapsulated ZnO NPs, (d) NSKE encapsulated ZnO NPs
The data presented in Table 1 revealed that the lowest (10.11) mean number of eggs per 100 g pods was observed in nanoscale ZnO particle formulation which was significantly different from all other treatments. The next best treatments were nanoscale chitosan encapsulated NSKE (12.31) and nanoscale chitosan particles (14.00).
The data on mean number of adult emergence showed that minimum C. serratus (12.5) adults were emerged from nanoscale chitosan encapsulated NSKE followed by nanoscale chitosan encapsulated neem oil at 3000 ppm (16.46). Maximum of 46.96 adults emerged in control.
The results on the per cent pod damage by count proved that minimum per cent pod damage (3.82) was noticed in nanoscale chitosan encapsulated NSKE and other treatments like nanoscale ZnO encapsulated NSKE (5.21) and nanoscale ZnO particles (4.34). In control treatment 49.33% mean pod damage was recorded. The per cent pod damage by weight though showed similar trend but the per cent pod damage values varied. The lowest damage was seen in nanoscale chitosan encapsulated NSKE (10.93) followed by nanoscale ZnO particle (16.95). The nanoscale ZnO encapsulated neem oil treatments at both doses (1000 and 3000 ppm) were on par with each other with respect to pod damage by weight. Whereas in contrast, the increase in the dose from 1000 to 3000 ppm in nanoscale chitosan encapsulated neem oil recorded 31.38 and 38.25, respectively, which was significant with each other and maximum per cent pod damage by weight of 47.34 was noticed in control treatment.
The per cent weight loss data at 180 days showed that there was significant variation between treatments. Relatively lowest per cent weight loss (54.61) was observed in nanoscale chitosan encapsulated NSKE. Maximum weight loss of 83.70 was recorded in untreated check. All the nanoscale formulations showed statistically insignificant data (55–64%).
The results pertaining to the efficacy of nanoscale ZnO and nanoscale chitosan particles revealed that these nanoscale particles were effective at all days of observation with respect to oviposition by C. serratus. These findings are in agreement with Stadler et al. [18], who reported that chitosan nanoscale particles arrested the egg laying of C. maculatus and C. chinensis under lab and semi‐storage conditions. Similarly the reports of Chandra et al. [19] also supported the findings of the present investigation who reported that the chitosan nanoscale particles coated fungal metabolites resulted in lower fecundity.
Sabbour and Abd El‐Aziz [20] also had similar findings in T. castaneum that nanoscale diatomaceous earth strongly suppressed the number of eggs deposited. Adult emergence was strongly suppressed by all the zinc and chitosan nanoscale particles up to 105 days after the treatment and nanoscale chitosan encapsulated NSKE has recorded the lowest no of emerged adults even up to 180 days. These results are best supported by the works of Yang et al. [21] who reported that the nanoscale particles coated with garlic oil then combined with polyethylene glycol using multi dispersion method caused 100% mortality of T. castaneum after 5 months which was mainly due to slow and continuous release of active components from nanoscale particles. Zahir et al. [22] reported that silver nanoscale particles synthesised by using aqueous leaves extracts of Euphorbia prostrate showed insecticidal activity against Sitophilus oryzae adults which also supports the present findings.
4 Conclusion
Nanobiotechnological interventions in agriculture and food science are leading to development of more reliable and cost‐effective methods for securing post‐harvest agricultural produce and their derivatives. In this study, it has been demonstrated that groundnut bruchid (C. serratus) was successfully controlled using the nanoscale materials‐based neem formulations. Among the treatments it has been observed that NSKE encapsulated chitosan NPs showed significant inhibitory effect on groundnut bruchid. This kind of approach to control the stored grain pest is relatively new and could be extended to other pests. Commercial production of the prepared nanoformulations is in progress.
5 Acknowledgment
Authors are thankful to the authorities of Acharya N G Ranga Agricultural University, for providing research facilities at Institute of Frontier Technology, Regional Agricultural Research Station, Tirupati to carryout part of the research work.
6 References
- 1. Singh V. Ansari S.U.: ‘Farmers level survey on insects and mites on stored groundnut in Andhra Pradesh’, Bull. Grain Technol., 1991, 29, (1), pp. 14 –21 [Google Scholar]
- 2. Dick K.M.: ‘Losses caused by insects to groundnuts stored in a warehouse in India’, Trop. Sci., 1987, 27, pp. 65 –75 [Google Scholar]
- 3. Kumari D.A. Vijay S. Sudhir Reddy V. et al.: ‘Quantitative and qualitative losses caused by pod bruchid, Caryedon serratus Olivier (Bruchidae: Coleoptera) in stored groundnut’, Indian J. Plant Prot., 2002, 30, (2), pp. 213 –214 [Google Scholar]
- 4. Subbaiah L.V. Prasad T.N.V.K.V. Krishna T.G. et al.: ‘Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.)’, J. Agric. Food Chem., 2016, 64, (19), pp. 3778 –3788 [DOI] [PubMed] [Google Scholar]
- 5. Prasad T.N.V.K.V. Adam S. Rao P.V. et al.: ‘Size dependent effects of antifungal phytogenic silver nanoparticles on germination, growth and biochemical parameters of rice (Oryza sativa L.), maize (Zea mays L.) and peanut (Arachishypogaea L.)’, IET Nanobiotechnol., 2016, 11, (3), pp. 277 –285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Supraja N. Prasad T.N.V.K.V. David E. et al.: ‘Antimicrobial kinetics of Alstoniascholaris’, Appl. Nanosci., 2016, 6, (5), pp. 779 –787 [Google Scholar]
- 7. Prasad T.N.V.K.V. Sudhakar P. Sreenivasulu Y. et al.: ‘Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut’, J. Plant Nutr., 2016, 35, (6), pp. 905 –927 [Google Scholar]
- 8. Calvo P. Remunan‐Lopez C. Vila‐Jato J.L. et al.: ‘Novel hydrophilic chitosan‐polyethylene oxide nanoparticles as protein carriers’, J. Appl. Polym. Sci., 1997, 63, (1), pp. 125 –132 [Google Scholar]
- 9. Janes K.A. Fresneau M.P. Marazuela A. et al.: ‘Chitosan nanoparticles as delivery systems for doxorubicin’, J. Control Release, 2001, 73, pp. 255 –267 [DOI] [PubMed] [Google Scholar]
- 10. Pan Y. Li Y. Zhao H. et al.: ‘Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo’, Int. J. Pharm., 2002, 249, pp. 139 –147 [DOI] [PubMed] [Google Scholar]
- 11. Bodmeier R. Chen H.G. Paeratakul O.: ‘A novel approach to the oral delivery of micro‐ or nanoparticles’, Pharm. Res., 1989, 6, pp. 413 –417 [DOI] [PubMed] [Google Scholar]
- 12. Xu Y. Du Y.: ‘Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles’, Int. J. Pharm., 2003, 250, (1), pp. 215 –226 [DOI] [PubMed] [Google Scholar]
- 13. Calvo P. Remunan‐Lopez C. Vila‐Jato J.L. et al.: ‘Chitosan and chitosan/ethylene oxide propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines’, Pharm. Res., 1997, 14, pp. 1431 –1436 [DOI] [PubMed] [Google Scholar]
- 14. Takahashi K. Kato H. Saito T. et al.: ‘Precise measurement of the size of nanoscaleparticles by dynamic light scattering with uncertainty analysis’, Part. Part. Syst. Charact., 2008, 25, (1), pp. 31 –38 [Google Scholar]
- 15. Davey P.M.: ‘The groundnut bruchid Caryedon gonagra F’, Bull. Entomol. Res., 1958, 49, pp. 384 –404 [Google Scholar]
- 16. Lal S.R.: ‘Studies on qualitative and quantitative losses in wheat due to insects at farm level storage’, Bull. Grain Technol., 1990, 28, pp. 210 –220 [Google Scholar]
- 17. Snedecor G.W. Cochran W.G.: ‘Statistical methods’ (Oxford and IBH Publishing Co., Culcatta, 1967, 6th edn.), pp. 194 –235 [Google Scholar]
- 18. Stadler T. Buteler M. Weaver D.K.: ‘Novel use of nanostructured alumina as an insecticide’, Pest Manag. Sci., 2010, 66, pp. 577 –579 [DOI] [PubMed] [Google Scholar]
- 19. Chandra J.H. Raj L.F.A.A. Namasivayam S.K.R. et al.: ‘Improved pesticidal activity of fungal metabolite from nomureaerileyi with chitosan nanoparticles’. Proc. of the Int. Conf. on Advanced Nanomaterials and Emerging Engineering Technologies, Chennai, 2013, pp. 387 –390 [Google Scholar]
- 20. Sabbour M. Abd El‐Aziz S.E.: ‘Efficacy of nano‐diatomaceous earth against red flour beetle, Triboliumcastaneum and confused flour beetle, Tribolium confusum (Coleoptera: Tenebrionidae) under laboratory and storage conditions’, Bull. Environ. Pharmacol. Life Sci., 2015, 4, pp. 54 –59 [Google Scholar]
- 21. Yang F.L Li X.G. Zhu F. et al.: ‘Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Triboliumcastaneum (Herbst) (Coleoptera: Tenebrionidae)’, J. Agric. Food Chem., 2009, 57, (21), pp. 10156 –10162 [DOI] [PubMed] [Google Scholar]
- 22. Zahir A.A. Bagavan A. Kamaraj C. et al.: ‘Efficacy of plant‐mediated synthesized silver nanoparticles against Sitophilus oryzae ’, J. Biopest., 2012, 5, pp. 95 –102 [Google Scholar]


