Abstract
Flower‐shaped copper nanoparticles were synthesised by a green and ecofriendly chemical reduction approach using copper sulphate and cytyltrimethal ammonium bromide. The UV–vis spectrophotometer analysis showed maximum absorption at about 552 nm, which is specifically reported for copper nanoparticles. The crystal lattice structure of copper nanoflowers was confirmed by X‐ray diffraction analysis. Further, the transmission electron microscopic studies revealed the flower shape copper nanoparticles in the size range of 100–500 nm. The stability of thus synthesised copper nanoflowers was assessed by zeta potential analysis, which was found to be 35 mV indicating the most stable nature of nanoflowers. The antifungal activity of these copper nanoflowers was evaluated by Kirby–Bauer disk diffusion method against selected common plant pathogenic fungi. It was found that the chemosynthesised copper nanoflowers demonstrated significant inhibitory activity against the plant pathogenic Aspergillus niger, Fusarium moniliforme, F. culmorum, F. oxysporum and F. tricinctum. The maximum antifungal activity was shown against A. niger followed by F. moniliforme, F. oxysporum and F. tricinctum, whereas the minimum activity was reported against F. culmorum. Similarly, the effect of the copper nanoflowers was also evaluated in combination with commercial antifungal agent (ketoconazole), which demonstrated the enhanced activity of antifungal agent.
Inspec keywords: copper, nanoparticles, reduction (chemical), nanofabrication, ultraviolet spectra, visible spectra, crystal structure, spectrochemical analysis, X‐ray diffraction, transmission electron microscopy, electrokinetic effects, nanobiotechnology
Other keywords: flower‐shaped copper nanoparticles, chemical reduction, antifungal agents, plant pathogenic fungi, copper sulphate, cytyltrimethal ammonium bromide, UV–vis spectrophotometer analysis, crystal lattice structure, X‐ray diffraction analysis, transmission electron microscopy, zeta potential analysis, Kirby–Bauer disk diffusion method, size 100 nm to 500 nm, Cu
1 Introduction
Among the various metal nanoparticles, copper nanoparticles have attracted attention of researchers all over the world due to their novel optical, mechanical, catalytic, electrical, thermal and antimicrobial properties; and most important is their low production cost as compared to expensive metals such as gold, silver or palladium [1, 2, 3]. Different physical chemical and biological approaches have been commonly used for the synthesis of copper and copper‐based nanoparticles [4]. Out of these, chemical approaches for the synthesis are found to be comparative convenient over the physical and biological approaches because in case of physical method there is involvement of hazardous radiation and lasers [5, 6]. However, biological synthesis of copper nanoparticles has been always a challenge for researchers due to the major problem of oxidation [2].
Generally, copper and copper‐based nanoparticles are synthesised; however, synthesis of copper nanoflowers was rarely reported. Wu and Chen [7] demonstrated the synthesis of copper nanoparticles by the reduction of cupric chloride with hydrazine in the aqueous cytyltrimethal ammonium bromide (CTAB) solution without giving input of extra inert gases. In this approach, ammonia solution was used to maintain pH and hydrazine as a reducing agent. In another study, copper nanoparticles were synthesised by reducing aqueous copper ions with NaBH4 in alkaline solution [8]. Gawande et al. [9] critically reviewed various chemical approaches for the synthesis of copper and various copper‐based nanoparticles. In addition, they have also discussed about their applications in catalysis. Chakraborty et al. [10] reported the synthesis of copper sulphide nanoparticles using chemical method and studied their antifungal activity against Mucor, Rhizopus, Fusarium oxysporum, Alternaria and Helminthosporium. The results obtained confirmed their potential antifungal activity. Similarly, Kanninen et al. [11], Kathad and Gajera [12] and Park et al. [13] also demonstrated various chemical approaches for the synthesis of copper nanoparticles.
There are few reports available on the synthesis of copper nanoflowers, Virk [14] demonstrated two different chemical approaches for the synthesis of copper nanoflowers‐one was electro‐deposition of copper in polymer and anodic alumina templates and another was CTAB‐assisted hydrothermal method, in both the approaches, there was formation of copper nanoflowers. In another study, Sampath et al. [15] reported the chemical synthesis of jasmine bud‐shaped copper nanoparticles using polyvinylpyrrolidone as a capping agent, l ‐ascorbic acid as a reducing agent and antioxidant agent, isonicotinic acid hydrazide as a reducing agent and water as a solvent at 60–70°C in the presence of air. The resulting jasmine nanobud was having average particle size 6.95 nm. Further, they also reported the potential antibacterial activity of these copper nanobuds against Escherichia coli and Staphylococcus aureus. Ghosh et al. [16] developed a method for the fabrication of hybrid organic–inorganic nanoflowers using calcium or copper ions as the inorganic component and elastin‐like polypeptide as the organic component. Recently, Somturk et al. [17] reported the synthesis of copper ion incorporated horse radish peroxidase‐based hybrid nanoflowers for enhanced catalytic activity and stability. Apart from these, Liao et al. [18] demonstrated the synthesis of copper oxide nanoplatelets and nanoflowers using facile hydrothermal approach. Further, they evaluated the catalytic activity of thus prepared nanoplatelets and nanoflowers, which was found to be comparatively higher than the simple CuO nanostructures.
Copper has been used as a novel biocidal agent for centuries. The ancient Greeks in the time of Hippocrates (400 BC) prescribed copper for pulmonary diseases and also for purification of drinking water. In the 1880s, mixture of copper sulphate, lime and water (Bordeaux mixture) and mixture of copper sulphate and sodium carbonate (Burgundy mixture) were used as potential fungicide all over the world. Apart from these applications, copper and their compounds have been used as effective antibacterial, antifungal and antiviral agents [19]. Unfortunately, bulk copper compounds in higher doses may be hazardous to the aquatic environment and other organisms. Therefore, instead of bulk copper compounds, their nanoparticles can be used to avoid toxicity to environment [4]. Recently, copper nanoparticles have been centre of attraction because for their significant bioactivities including antimicrobial potential. Ingle et al. [4] critically reviewed different broad spectrum bioactivities of copper and copper‐based nanoparticles, which include antibacterial, antifungal, antiviral, antiparasital and anticancerous activities. Das et al. [20] studied the growth inhibiting activity of copper nanoparticles against three bacteria namely S. aureus, Bacillus subtilis and E. coli by disc diffusion method. Similarly, Ramyadevi et al. [21] demonstrated the potential antibacterial activity of copper nanoparticles against Micrococcus luteus, S. aureus, E. coli, Klebsiella pneumoniae and Pseudomonas aeruginosa. They reported that E. coli was the most susceptible bacterium followed by S. aureus, M. luteus and K. pneumoniae, whereas, P. aeruginosa was found to be comparatively resistant. Also, there were some other reports on antibacterial activity of copper‐based nanoparticles, which includes activity against E. coli and B. subtilis [22], E. coli and S. aureus [23, 24], E. coli [25, 26, 27], E. coli, B. subtilis and S. aureus [28], E. coli and M. luteus [29], E. coli and Bacillus megatherium [30], S. aureus, B. subtilis, P. aeruginosa and Salmonella choleraesuis [31] etc.
When compared with antibacterial activity, antifungal activity of copper nanoparticles is least studied specifically against plant pathogenic fungi. According to recent reports, fungal infections possess major health concerns in human beings as well as animals and also infects variety of plants including crop plants which leads to notable loss in yield. The available reports confirmed the antifungal potential of copper nanoparticles. Petranovskii et al. [32] evaluated antifungal activity of copper‐based zeolites against three different fungi such as Cladosporium cladosporoides, Phaeococcomyces chersonesos and Ulocladium chartarum. In another study, Kim et al. [33] reported that copper nanocomposite with SiO2 showed significant activity against fungi like Candida albicans and Penicillium citrinum. Considering the strong antifungal activity of copper nanoparticles, these have been used in textile fabrics by Usha et al. [24] and also demonstrated the activity of copper oxide‐coated fabrics against Aspergillus niger which showed 100% inhibition of fungus within 48 h of incubation. Similarly, in another study, Ramyadevi et al. [21] reported the antifungal activity of copper nanoparticles against A. flavus, A. niger and C. albicans. The maximum activity was reported against C. albicans, while minimum activity was observed against A. flavus. Shende et al. [2] demonstrated antifungal activity of biologically synthesised copper nanoparticles against plant pathogenic fungi including F. culmorum, F. oxysporum and F. graminearum. Recently, Viet et al. [34] proposed the chemical approach for the synthesis of copper nanoparticles using CTAB as reducing agents. They reported the synthesis of nanoparticles in the range of 20–50 nm and further they evaluated their antifungal efficacy against plant pathogenic fungus, Fusarium species recovered from tomato and dragon fruit plants. The results showed that the synthesised copper nanoparticles significantly inhibited (93.98%) the growth of Fusarium at the concentration of 450 ppm in 9‐day incubation. Similarly, in another study, Bramhanwade et al. [35] demonstrated the chemical synthesis of copper nanoparticles and tested their efficacy against various species of Fusarium. Among the three different test Fusaria Fusarium equiseti showed maximum susceptibility (25 mm zone of inhibition) followed by F. oxysporum (20 mm) and F. culmorum (19 mm).
The main aim of the present study was to develop a green, ecofriendly and cost‐effective method for the synthesis of novel copper nanoflowers and evaluation of their efficacy against selected plant pathogenic fungi such as A. niger, F. moniliforme, F. culmorum, F. oxysporum, F. tricinctum etc.
2 Experimental
2.1 Synthesis of copper nanoflowers
Rapid synthesis of copper nanoparticles (nanoflowers) was carried out by reducing aqueous copper ions with reducing reagent in the presence of stabilising agent. 0.09 M CTAB prepared in isopropyl alcohol (IPA) was drop wise added to 0.003 M copper nitrate prepared in IPA under vigorous stirring on magnetic stirrer at room temperature till the colour changed from colourless to dark‐brown. After the colour change, a few drops of l ‐ascorbic acid (1 M) was added to the reaction mixture for the stabilisation of nanoparticles and to prevent agglomeration of the particles.
2.2 Visual detection of copper nanoflowers
The primary detection was made by visual observation. The change in colour of reaction mixture (100 mM copper nitrate in IPA) from colourless to dark‐brown after drop wise addition of CTAB with constant stirring on magnetic stirrer evidences the formation of copper nanoflowers.
2.3 Characterisation of copper nanoflowers
Ultraviolet–visible (UV–vis) spectroscopy analysis: Further, copper nanoflowers were characterised by using UV–vis spectrophotometer (Shimadzu UV‐1700, Japan), for which the dark colour reaction mixture was subjected to optical analysis and the spectra were obtained at the resolution of 1 nm from 200 to 800 nm for each sample.
X‐ray diffraction (XRD) studies : XRD analysis of copper nanoflower was carried out using Phillips PW 1830 instrument operating at a voltage of 40 kV and current of 20 mA with Cu K(α) radiation of 1.54187 nm wavelength. XRD data analysis was done using ISDD software JCPDS.
Transmission electron microscopy (TEM) analysis : The copper nanoflowers were also characterised by TEM (Philips, CM 12) on conventional carbon‐coated copper grids (400 meshes, Plano Gmbh, Germany). A5 μl of sample was taken for the characterisation and three images of each sample were taken for the clarification of the composition.
Zeta potential analysis : The samples of reaction mixture were subjected to zeta potential analysis for the determination of stability of synthesised copper nanoflowers with the help of Malvern Zetasizer instrument (Malvern Zetasizer NanoZS90, UK) using zeta dip cell.
2.4 The test fungi
The test plant pathogenic fungi, namely A. niger (MTCC‐478), F. moniliforme (MTCC‐2088), F. culmorum (MTCC 349), F. oxysporum (MTCC 1755) and F. tricinctum (MTCC‐2080), were procured from Microbial Type Culture Collection Center, Chandigarh, Punjab, India. These cultures were grown on potato dextrose agar (PDA) medium at 28 ± 2°C for 3 days and maintained at 4°C in a refrigerator.
2.5 In vitro evaluation of antifungal activity of copper nanoflowers
The in vitro antifungal activity of copper nanoflowers was evaluated using disc diffusion method proposed by Bauer et al. [36] singly and in combination with commercial antifungal agent. For this, a loop culture of test fungi was inoculated in each 250 mL Erlenmeyer flask containing 100 mL of presterilised and cooled potato dextrose broth and the flasks were then incubated at 28 ± 2°C for 2–3 days in orbital shaking incubator at 150 rpm. After the proper growth of test fungi, 20 µL of spore suspension of each test fungus was spread on to the surface of sterile PDA medium plates with the help of cotton swab (purchased from Hi‐Media Laboratories Pvt. Ltd., Mumbai, India). Then total five discs, three sterile discs (purchased from Hi‐Media Laboratories Pvt. Ltd., Mumbai, India) each containing 20 µL of copper nanoflowers, copper nitrate solution (100 mM) and IPA and two commercial antifungal agent discs (KT50 = ketoconazole 50 µg/disc, purchased from Hi‐Media Laboratories Pvt. Ltd., Mumbai, India) out of which one was impregnated with 20 µL copper nanoflower to study the synergistic effect were placed on the surface of inoculated media plates. After inoculation, all the plates were incubated at 28 ± 2°C for 2–3 days followed and the by measurement of the zones of inhibition. The antimicrobial assays were performed in triplicate.
3 Results and discussion
The present study was aimed to synthesise copper nanoflowers using green and ecofriendly chemical approach, in which the reduction of aqueous copper ions (copper nitrate) was carried using CTAB as reducing agents and l ‐ascorbic acid as stabilising as well as capping agent to prevent agglomeration and precipitation of the nanoparticles. As mentioned earlier, the change in colour of reaction mixture from colourless to dark‐brown (Fig. 1) was reported after the drop wise addition of CTAB solution with constant stirring on magnetic stirrer within 5–10 min; later, a few drops of l ‐ascorbic acid (1 M) was added for the stabilisation of nanoparticles. The appearance of brown colour indicates the synthesis of copper nanoparticles [3]. Umer et al. [3] demonstrated the role of l ‐ascorbic acid in capping and stabilisation of copper nanoparticles synthesised by chemical approach.
Fig. 1.

Visual observation of formation copper nanoflowers: (I) CTAB solution, (II) Copper nitrate solution and (III) Copper nitrate solution after addition of CTAB, i.e. copper nanoflowers
The collective oscillations of conduction electrons at the surface of nanosized materials absorb visible electromagnetic waves and the phenomenon is known as surface plasmon resonance (SPR) [37]. In this context, after visual detection, the reaction mixture was subjected to optical analysis to study the SPR of synthesised copper nanoflowers using UV–vis spectrophotometer. The absorption maxima was reported at 552 nm (Fig. 2) which is specific for copper nanoparticles. Usually, SPR effect is studied to estimate the formation of nanosized metal particles in the solution through UV–vis spectrophotometry analysis [38].
Fig. 2.
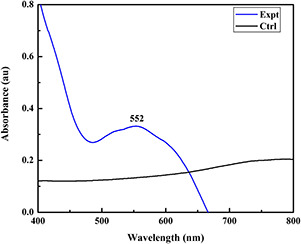
UV–visible spectra of chemically synthesised copper nanoflowers showing peak at 552 nm. (Expt.) = copper nanoflowers and (Ctrl) = copper nitrate
The phase composition of crystal structures of the synthesised copper nanoflowers was analysed by XRD (Fig. 3). The diffraction data presented inferred the formation of pure crystalline metallic phase copper nanoparticles (nanoflowers) with face centred cubic (FCC) structures having characteristic peaks indexed to (111), (200) and (220) at corresponding 2θ value of 43.56°, 50.70° and 74.28°, respectively. The experimental and standard diffraction angles for copper nanoparticles obtained by XRD have been presented in Table 1 [3]. These results were found in a close relation with many previous reports [2, 13, 15, 28]. In the present study, experiments were performed at room temperature in constant stirring condition on magnetic stirrer. According to Sampath et al. [15] moderate temperature results in intensifying and sharpening of the diffraction peaks which affects quality of nanoparticles (nanobuds) formed. They also studied the effect of different temperatures and precursor salts used in chemical reduction method and concluded that changing treatment temperatures and precursor salts, the size of copper nanobuds can be controlled.
Fig. 3.
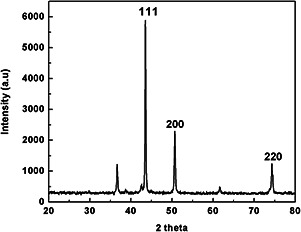
XRD pattern of copper nanoflowers showing the FCC crystallite structure
Table 1.
Experimental and standard diffraction angles of copper nanoparticles (nanoflowers)
| Experimental diffraction angle degree (2θ) | Standard 2θ degree angle in JCPD: 04‐0836 | diffraction hkl (planes) angle 2θ in copper |
|---|---|---|
| 43.56 | 43.297 | (111) |
| 50.70 | 50.433 | (200) |
| 74.28 | 74.130 | (220) |
TEM analysis was performed to determine the size and shape of synthesised nanoparticles. TEM micrographs for copper nanoparticles synthesised from copper nitrate are illustrated in Fig. 4. These micrographs confirmed that the synthesised copper nanoparticles were flower shaped with well‐defined morphology. The diameter of these nanoparticles was found to be between 100 and 500 nm. The size of nanoflowers reported here is quite large, but as proposed by Sampath et al. [15], the size can be manipulated by altering treatment temperature and precursor salt. Further, the stability and charge on the surface of synthesised copper nanoflowers were assessed by analysis of zeta potential. Zeta potential is a measure of the magnitude of the repulsion or attraction between particles. Colloidal nanoparticles develop surface charges in aqueous solutions and dispersion is electrostatically stabilised by inter‐particle repulsion due to their ionic characteristics. Surface potential of nanoparticles is responsible for their behaviour [39, 40]. The zeta potential reported for these copper nanoflowers was found to be 35 mV which indicates that the synthesised copper nanoflowers are highly stable (Fig. 5). These results showed resemblance with results obtained by Somturk et al. [17], whereas, zeta potentials may differ depending upon the chemicals used in the synthesis.
Fig. 4.
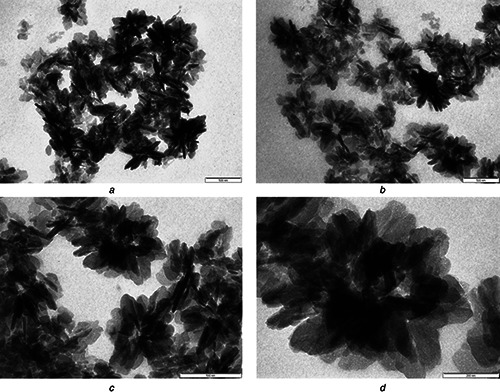
TEM micrographs of copper nanoflowers having size range of 100–500 nm at various magnification
(a) , (b) and, (c) At 500 nm, (d) At 200 nm
Fig. 5.
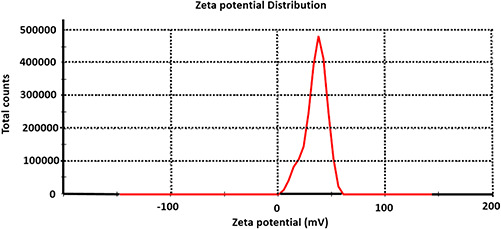
Zeta potential analysis of chemosynthesised copper nanoflowers
It is widely known that metals like copper have been used since ancient time in the form of coins, jewellery and utensils etc. The important reason for the use of such metals in daily life is their inherent antimicrobial nature. Before the development of antibiotics and other chemotherapeutics, the formulations of inorganic antimicrobials (copper) were commonly used to treat microbial infections [41]. In the modern era of nanotechnology, these bulk copper compounds can be replaced by copper nanoparticles. Considering these facts, antifungal potential of chemically synthesised copper nanoflowers was evaluated against common plant pathogenic fungi, namely F. oxysporum, F. moniliforme, F. culmorum, F. tricinctum and A. niger. The maximum antifungal activity was demonstrated against A. niger followed by F. moniliforme, F. oxysporum and F. t ricinctum, whereas the minimum activity was found against F. culmorum. Similarly, the effect of these copper nanoflowers was also tested in combination with commercial antifungal agent (ketoconazole). The results proved that the efficacy of the antifungal agent used was enhanced when used in combination with copper nanoflowers (Fig. 6). These results showed the close relationship with the results reported in previous study by Petranovskii et al. [32] who demonstrated antifungal activity of copper‐based zeolites against C. cladosporoides, P. chersonesos and U. chartarum. Similarly, Ramyadevi et al. [21], Usha et al. [24] and Kim et al. [33] reported efficacy of different copper‐based nanoparticles against C. albicans, P. citrinum, A. niger and A. flavus. More recently, Shende et al. [2] reported that biologically synthesised copper nanoparticles demonstrated the significant antifungal activity against plant pathogenic fungi such as F. culmorum, F. oxysporum and F. graminearum. It was reported that copper nanoparticles and the type of microorganisms play an important role in the antimicrobial activity. The efficacy of copper nanoparticles also depends on the combination of several factors such as temperature, concentration of nanoparticles, pH and concentration of test organism [15].
Fig. 6.
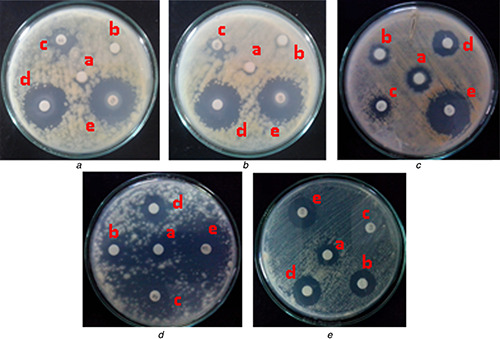
Antifungal activity of chemically synthesised copper nanoflowers against some common plant pathogenic fungi
(A) A. niger, (B) F. moniliforme, (C) F. culmorum, (D) F. oxysporum, (E) F. tricinctum. (a) Copper nitrate, (b) IPA, (c) Antifungal agent (ketoconazole), (d) Copper nanoflowers, (e) Copper nanoflowers + Antifungal agent (AF)
4 Conclusions
The chemical approach used in the present study is very rapid, green and ecofriendly for the synthesis of novel copper nanoflowers using copper nitrate as a precursor. Further, the in vitro assessment of antifungal efficacy of synthesised copper nanoflowers against common plant pathogenic fungi confirmed their high efficacy in the management of crop pathogens tested here. Considering the significant antifungal efficacy, these nanoflowers can be used as next generation nano‐based fungicides after extensive studies of toxicity to the environment and other beneficial organisms.
5 Acknowledgment
Avinash P. Ingle is highly thankful to the Science and Engineering Research Board, New Delhi for providing financial assistance to carry out the present research work in the form of Fast Track Research Project for Young Scientist. We also thank University Grants Commission, New Delhi for providing SAP (DRS I) to Department of Biotechnology, SGB Amravati University, Amravati.
6 References
- 1. Lee B. Kim Y. Yang S. et al.: ‘A low‐cure temperature copper nano ink for highly conductive printed electrodes’, Curr. Appl. Phys., 2009, 9, pp. 157 –160 [Google Scholar]
- 2. Shende S. Ingle A.P. Gade A. et al.: ‘Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity’, World J. Microbiol. Biotechnol., 2015, 31, pp. 865 –873 [DOI] [PubMed] [Google Scholar]
- 3. Umer A. Naveed S. Ramzan N. et al.: ‘A green method for the synthesis of copper nanoparticles using L‐ascorbic acid’, Rev. Mater., 2014, 19, pp. 197 –203 [Google Scholar]
- 4. Ingle A.P. Duran N. Rai M.: ‘Bioactivity, mechanism of action, and cytotoxicity of copper‐based nanoparticles: a review’, Appl. Microbiol. Biotechnol., 2014, 98, pp. 1001 –1009 [DOI] [PubMed] [Google Scholar]
- 5. Joshi S.S. Patil S.F. Iyer V. et al.: ‘Radiation induced synthesis and characterization of copper nanoparticles’, Nanostruct. Mater., 1998, 10, pp. 1135 –1144 [Google Scholar]
- 6. Wang H. Xu J.Z. Zhu J.J. et al.: ‘Preparation of CuO nanoparticles by microwave irradiation’, J. Crystal Growth, 2002, 244, pp. 88 –94 [Google Scholar]
- 7. Wu S.H. Chen D.H.: ‘Synthesis of high‐concentration Cu nanoparticles in aqueous CTAB solutions’, J. Colloid Interface Sci., 2004, 273, pp. 165 –169 [DOI] [PubMed] [Google Scholar]
- 8. Qing‐Ming L. De‐Bi Z. Yamamoto Y. et al.: ‘Preparation of Cu nanoparticles with NaBH4 by aqueous reduction method’, Trans. Nonferrous. Met. Soc. China, 2012, 22, pp. 117 –123 [Google Scholar]
- 9. Gawande M.B. Goswami A. Felpin F.X. et al.: ‘Cu and Cu‐based nanoparticles: synthesis and applications in catalysis’, Chem. Rev., 2016, 116, pp. 3722 –3811 [DOI] [PubMed] [Google Scholar]
- 10. Chakraborty P. Adhikary J. Chatterjee S. et al.: ‘Facile synthesis of copper sulfide nanoparticles: antibacterial and antifungal activity study’, Rasayan J. Chem., 2016, 9, (1), pp. 77 –83 [Google Scholar]
- 11. Kanninen P. Johans C. Merta J. et al.: ‘Influence of ligand structure on the stability and oxidation of copper nanoparticles’, J. Colloid Interface Sci., 2008, 318, pp. 88 –95 [DOI] [PubMed] [Google Scholar]
- 12. Kathad U. Gajera H.P.: ‘Synthesis of copper nanoparticles by two different methods and size comparison’, Int. J. Pharm. Biol. Sci., 2014, 5, (3), pp. 533 –540 [Google Scholar]
- 13. Park B.K. Jeong S. Kim D. et al.: ‘Synthesis and size control of monodisperse copper nanoparticles by polyol method’, J. Colloid Interface Sci., 2007, 311, pp. 417 –424 [DOI] [PubMed] [Google Scholar]
- 14. Virk H.S.: ‘Fabrication and characterization of metallic copper and copper oxide nanoflowers’, Pak. J. Chem., 2011, 1, (4), pp. 148 –154 [Google Scholar]
- 15. Sampath M. Vijayan R. Tamilarasu E. et al.: ‘Green synthesis of novel jasmine bud‐shaped copper nanoparticles’, J. Nanotechnol., 2014, 2014, p. 7, doi: 10.1155/2014/626523 [Google Scholar]
- 16. Ghosh K. Balog E.R.M. Sista P. et al.: ‘Temperature‐dependent morphology of hybrid nanoflowers from elastin‐like polypeptides’, APL Mater., 2014, 2, p. 021101 [Google Scholar]
- 17. Somturk B. Hancer M. Ocsoy I. et al.: ‘Synthesis of copper ion incorporated horseradish peroxidase‐based hybrid nanoflowers for enhanced catalytic activity and stability’, Dalton Trans., 2015, 44, pp. 13845 –13852 [DOI] [PubMed] [Google Scholar]
- 18. Liao J. Li H. Zhang X. et al.: ‘Copper oxide nanoplatelets and nanoflowers: facile synthesis and catalytic activity in oxidative degradation of methylene blue’, Micro & Nano Lett., 2014, 9, (7), pp. 432 –436 [Google Scholar]
- 19. Borkow G. Gabbay J.: ‘Copper: an ancient remedy returning to fight microbial, fungal and viral infections’, Curr. Chem. Biol., 2009, 3, pp. 272 –278 [Google Scholar]
- 20. Das R. Gang S. Nath S.S. et al.: ‘Linoleic acid capped copper nanoparticles for antibacterial activity’, J. Bionanosci., 2010, 4, pp. 82 –86 [Google Scholar]
- 21. Ramyadevi J. Jeyasubramanian K. Marikani A. et al.: ‘Synthesis and antimicrobial activity of copper nanoparticles’, Mater. Lett., 2012, 71, pp. 114 –116 [Google Scholar]
- 22. Yoon K.Y. Byeon J.H. Park J.H. et al.: ‘Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles’, Sci. Total Environ., 2007, 373, pp. 572 –575 [DOI] [PubMed] [Google Scholar]
- 23. Esteban‐Cubillo A. Pecharroman C. Aguilar E. et al.: ‘Antibacterial activity of copper monodispersed nanoparticles into sepiolite’, J. Mater. Sci., 2006, 41, pp. 5208 –5212 [Google Scholar]
- 24. Usha R. Prabu E. Palaniswamy M. et al.: ‘Synthesis of metal oxide nanoparticles by streptomyces sp. for development of antimicrobial textiles’, Global J. Biotechnol. Biochem., 2010, 5, (3), pp. 153 –160 [Google Scholar]
- 25. Gopalakrishnan K. Ramesh C. Ragunathan V. et al.: ‘Antibacterial activity of Cu2 O nanoparticles on E. coli synthesized from Tridax procumbens leaf extract and surface coating with polyaniline.’, Dig. J. Nanomater. Biol., 2012, 7, (2), pp. 833 –839 [Google Scholar]
- 26. Lee H.J. Song J.Y. Kim B.S.: ‘Biological synthesis of copper nanoparticles using Magnolia kobus leaf extract and their antibacterial activity’, J. Chem. Technol. Biotechnol., 2013, 88, (11), pp. 1971 –1977 [Google Scholar]
- 27. Raffi M. Mehrwan S. Bhatti T.M. et al.: ‘Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli ’, Ann. Microbiol., 2010, 60, pp. 75 –80 [Google Scholar]
- 28. Ruparelia J.P. Chatterjee A.K. Duttagupta S.P. et al.: ‘Strain specificity in antimicrobial activity of silver and copper nanoparticles’, Acta Biomater., 2008, 4, pp. 707 –716 [DOI] [PubMed] [Google Scholar]
- 29. Esteban‐Tejeda L. Malpartida F. Esteban‐Cubillo A. et al.: ‘Antibacterial and antifungal activity of a sodalime glass containing copper nanoparticles’, Nanotechnology, 2009, 20, p. 505701 [DOI] [PubMed] [Google Scholar]
- 30. Theivasanthi T. Alagar M.: ‘Studies of copper nanoparticles effects on microorganisms’, Ann. Biol. Res., 2011, 2, (3), pp. 368 –373 [Google Scholar]
- 31. Usman M.S. El‐Zowalaty M.E. Shameli K. et al.: ‘Synthesis, characterization and antimicrobial properties of copper nanoparticles’, Int. J. Nanomed., 2013, 8, pp. 4467 –4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petranovskii V. Panina L. Bogomolova E. et al.: ‘Microbiologically active nanocomposite media’, Proc. SPIE, 2003, 5218, pp. 244 –255 [Google Scholar]
- 33. Kim Y.H. Lee D.K. Cha H.G. et al.: ‘Preparation and characterization of the antibacterial Cu nanoparticle formed on the surface of SiO2 nanoparticles’, J. Phys. Chem. B, 2006, 110, pp. 24923 –24928 [DOI] [PubMed] [Google Scholar]
- 34. Viet P.V. Nguyen H.T. Cao T.M. et al.: ‘Fusarium antifungal activities of copper nanoparticles synthesized by a chemical reduction method’, J. Nanomater., 2016, 2016, p. 7 [Google Scholar]
- 35. Bramhanwade K. Shende S. Bonde S. et al.: ‘Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases’, Environ. Chem. Lett., 2016, 14, (2), pp. 229 –235 [Google Scholar]
- 36. Bauer A.W. Kirby M. Sherris J.C. et al.: ‘Antibiotic susceptibility testing by a standardized single disk method’, Am. J. Clin. Pathol., 1996, 45, pp. 493 –496 [PubMed] [Google Scholar]
- 37. Dang T.M.D. Le T.T.T. Fribourg‐Blanc E. et al.: ‘The influence of solvents and surfactants on the preparation of copper nanoparticles by a chemical reduction method’, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2011, 2, p. 015009 [Google Scholar]
- 38. Soomro R.A. Sherazi S.T.H. Sirajuddin et al.: ‘Synthesis of air stable copper nanoparticles and their use in catalysis’, Adv. Mat. Lett., 2014, 5, (4), pp. 191 –198 [Google Scholar]
- 39. Hunter K.A. Liss P.S.: ‘The surface charge of suspended particles in estuarine and coastal waters’, Nature, 1979, 282, pp. 823 –825 [Google Scholar]
- 40. Guzman K.A.D. Taylor M.R. Banfield J.F.: ‘Environmental risks of nanotechnology: national nanotechnology initiative funding, 2000‐2004’, Environ. Sci. Technol., 2006, 40, pp. 1401 –1407 [DOI] [PubMed] [Google Scholar]
- 41. Lin Y.E. Vidic R.D. Stout J.E. et al.: ‘Inactivation of Mycobacterium avium by copper and silver ions’, Water Res., 1998, 32, (7), pp. 1997 –2000 [Google Scholar]


