Abstract
In the present study, silver nanoparticles (AgNPs) were synthesised by adding 1 mM Ag nitrate solution to different concentrations (1%, 2.5%, 5%) of branch extracts of Eurycoma longifolia, a well known medicinal plant in South–East Asian countries. Characterisation of AgNPs was carried out using techniques such as ultraviolet–visible spectrophotometry, X‐ray diffractrometry, Fourier transform infrared–attenuated total reflection spectroscopy (FTIR–ATR), scanning electron microscopy. XRD analysis revealed face centre cubic structure of AgNPs and FTIR–ATR showed that primary and secondary amide groups in combination with the protein molecules present in the branch extract were responsible for the reduction and stabilisation of AgNPs. Furthermore, antioxidant [2,2‐diphenyl‐1‐picrylhydrazyl and 2,2′‐Azino‐bis(3‐ethylbenzthiazoline‐6‐sulphonic acid)], antimicrobial and anticancer activities of AgNPs were investigated. The highest bactericidal activity of these biogenic AgNPs was found against Escherichia coli with zone inhibition of 11 mm. AgNPs exhibited significant anticancer activity against human glioma cells (DBTRG and U87) and human breast adenocarcinoma cells (MCF‐7 and MDA‐MB‐231) with IC50 values of 33, 42, 60 and 38 µg/ml.
Inspec keywords: biomimetics, cancer, antibacterial activity, nanoparticles, silver, microorganisms, cellular biophysics, biomedical materials, nanomedicine, nanofabrication, X‐ray diffraction, Fourier transform infrared spectra, attenuated total reflection, ultraviolet spectra, visible spectra, proteins, molecular biophysics, biochemistry
Other keywords: Biomimetic synthesis; anticancer activity; Eurycoma longifolia branch extract‐mediated silver nanoparticles; nitrate solution; medicinal plant; ultraviolet‐visible spectrophotometry; X‐ray diffractometry; Fourier transform infrared‐attenuated total reflection spectroscopy; FTIR‐ATR spectroscopy; scanning electron microscopy; XRD; face centre cubic structure; primary amide groups; secondary amide groups; protein molecules; antioxidant; 2,2‐diphenyl‐1‐picrylhydrazyl; 2,2′‐azino‐bis(3‐ethylbenzthiazoline‐6‐sulphonic acid); antimicrobial activity; bactericidal activity; biogenic silver nanoparticles; Escherichia coli; zone inhibition; DBTRG human glioma cells; U87 human glioma cells; MCF‐7 human breast adenocarcinoma cells; MDA‐MB‐231 human breast adenocarcinoma cells; Ag
1 Introduction
There is no qualm that nanotechnology is one of the fast developing fields in the area of multidisciplinary research, biotechnology in particular [1]. Present research trends focuses on biosynthesis of metallic nanoparticles (NPs), mostly silver NPs (AgNPs), due to the renowned features such as ease and rapidness, usage of natural resources, eco‐friendliness, well‐defined and control over the size of the NPs. These emerging features are important in medical applications [2]. Since ancient times, bactericidal effects and oligodynamic properties of Ag salts enable them to react as antimicrobial agents [3]. However, Ag salts result in improper adsorption of ions in sweat glands and epidermal cells [4, 5]. To circumvent this, AgNPs were chosen to replace Ag salts and complexes. AgNPs (measured size is <100 nm in at least one dimension) were produced using different methods including biosynthesis (using biotemplates such as plant materials, microbes, proteins etc.). Biogenically synthesised AgNPs with diverse properties such as antimicrobial, antioxidant, antifungal, anticancer, anti‐inflammatory and catalytic properties are used in home water purification system, electronic devices, open wound treatment and house hold applications. There are several plant sources which act as biogenic agent such as Terminalia species [6], Canthium coromandelicum [7], Trianthema decandra [8], Olea europaea [9], Rhinacanthus nasutus [10], Cinnamon zeylanicum [11], Musa balbisiana [12], Ulva lactuca [13] and Ocimum bacillicum [14].
Oxidation is one of the important processes in living organisms for energy generation in order to stimulate biological activities. In spite of that, excessive amounts of reactive oxygen species (ROS) including superoxides, nitrous oxide, peroxides and hydroxyl radicals are formed in the living organisms by cellular metabolism that could lead to cellular damage. This eventually induces numerous diseases including cancer, Parkinson's and disorders such as ageing in living organisms [15, 16]. Herbicidal plants are enriched with effective phytochemicals which act as natural antioxidant agents. Antioxidants are the compounds that react with free radicals to prevent damage to the structure of biomolecules in the cell. Antioxidant compounds pave way to improve the immune defence level and reduce the risk of degenerative diseases and cancer [17]. To date, several studies have proven that biogenically synthesised AgNPs have significant antioxidant activity compared with normal plant extracts [18].
Antimicrobial agents have provided strong protection against various communicable diseases [19]. Nevertheless, multidrug‐resistant bacteria such as methicillin‐resistant Staphylococcus aureus have become more common and transformed themselves to be resistant to almost all current available antibiotics and antibiotic agents. This transformation can be due to many biochemical and physiological mechanisms [20]. New methods and techniques are acquired to safeguard against developing infections and drug‐resistant bacterial pathogens [21]. Currently, nanotechnology is creating ways to discover effective antimicrobial agents in nano range that could solve this emerging problem effectively. Ag is a safe inorganic, non‐toxic antibacterial agent which possesses the ability to inhibit about 650 types of disease‐causing microorganisms [22].
Among the human diseases, cancer cases have been increasing at alarming rate and are expected to rise by 75% (25 million) in the next two decades. The most common types of cancers are lung (1.6 million), liver (0.8 million) and stomach (0.7 million) cancer. Cancer results from abnormal proliferation of cells and affects digestive, nervous and circulatory systems [23]. Drug resistance and uncontrolled side effects of chemotherapy are major drawbacks of therapeutic management of cancer patients. Many techniques, devices and nanomaterials have been improved in cancer treatment [24, 25]. Phytochemicals derived from plant sources and biogenically synthesised NPs play a vital role in different types of cancers [26]. Previous studies reported on the antitumour efficacy of AgNPs and have gained marginal attention among researchers. Sulaimain et al. [24] found that biogenically synthesised AgNPs using Eucalyptus chapmaniana leaves exhibited anticancerous activity against human acute promyelocytic leukaemia (HL‐60) cell line. Colloidal AgNPs significantly reduced the proliferation of human glioblastoma cells [25] and Jeyaraj et al. [27] reported that Sesbania grandiflora (L)‐mediated AgNPs showed potential anticancer efficacy against MCF‐7 breast cancer cells.
In this context, herein, different concentrations of branch extracts of Eurycoma longifolia were used to synthesise biogenic AgNPs. E. longifolia, well known by its local traditional name ‘Tongkat Ali’, is usually found in South–East Asian forests such as in Java, Malaysia, Sumatra, Thailand and Vietnam. This is a flowering plant which belongs to Simaroubaceae family and Eurycoma genus. In Malaysia, E. longifolia is found in primary and secondary forests [28]. Several studies have reported that different parts of E. longifolia have a conglomerate of biological efficacies such as anti‐malarial [29], antimicrobial [30, 31], anticancer [32], antioxidant [33], antianxiety [34] and antidiabetic activity [35]. Different parts of the plant react diversely in biogenic synthesising process. The main objectives of the present paper were to synthesise one pot, rapid and eco‐friendly AgNPs by green chemistry method using branch extract of E. longifolia and to study their biological activities. To the best of our knowledge, no study had been reported on the synthesis of AgNPs using branch extracts of E. longifolia.
2 Material and methods
2.1 Materials
Ag nitrate and 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH) were purchased from Sigma‐Aldrich (USA). All chemicals were of analytical grade and used as received without further modifications.
2.2 Preparation of plant extracts
Fresh branches of E. longifolia were collected and washed with tap water to eliminate all contaminants. The plant parts were dried under the shade at room temperature. The dried plant parts were powdered. Different concentrations (1%, 2.5%, 5%) of dried plant parts were prepared by adding different amounts of the powder (1 g, 2.5 g, 5 g) in 100 ml of sterile distilled water. The solution was boiled in a water bath at 100°C for 30 min. The solution was refined through Whatman No. 1 filter paper. The extracts were kept at 4°C for further studies. The pH of the extracts was adjusted to pH 8 by adding 0.1 M sodium hydroxide.
2.3 Synthesis of AgNPs
A stock solution of 1 mM AgNO3 was prepared as 1 : 1 solution of both Ag nitrate and E. longifolia branch extracts in falcon tubes. The falcon tubes were wrapped with aluminium foil to prevent exposure to light which could oxidise the AgNPs. The colour change and ultraviolet–visible (UV–vis) spectroscopy reading were observed for every 30 s intervals for 2.5 min. The synthesised AgNPs were incubated at room temperature for 24 h and purified by using centrifugation process at 8000 rpm for 10 min. The AgNP pellets were then collected, washed and dried at room temperature.
2.4 UV–vis spectrum analysis
UV–vis spectrum of the colloidal solution was measured in the range of 200–700 nm (Spectroquant Pharo 300, Optizen 3220 UV, MERCK) at different functional times to monitor the reduction and stabilisation process of Ag ions. Distilled water was used to dilute a small aliquot of the samples to ten‐fold dilution.
2.5 X‐ray diffraction (XRD) analysis
The crystalline structure of the synthesised AgNPs was determined by using X‐ray diffractometer (D2 PHASER, 2nd Gen, Brucker) at 2θ ranges from 0° to 100°.
The average particular size of the AgNPs synthesised was analysed by using Debye–Scherrer's formula
where β is the width of peak at half maximum intensity of a specific phase in radians, t is the mean crystalline size, θ is the centre angle of the peak in radian and λ is the wavelength of incident rays.
2.6 Fourier transform infrared spectroscopy (FTIR)–attenuated total reflection
The functional biomolecules associated with AgNPs were analysed by FTIR (Nicolet 6700, Thermo Nicolet Corp., Madison, WI). The spectrum was measured at adsorption range of 500–4000 cm−1.
2.7 Scanning electron microscopy (SEM) analysis
The external morphology (texture), crystalline structure and orientation of AgNPs were analysed by using SEM under standard conditions (TM3030, Hitachi, USA). A small quantity of the sample was located on the copper membraned grid to prepare thin film layers. Extra solution of the sample was eliminated by using bloating paper. Then, the thin film layers were dried under mercury for 5 min.
2.8 DPPH antioxidant assay
Antioxidant property of plant extract and biogenically synthesised AgNPs were determined by DPPH assay. Various concentrations of AgNPs and plant extracts (50, 100, 150, 200, 250 and 300 µg/ml) were prepared by serial dilution of the stock solution (1 mg/ml). The assay was done triplicates. The absorbance was read using a spectrophotometer at 517 nm wavelength. Ascorbic acid was used as reference standard. The free radical scavenging activity [proportional–integral (PI)] was calculated based on the following equation:
2.9 2,2′‐Azino‐bis(3‐ethylbenzthiazoline‐6‐sulphonic acid) (ABTS) antioxidant assay
The free radical scavenging activity of the AgNPs and plant extracts were determined by using ABTS radical scavenging assay. UV–vis spectrophotometer was set to 734 nm to record absorbance after 6 min. The control ABTS solution was used to compare the results obtained. The percentage inhibition was determined by using formula stated below:
2.10 Antimicrobial activity
Kirby–Bauer technique was selected to study in vitro bactericidal activity of the synthesised NPs. Three Gram‐positive bacteria [Staphylococcus aureus, Streptococcus faecalis (SF), Bacillus subtilis (BS)] and two Gram‐negative bacteria [Escherichia coli (EC), Shigella boydii (SB)] were used in this paper. Briefly, 20 µl of both branch extract and AgNPs were pipetted into 6 mm diameter sterile diffusion discs. All filled discs were dried at 30°C for 30 min. Subcultures of all bacteria were prepared in Luria broth broth and incubated for 24 h. Bacteria subcultures (1 µl) were dissolved in sterile saline and 0.5 McFarland turbidity standard was used as standard to fix bacterial colony to 1.5 × 108 colony forming unit per ml. The nutrient agar medium was fully spread with prepared suspension of bacterium by using steeped cotton swab. The dried saturated discs were placed on inoculated medium. The medium was left to incubate at 37°C for 18–24 h. Mean ± standard deviation (SD) of zone of inhibition of the triplicate experiments was measured in millimetres. Ampicillin was used as positive control for comparison.
2.11 Anticancer activity
2.11.1 Cell culture condition
Human breast adenocarcinoma cells (MCF‐7 and MDA‐MB‐231) and human glioma cells (DBTRG and U87) were propagated in Dulbecco's modified Eagle's medium, supplemented with 10% foetal bovine serum and 1 unit/ml penicillin/streptomycin. The cell lines were maintained at 37°C in 5% carbon dioxide humidified atmosphere.
2.11.2 Preparation of AgNPs stock solution
Synthesised AgNPs using E. longifolia (EBAgNPs) branch extracts were dissolved in dimethyl sulphoxide to produce a 10 mg/ml stock concentration. Prior to experiment, (EBAgNPs) extract stock concentration was prepared in concentrations of 10, 25, 50, 75 and 100 µg/ml in culture medium.
2.11.3 Determination of cell viability by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5 diphenyltetrazolium bromide (MTT) assay
Cells were seeded at the concentration of 0.5−1 × 104 cells in 96‐well culture plates and allowed to attach overnight. The cells were then cultured with EBAgNPs for 24 h. The number of viable cells in each sample was determined using MTT assay kit (Sigma‐Aldrich, USA) according to manufacturer's instructions and the absorbance of the colorimetric assay was measured using a spectrophotometer at a wavelength of 570 nm. The percentages of cell viability against EBAgNPs concentrations were plotted separately, and from these curves the inhibitory concentration that caused 50% cell growth inhibition (IC50) were determined.
3 Results and discussion
3.1 UV–vis spectrum
The E. longifolia branch extract expressed notable colour change from brown to dark brown (Fig. 1), confirming the formation of AgNPs in the colloidal solutions. The colour change is triggered by bioreduction process of Ag+ ions to Ag0 atoms. Abundant biomolecules in E. longifolia branch extracts reacted with reducing agents and the overall bioreduction process can be simplified as
Fig. 1.

UV–vis spectrum graph of synthesised AgNPs using E. longifolia branch at different concentrations (1, 2.5 and 5%) (inset: synthesis of NPs from E. longifolia)
Logeswari et al. [36] reported the colour change to dark brown in AgNPs synthesis using leaf extracts of Syzgium cumini, Citrus sinesis, Solanum tribatum and Centella asiatica. Synthesis of the AgNPs was found to be concentration dependent. The colour intensity increased as the concentration of the plant extract increased from 1 to 5%. Large quantity of the biomolecules present in branch of E. longifolia facilitates Ag reduction process and colour change results in synthesis of NPs. Similar results were observed with the synthesis of AgNPs using the leaves of Cinnamomum camphora and bark extract of C. zeylanicum [37].
Time intervals of 30, 60, 90, 120 and 150 s were used to measure the adsorption peak. At 150 s, all concentrations exhibited the highest adsorption. The adsorption peaks of biogenically synthesised AgNPs using 1, 2.5 and 5% of E. longifolia branch extracts were 397, 414 and 429 nm, respectively at 150 s (Fig. 1). It is observed that surface plasmon resonance (SPR) adsorption band triggered high adsorption peak of AgNPs. Free electrons of biogenically synthesised AgNPs promote the generation SPR band by combining electron vibration in resonance with the light wave [38]. Several factors such as type of biomolecules present in the plant extracts, shape of the NPs, amount of extracts and Ag nitrate concentration play major role in forming SPR banding patterns. Femila et al. [37] reported that polyphenols and protocatechuic acid‐type phenolic compound were involved in the capping process and synthesis of AgNPs. Branch extracts of E. longifolia‐ mediated AgNPs were adjusted to pH 8. This is supported by Vanaja et al. [39] who reported on the synthesis of small‐sized AgNPs in alkaline pH due to the availability of large amount of positive functional groups in the leaf extract which allows Ag ions to get more binding sites.
3.2 XRD
XRD analysis is an improved and sophisticated way to identify the crystalline structure of synthesised AgNPs by interpreting the penetration of particular X‐rays wavelength. The peaks at 2θ values of 38°, 44°, 64° and 77° in Fig. 2 denoted (111), (200), (220) and (311) lattice plans of Ag, respectively, illustrating that the biogenically synthesised AgNPs are face centre cubic (FCC)‐shaped and crystalline. These results are in accordance with the previous studies [9, 40]. The approximate crystalline size for AgNPs synthesised from E. longifolia branch is 88 nm by formula. This clearly illustrates that the AgNPs synthesised in the present paper are monocrystalline. Similar results had been reported in previous studies. Firdhouse and Lalitha [40] stated that the average crystalline size of synthesised AgNPs from Pisonia grandis plant extracts was 56.86 nm which is consistent with the present paper. Generally, biogenically synthesised AgNPs were reported to show significant size variability that is widely dependent on factors such as pH, type of plant extracts and temperature [41]. Different size ranges of AgNPs such as 5–30, 15–150, 16–40, 25–40 and 50–100 nm have been synthesised by using leaf extracts of Azadirachta indica, Mentha piperita, Eucalyptus hybrida and Pelargonium graveolens, respectively[42, 43, 44, 45].
Fig. 2.

XRD micrograph of E. longifolia branch‐mediated (5%) AgNPs clearly showing the predominant (111) lattice space with Bragg's reflections of FCC structure
3.3 FTIR–attenuated total reflection (ATR) analysis
The interaction between biogenically synthesised NPs and bioorganics of aqueous branch extracts of E. longifolia can be distinguished by interpreting FTIR–ATR spectrum. In Fig. 3, the adsorption at 2970.96 cm−1 indicates the C–H stretching of the alkyl group in AgNPs [9]. The band appears at 2370.42, 2342.75 cm−1 reflects the presence of –C–H– group [17, 37]. The adsorption band at 2289.4 and 1717.03 cm−1 denotes –C = C– group and ketones, respectively [12, 46]. Furthermore, the adsorption peak at 1602.87 and 1508.87 cm−1 shows the functional groups of C–C in alkene rings and aromatic ring C = C, respectively [46, 47]. Bands appear at 1364.28 and 1216.99 cm−1 reflect the presence of C–H scissoring bending (alkenes) and C–N stretch (aliphatic amines), respectively [48]. Peaks at 816 and 673.05 cm−1 denote catechins and C–H band of alkene bonds, respectively [46, 48].
Fig. 3.
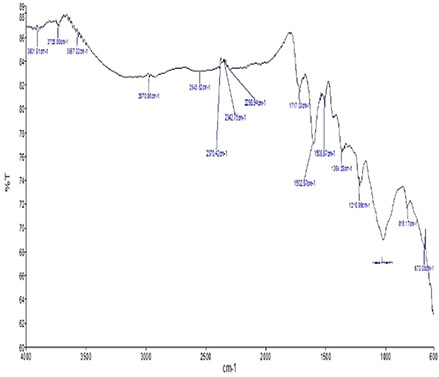
FTIR–ATR micrograph of E. longifolia branch‐mediated (5%) AgNPs representing the functional groups involved in the reduction and stabilisation process of AgNPs
Plant extracts play both the roles as reducing and capping agents in biogenic synthesis of AgNPs. In the present paper, the stretching vibrations of plant biological molecules mediated synthesised AgNPs indicate the presence of different proteins and terpenoids in the aqueous extracts which enhanced the bioreduction of Ag ions. Previous studies reported that E. longifolia is enriched with phenolic compounds such as 2, 6‐dimethoxy‐phenol, 2‐methoxy‐4‐(1‐propenyl)‐(E)‐phenol and 3,4,5‐ trimethoxy‐phenol [33]. These functional groups might be the causative agents to promote capping and reduction process of Ag ions in the present paper.
3.4 SEM analysis
SEM analysis showed high‐density AgNPs synthesised using 5 and 2.5% of E. longifolia branch extract (Fig. 4). The approximate size of biogenically synthesised AgNPs is 30 μm (2000× magnification). The synthesised AgNPs have sharp edges and are coated with crystalline surfaces. The AgNPs are agglomerated and overlapped. Different sizes of spherical and cuboidal shaped AgNPs are observed in Fig. 4 which could be due to availability of different quantities of capping agents present in the plant extracts. The differences and shifts in areas of peaks obtained in FTIR analysis support these results. In addition, similar results were also observed by Awwad et al. [49], whereby the biosynthesised AgNPs from Ceratonia siliqua leaves showed mixed sizes of combined shapes in SEM analysis. According to Banerjee et al. [12], three different shapes (triangular, spherical and cuboidal) of biogenically synthesised AgNPs from M. balbisiana, A. indica and O. tenuiflorum were obtained, respectively, in SEM analysis.
Fig. 4.
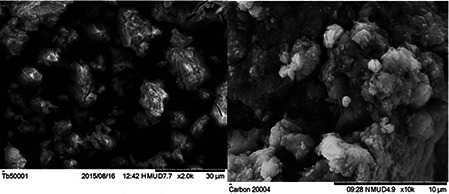
SEM micrograph (left) of E. longifolia branch‐mediated (5%) AgNPs (30 µm bar scale) (right)of E. longifolia branch‐mediated (2.5%) AgNPs (10 µm bar scale) showing slightly agglomerated AgNPs with roughly spherical in shape with an average size of 88 nm
The SEM image of AgNPs is the result of electrostatic interactions between the bioorganic capping molecules bound to the AgNPs and the hydrogen bonds. The larger AgNPs could be due to the amalgamation of the smaller AgNPs [49].
3.5 DPPH assay
DPPH is a well known lipophilic free radical due to its strong stability and tendency to accept electrons from nearby antioxidant compounds. It converts colour from violet to yellow which is detected at 517 nm [38]. In the present paper, the E. longifolia branch extract exhibited potential free radical scavenging activity ranging from 35.4 to 76.2% at concentrations ranging from 50 to 300 µg/ml (Fig. 5). Furthermore, biogenically synthesised AgNPs using E. longifolia branch extract exhibited significant free radical scavenging activity from 39.8 to 81% at concentrations of 50 to 300 µg/ml (Fig. 5). The standard, ascorbic acid, showed the highest activity at all concentrations, in the range of 75.7–90.4% (Fig. 6). Similarly, Sangeetha and Singaravelu [50] reported that biogenic AgNPs from excoecaria agallocha exhibited significant DPPH radical inhibition activity (more than 80%) compared with E. agallocha leaf extracts (<80%) in a concentration‐dependent manner. In this context, the present result is warranted by previous studies [51, 52]. Similar trend was also observed in the biosynthesised AgNPs from raphanus sativus var. longipinnatus [53] and chrysophyllum oliviforme [54] leaf extracts.
Fig. 5.

DPPH free radical scavenging activity of E. longifolia branch, E. longifolia branch AgNPs and ascorbic acid. The values represented are the mean ± SD of triplicate samples significant level at (p < 0.05)
Fig. 6.

ABTS free radical scavenging activity of E. longifolia branch, E. longifolia branch‐mediated AgNPs and ascorbic acid. The values represented are the mean ± SD of triplicate samples significant level at (p < 0.05)
3.6 ABTS+ assay
ABTS+ ·stable radicals readily accepts an electron to reach a stable diamagnetic structure [55]. ABTS free radical scavenging activity of branch extract showed significant activity ranging from 41.6 to 81.3% at concentrations of 50–300 µg/ml (Fig. 6). Biogenically synthesised AgNPs showed 47.8–84.8% activity at concentrations of 50–300 µg/ml (Fig. 6). Ascorbic acid showed the highest free radical scavenging activity (51.5–89.8%) at all concentrations (Fig. 6). Similarly, Kokila et al. [50] reported that the ABTS radical scavenging activity of synthesised AgNPs from Cavendish banana (M. cavendishii) peel was high (84.71%) compared with the peel extract alone(42.47%) [56]. The same research group also reported higher activity of synthesised AgNPs from C. sinesis peel (79.03%) compared with C. sinesis peel extract (54.83%). Similarly, Psidium guajava extract and AgNPs from P. guajava possess similar kind of activity [57].
3.7 Antimicrobial activity
Since ancient times, Ag and Ag‐based compounds have been well known for their antimicrobial efficacies [8]. It is important to invent new substitutes to replace available antimicrobial agents and antibiotics due to multidrug‐resistance issues [58]. To combat this, nanomedicine has discovered nano weapons against multidrug‐resistant bacteria in the form of AgNPs. The present paper showed potential antimicrobial activity of AgNPs against different types of bacteria such as BS, Staphyloccous aureus (SA), SF, EC and SB were selected to investigate the inhibitory effects of biogenically synthesised AgNPs.
The branch extracts of E. longifolia showed potential bactericidal efficacy against both Gram‐positive and Gram‐negative bacteria. E. longifolia branch extract showed high zone of inhibition in S. aureus with 7.9 mm, followed by BS (7.3 mm), EC (7.2 mm), SF (7 mm) and S. boydii (6.8 mm) (Table 1). The growth of EC was inhibited with highest zone of inhibition (11 mm). E. longifolia branch‐mediated AgNPs also expressed significant bactericidal efficacy against BS (10.9), SF (10.8 mm), S. boydii (10.7 mm) and S. aureus (10.1 mm). Ampicillin was used as positive control in order to compare the activity with the AgNPs and plant extract.
Table 1.
Evaluation of antimicrobial activity of AgNPs against an array of microorganisms and antibiotics
| Microorganisms | Antimicrobial agents | ||
|---|---|---|---|
| E. longifolia branch | AgNPs | Ampicillin | |
| S. aureus | 7.4 ± 0.8 | 10.1 ± 0.1 | 12.5 ± 0.3 |
| BS | 7.3 ± 0.4 | 10.9 ± 0.1 | 12.2 ± 1.0 |
| SF | 7 ± 0.0 | 10.8 ± 0.4 | 11.5 ± 1.2 |
| EC | 7.2 ± 0.8 | 11 ± 0.6 | 12.0 ± 0.0 |
| SB | 6.8 ± 0.1 | 10.7 ± 0.5 | 11.1 ± 0.2 |
Notably, the inhibition of EC was found to be the highest when compared with the other bacteria used in this paper, which shows that EC is more sensitive among the others. This outcome is consistent with the report by Kim and co‐researchers [59], whereby this group of researchers suggested that the inhibitory efficacy of AgNPs is attributed mainly by the characteristics of specific bacterial groups. In general, both Gram‐negative and Gram‐positive bacteria differ in terms of peptidoglycan layer structure which functions to safeguard bacteria from antimicrobial agents such as toxins, antibiotics, degradative enzymes and chemicals [58]. Gram‐positive bacteria are shielded with thick peptidoglycan layer which is made up of linear polysaccharide chains crossly joined by short peptides. This forms complex rigid membrane structure and causes tough penetration of AgNPs. However, the cell wall of Gram‐negative bacteria consists of thinner peptidoglycan layer and promotes easy penetration of AgNPs [60]. Similarly, Gram‐positive bacteria, S. aureus are coated by cytoplasmic membrane comprised of bi‐layered, thick and bag‐patterned cell wall [61]. On the basis of the existing research reports, the Gram‐positive bacterium possesses two distinct sortases that direct proteins to peptidoglycan. The mediated proteins promote the manifestation of infection. Thus, the difference in pathogenicity of S. aureus is supported by genome islands releasing a lot of toxins [62].
The exact mechanism and principle of bacterial inhibitory effects of AgNPs are not known. However, several possible mechanisms of action of AgNPs have been proposed in connection with structural and morphological alteration that happened in the bacterial cells [63]. The schematic diagram (Fig. 7) portrays AgNPs synthesis and the different antimicrobial mechanisms. The overall reaction starts with synthesis of AgNPs using selected plant extracts and Ag nitrate.
Fig. 7.

Schematic representation of possible biocidal mechanisms of AgNPs
The biogenically synthesised AgNPs anchor into bacterial cell wall and accumulate at membrane side. The accumulated AgNPs induce development of irregular shaped of ‘pits’ at the outer membrane of microbial cells. This eventually alters the cell membrane permeability due to excessive release of membrane proteins and lipopolysaccharide molecules [64]. Furthermore, AgNPs form ROS, a type of free radicals such as anion (O2 −−) hydroxyl radical (OH) and singlet oxygen (1 O2) in microbial cells by reacting on respiratory enzymes and lead to cell structure damage which eventually hampers cell death by synthesising pits [65]. Free radicals tend to react with the functional group of enzymes and change the specific structure of biomolecules. The ROS could react with DNA strands and cause disruption of signal transduction.
Furthermore, dephosphorylation of tyrosine protein mechanism can be another mode of antimicrobial mechanism of AgNPs. The Ag+ released from the AgNPs surface could react with thiol groups in tyrosine protein which carries soft base groups and eventually inactivates them [66, 67]. The Ag ions, also known as soft ions, react with thiol groups of multiple enzymes and cause dephosphorylation of protein molecules. Dephosphorylation of tyrosine molecules inhibits proper signal transduction and may suppress division of bacterial cells [68]. Next, the reactivity of Ag+ with sulphur and phosphate groups of DNA double strand is proposed to be another inhibitory mechanism. The negatively charged sulphur and phosphate groups of DNA can react with positively charged Ag ions of AgNPs causing changes to the overall structure of DNA and signal transduction leading to microbial cell disruption and cell death [60, 69]. Furthermore, AgNPs could react with cellular building blocks such as mitochondria, golgi body, vacuoles and supports cell lysis [70]. The accumulation of pits on cellular membrane depicts membrane damage and cellular death. Formation of pits on cellular membrane is influenced by the concentration of AgNPs present in the microbial cells [71]. Accumulation of negatively charged AgNPs in bacterial membrane facilitates the permeability of the membrane [52]. The size of AgNPs is important in antimicrobial activity. Smaller sized AgNPs with larger surface area can penetrate into microbial cell membranes effectively and produce strong inhibitory effect [72, 73].
3.8 Anticancer activity
The effects of EBAgNPs on cell proliferation were evaluated in MCF‐7, MDA‐MB‐231, DBTRG and U87 cell lines. The growth of cells treated with EBAgNPs at 10, 25, 50, 75 and 100 µg/ml were inhibited in a dose‐dependent manner (Fig. 8 a –d) with various IC50 values as indicated in Table 2. EBAgNPs was found to be more potent in cancer cell lines with IC50 values ranging from 33 to 60 µg/ml at 24 h. MCF‐7 cells were observed to be most affected in response to EBAgNPs followed by U87, MDA‐MB‐231 and DBTRG cells.
Fig. 8.

Cellular proliferation of
(a) MCF‐7, (b) MDA‐MB‐231, (c) DBTRG, (d) U87 cells treated ELB + AgNP. Cells were treated with 10, 25, 50, 75 and 100 mg/ml of ELB + AgNP for 24 h. The percentages of cell viability were determined by MTT assay. Results represent the mean of triplicate determination
Table 2.
IC50 values (μg/ml) of EBAgNPs determined in MCF‐7, MDA‐MB‐231, DBTRG and U87 cells. Cells were treated with various EBAgNPs (10–100 μg/ml) for 24 h and percentages of cell viability were measured using the MTT assay (each value represents the mean of triplicate determinations)
| IC50, µg/ml | ||||
|---|---|---|---|---|
| cell lines | MCF‐7 | MDA‐MB‐231 | DBTRG | U87 |
| EBAgNPs | 33 | 42 | 60 | 38 |
Problems associated with conventional chemotherapy include poor specificity, development of multiple drug resistance and severe side effects. As such, many cancer patients are choosing herbal medicines that are thought to be comparatively safer. Cumulating evidence shows that the root extract of E. longifolia is cytotoxic to various human cancer cell lines including MCF‐7 breast cancer cells [74]. It induced MCF‐7 apoptosis via Bcl‐2‐regulated and caspase‐7‐dependent pathway [75]. The extract also induced apoptotic cell death of chronic myelocytic leukaemia cell line, K‐562 and inhibited tumour growth in the K‐562 xenograft model [76]. These anti‐leukaemic effects could be attributed to the active compounds such as eurycomanone and eurycomanol, which act via inhibition of the mitogen‐activated protein kinase signalling pathway [77]. On the other hand, p53‐dependent mechanism was reported in eurycomanone‐induced apoptosis of the human hepatocarcinoma HepG2 cells [78]. The branch of E.longifolia‐ mediated biogenically synthesised AgNPs might also able to have cytotoxic efficacy on different cancerous cell lines in similar way.
4 Conclusion
In the present paper, a green synthesis approach of stable AgNPs using branch extracts of E. longifolia was demonstrated. Several bioactive compounds such as alcohols, amines and phenols in the branch extract promoted the bioreduction and capping process of NPs rapidly at ambient conditions. Nano‐sized AgNPs promoted the bacterial inhibition and also free radical scavenging activity. Furthermore, the potential and significant cytotoxic effects of E. longifolia branch‐mediated AgNPs have been observed. Clear differences were also spotted in the biological efficacy between plant extract and plant extract‐mediated synthesised AgNPs, which clearly explains the smaller particles with the large surface volume possess higher activity. Furthermore, an extended research is warranted which focuses on the mechanisms of action of these plant‐mediated AgNPs in in vivo systems.
5 Acknowledgments
The authors acknowledge the financial supports from FRGS (R/FRGS/A07.00/00295A/002/2014/000183) and RU Grant No: (1001/PPSP/853002).
6 References
- 1. Natarajan K. Selvaraj S. Ramachandra M.V.: ‘Microbial production of silver nanoparticles’, Dig. J. Nanomater. Biostruct., 2010, 5, pp. 135 –140 [Google Scholar]
- 2. Mittal A.K. Chisti Y. Banerjee U.C.: ‘Synthesis of metallic nanoparticles using plant extracts’, Biotechnol. Adv., 2013, 31, pp. 346 –356 [DOI] [PubMed] [Google Scholar]
- 3. Maity D. Pattanayak S. Mollick M.R. et al.: ‘Green one step morphosynthesis of silver nanoparticles and their antibacterial and anticancerous activities’, New J. Chem., 2016, 40, pp. 2749 –2762 [Google Scholar]
- 4. Russell A.D. Hugo W.B.: ‘7 antimicrobial activity and action of silver’, Prog. Med. Chem., 1994, 31, pp. 351 –370 [DOI] [PubMed] [Google Scholar]
- 5. Silver S.: ‘Bacterial silver resistance: molecular biology and uses and misuses of silver compounds’, FEMS Microbiol. Rev., 2003, 27, (2– 3), pp. 341 –353 [DOI] [PubMed] [Google Scholar]
- 6. Mohamed EL‐Rafie H. Abdel‐Aziz Hamed M.: ‘Antioxidant and anti‐inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species’, Adv. Nat. Sci., Nanosci. Nanotechnol., 2014, 5, (3), p. 035008 [Google Scholar]
- 7. Mohan S.C. Sasikala K. Anand T. et al.: ‘Green synthesis, antimicrobial and antioxidant effects of silver nanoparticles using Canthium coromandelicum leaves extract’, Res. J. Microbiol., 2014, 9, (3), p. 142 [Google Scholar]
- 8. Geethalakshmi R. Sarada D.: ‘Gold and silver nanoparticles from Trianthema decandra: synthesis, characterization and antimicrobial properties’, Int. J. Nanomed., 2012, 7, pp. 5375 –5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khalil M.M.H. Ismail E.H. El‐Baghdady K.Z. et al.: ‘Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity’, Arab. J. Chem., 2014, 7, (6), pp. 1131 –1139 [Google Scholar]
- 10. Pasupuleti V.R. Prasad T.N.V.K.V. Shiekh R.A. et al.: ‘Biogenic silver nano particles using Rhinacanthus nasutus leaf extract: synthesis, spectral analysis, and antimicrobial studies’, Int. J. Nanomed., 2013, 8, pp. 3355 –3364 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Sathishkumar M. Sneha K. Won S.W. et al.: ‘ Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano‐crystalline silver particles and its bactericidal activity’, Colloids Surf. B, Biointerfaces, 2009, 73, (2), pp. 332 –338 [DOI] [PubMed] [Google Scholar]
- 12. Banerjee P. Satapathy M. Mukhopahayay A. et al.: ‘Leaf extract mediated green synthesis of silver nano particles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis’, Bioresour. Bioprocess., 2014, 1, (1), pp. 1 –10 [Google Scholar]
- 13. Kumar P. Govindaraju M. Senthamilselvi S. et al.: ‘Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca ’, Colloids Surf. B, Biointerfaces, 2013, 103, pp. 658 –661 [DOI] [PubMed] [Google Scholar]
- 14. Sivaranjini K. Meenakshisundaram M.: ‘Biological synthesis of silver nanoparticles using Ocimum basillicum leaf extract and their antimicrobial activity’, Int. Res. J. Pharm., 2013, 4, (1), pp. 225 –229 [Google Scholar]
- 15. Durgasani S. Pichika M.R. Nadarajah V.D. et al.: ‘Comparative antioxidant and anti‐inflammatory effect of [6]‐gingerol,[8]‐gingerol and [6]‐shagol’, J. Ethnopharmacol., 2010, 127, (2), pp. 515 –520 [DOI] [PubMed] [Google Scholar]
- 16. Mukherjee S. Pawar N. Kulkarni O. et al.: ‘Evaluation of free‐radical quenching properties of standard ayurvedic formulation vayasthapana Rasayana’, BMC Complement. Altern. Med., 2011, 11, (1), p. 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pham‐Huy L.A. He H. Pham‐Huy C.: ‘Free radicals, antioxidants in disease and health’, Int. J. Biomed. Sci., 2008, 4, (2), pp. 89 –96 [PMC free article] [PubMed] [Google Scholar]
- 18. Mittal A.K. Kaler A. Banerjee U.C.: ‘Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of Rhododendron dauricum ’, Nano Biomed. Eng., 2012, 4, (3), pp. 118 –124 [Google Scholar]
- 19. Rad J.S. Alfatemi S.M.H. Rad M.S. et al.: ‘In‐vitro antioxidant and antibacterial activities of Xanthium strumarium L. extracts on methicillin‐susceptible and methicillin‐ resistant Staphylococcus aureus ’, Anc. Sci. Life, 2013, 33, (2), p. 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moteriya P. Chanda S.: ‘Low cost and ecofriendly photosynthesis of silver nanoparticles using Cassia roxburghii stem extract and its antimicrobial and antioxidant efficacy’, Open J. Adv. Drug Deliv., 2014, 2, (4), pp. 557 –575 [Google Scholar]
- 21. Tillotsan G.S. Theriault N.: ‘New and alternative approaches to tackling antibiotics resistance’, F1000prime Rep., 2013, 5, (51), pp. 1 –9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeong S.H. Yeo S.Y. Yi S.C.: ‘The effect of filter particle size on the antibacterial properties of compounded polymer/silver fibers’, J. Mater. Sci., 2005, 40, pp. 5407 –5411 [Google Scholar]
- 23. Wellington Kevin W.: ‘Understanding cancer and the anticancer activities of naphthoquinones – a review’, RSC Adv., 2015, 5, (26), pp. 20309 –20338 [Google Scholar]
- 24. Sulaiman G.M. Mohammed W.H. Marzoog T.R. et al.: ‘Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract’, Asian Pac. J. Trop. Biomed., 2013, 3, (1), pp. 58 –63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. AshaRani P.V. Low KahMun G. Hande M.P. et al.: ‘Cytotoxicity and genotoxicity of silver nanoparticles in human cells’, ACS Nano, 2008, 3, (2), pp. 279 –290 [DOI] [PubMed] [Google Scholar]
- 26. Rao P.V. Nallappan D. Madhavi K. et al.: ‘Phytochemicals and biogenic metallic nanoparticles as anticancer agents’, Oxidative medicine and cellular longevity., 2016, pp. 1 –15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jeyaraj M. Sathishkumar G. Sivanandhan G. et al.: ‘Biogenic silver nanoparticles for cancer treatment: an experimental report’, Colloids Surf. B., 2013, 106, pp. 86 –92 [DOI] [PubMed] [Google Scholar]
- 28. Varghese C.P. Ambrose C. Jin S.C. et al.: ‘Antioxidant and anti‐inflammatory activity of Eurycoma longifolia Jack a traditional medicinal plant in Malaysia’, Int. J. Pharma. Sci. Nanotechnol., 2013, 5, (4), pp. 1875 –1878 [Google Scholar]
- 29. Mohd‐Fuat A.R . Kofi E.A. et al.: ‘Mutagenic and cytotoxic properties of three herbal plants from Southeast Asia’, Trop. Biomed., 2007, 24, (2), pp. 49 –59 [PubMed] [Google Scholar]
- 30. Danial M. Saghal G. Ahmad Mubbarakh S. et al.: ‘Antibacterial studies on in vitro plant parts of medicinally important Eurycoma longifolia (Tongkat Ali)’, Pakistan J. Bot., 2013, 45, (5), pp. 1693 –1700 [Google Scholar]
- 31. Farouk A.E. Benafri A.: ‘Antibacterial activity of Eurycoma longifolia Jack. A Malaysian medicinal plant’, Saudi Med. J., 2007, 28, (9), pp. 1422 –1424 [PubMed] [Google Scholar]
- 32. Jiwajinda S. Santisopasri V. Murakami A. et al.: ‘ In vitro anti‐tumor promoting and anti‐ parasitic activities of the quassinoids from Eurycoma longifolia, a medicinal plant in Southeast Asia’, J. Ethnopharmacol., 2002, 82, (1), pp. 55 –58 [DOI] [PubMed] [Google Scholar]
- 33. Purwantiningsih Hussin A.H. Chan K.L.: ‘Free radical scavenging activity of the standardized ethanolic extract of Eurycoma longifolia (TAF‐273)’, Int. J. Pharm. Pharma. Sci., 2011, 3, (4), pp. 343 –347 [Google Scholar]
- 34. Ang H.H. Cheang H.S.: ‘Studies on the anxiolytic activity of Eurycoma longifolia jack roots in mice’, Jpn. J. Pharmacol., 1999, 79, (4), pp. 497 –500 [DOI] [PubMed] [Google Scholar]
- 35. Husen R. Pihie A.H.L. Nallappan M.: ‘Screening for antihyperglycaemic activity in several local herbs of Malaysia’, J. Ethnopharmacol., 2004, 95, (2), pp. 205 –208 [DOI] [PubMed] [Google Scholar]
- 36. Logeswari P. Silambarasan S. Abraham J.: ‘Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties’, Sci. Iranica, 2013, 20, (3), pp. 1049 –1054 [Google Scholar]
- 37. Femila E.E. Srimathi R. Deivasigamani C.: ‘Removal of malachite green using silver nanoparticles via adsorption and catalytic degradation’, Int. J. Pharm. Pharma. Sci., 2014, 6, (8), pp. 579 –583 [Google Scholar]
- 38. Johnson A.S. Obota I.B. Ukponga U.S.: ‘Green synthesis of silver nanoparticles using Artemisia annua and Sidaacuta leaves extract and their antimicrobial, antioxidant and corrosion inhibition potentials’, J. Mater. Environ. Sci., 2014, 5, (3), pp. 899 –906 [Google Scholar]
- 39. Vanaja M. Gnanajobitha G. Paulkumar K. et al.: ‘Phytosynthesis of silver nanoparticles by Cissusquadrangularis: influence of physiochemical factors’, J. Nanostruct. Chem., 2013, 3, (1), p. 17 [Google Scholar]
- 40. Firdhouse M.J. Lalitha P.: ‘Green synthesis of silver nanoparticles using the aqueous extract of Portulacaoleracea (L.) ’, Asian J. Pharma. Clin. Res., 2012, 6, (1), pp. 92 –94 [Google Scholar]
- 41. Musarrat J. Dwivedi S. Singh B.R. et al.: ‘Production of antimicrobial silver nanoparticles in water extracts of the fungus Amylomyces rouxii strain KSU‐09’, Bioresour. Technology., 2010, 101, (22), pp. 8772 –8776 [DOI] [PubMed] [Google Scholar]
- 42. Ali K. Ahmed B. Dwivedi S. et al.: ‘Microwave accelerated green synthesis of stable silver nanoparticles with Eucalyptus globulus leaf extract and their antibacterial and antibiofilm activity on clinical isolates’, PloS One, 2015, 10, (7), p. e0131178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shankar S.S. Ahmad A. Sastry M.: ‘Geranium leaf assisted biosynthesis of silver nanoparticles’, Biotechnol. Prog., 2003, 19, (6), pp. 1627 –1631 [DOI] [PubMed] [Google Scholar]
- 44. Parashar U.K. Saxena P.S. Srivastava A.: ‘Bioinspired synthesis of silver nanoparticles’, Dig. J. Nanomater. Biostruct., 2009, 4, (1), pp. 159 –166 [Google Scholar]
- 45. Reddy M.C. Rama Murthy K.S. Srilakshmi A. et al.: ‘Phytosynthesis of eco‐friendly silver nanoparticles and biological applications – a novel concept in Nanobiotechnology’, Afr. J. Biotechnol., 2015, 14, (3), pp. 222 –247 [Google Scholar]
- 46. Bunghez I.R. Patrascu M.E.B. Badea N. et al.: ‘Antioxidant silver nanoparticles green synthesized using ornamental plants’, J. Optoelectron. Adv. Mater., 2012, 14, (11– 12), pp. 1016 –1022 [Google Scholar]
- 47. Ramteke C. Chakrabarti T. Sarangi B.K. et al.: ‘Synthesis of silver nanoparticles from the aqueous extracts of leaves of Ocimum sanctum for enhanced antibacterial activity’, J. Chem., 2013, pp. 1 –8 [Google Scholar]
- 48. Selvam G.G. Sivakumar K.: ‘Phycosynthesis of silver nanoparticles and photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Hypnea musciformis (Wulfen) JV Lamouroux ’, Appl. Nanosci., 2014, 5, (5), pp. 617 –622 [Google Scholar]
- 49. Awwad A.M. Salem N.M. Abdeen A.O.: ‘Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity’, Int. J. Ind. Chem., 2013, 4, (1), p. 29 [Google Scholar]
- 50. Sangeetha A. Singaravelu S.U.: ‘Investigation of free radical scavenging activity of biogenic silver nano particles’, Int. J. Pharma. Biosci., 2015, 6, (2), pp. 439 –445 [Google Scholar]
- 51. Kokila T. Ramesh P.S. Geetha D.: ‘A biogenic approach for green synthesis of silver nanoparticles using peel extract of Citrus sinensis and its application’, Int. J. ChemTech Res., 2015, 7, (2), pp. 804 –813 [Google Scholar]
- 52. Chandra Mohan S. Sasikala K. Anand T. et al.: ‘Green synthesis, antimicrobial and antioxidant effects of silver nanoparticles using Canthium coromandelicum leaves extracts’, Res. J. Microbiol., 2014, 9, (3), pp. 142 –150 [Google Scholar]
- 53. Koyyati R. Nagati V. Merugu R. et al.: ‘Biological synthesis of silver nanoparticles using Raphanus sativus var. longipinnatus leaf extract and evaluation of their antioxidant and antibacterial activity’, Int. J. Med. Pharma. Sci., 2013, 3, (4), pp. 89 –100 [Google Scholar]
- 54. Anju Varghesee R. Anandhi P. Arunadevi R. et al.: ‘Satin leaf (Chrysophyllum oliviforme) extract mediated green synthesis of silver nanoparticles: antioxidant and anticancer activities’, J. Pharma. Sci. Res., 2015, 7, (6), pp. 266 –273 [Google Scholar]
- 55. Valantina R.: ‘Selective ABTS and DPPH –radical scavenging activity of peroxide from vegetable oils’, Int. Food Res. J., 2015, 22, (1), pp. 289 –294 [Google Scholar]
- 56. Kokila T. Ramesh P.S. Geetha D.: ‘Biosynthesis of silver nanoparticles from Cavendish banana peel extract and its antibacterial and free radical scavenging assay: a novel biological approach’, Appl. Nanosci., 2015, 5, (8), pp. 911 –920 [Google Scholar]
- 57. Sathiyapriya D.G. R P.S.: ‘Antioxidant activity of biosynthesised Psidium guajava leaf extract mediated’, Int. J. Recent Sci. Res., 2014, 5, (9), pp. 1689 –1692 [Google Scholar]
- 58. Tenover F.C.: ‘Mechanisms of antimicrobial resistance in bacteria’, Am. J. Med., 2006, 119, (6 Suppl. 1), pp. S3 –S10, discussion S62‐S70 [DOI] [PubMed] [Google Scholar]
- 59. Kim J.S. Kuk E. Yu K.N. et al.: ‘Antimicrobial effects of silver nanoparticles’, Nanomed. Nanotechnol. Biol. Med., 2007, 3, (1), pp. 95 –101 [DOI] [PubMed] [Google Scholar]
- 60. Shrivastava S. Bera T. Roy A. et al.: ‘Characterization of enhanced antibacterial effects of novel silver nanoparticles’, Nanotechnology, 2007, 18, (22), p. 225103 [DOI] [PubMed] [Google Scholar]
- 61. Bhagavan N.V.: ‘Medical biochemistry’ (Academic Press, San Diego, 2002) [Google Scholar]
- 62. Petica A. Gavriliu S. Lungu M. et al.: ‘Colloidal silver solutions with antimicrobial properties’, Mater. Sci. Eng. B, 2008, 152, (1), pp. 22 –27 [Google Scholar]
- 63. Umadevi M. Rani T. Balakrishnan T. et al.: ‘Antimicrobial activity of silver nanoparticles prepared under an ultrasonic field’, Int. J. Pharma. Sci. Nanotechnol., 2011, 4, pp. 1491 –1496 [Google Scholar]
- 64. Sondi I. Salopek‐Sondi B.: ‘Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram‐negative bacteria’, J. Colloid Interface Sci., 2004, 275, (1), pp. 177 –182 [DOI] [PubMed] [Google Scholar]
- 65. Danilczuk M. Lund A. Sadio J. et al.: ‘Conduction electron spin resonance of small silver particles’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2006, 63, (1), pp. 189 –191 [DOI] [PubMed] [Google Scholar]
- 66. Matsumura Y. Yoshikata K. Kunisaki S.I. et al.: ‘Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate’, Appl. Environ. Microbiol., 2003, 69, (7), pp. 4278 –4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Feng Q.L. Wu J. Chen G.Q. et al.: ‘A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus ’, J. Biomed. Mater. Res., 2000, 52, (4), pp. 662 –668 [DOI] [PubMed] [Google Scholar]
- 68. Morones J.R. Elechiguerra J.L. Camacho A. et al.: ‘The bactericidal effect of silver nanoparticles’, Nanotechnology, 2005, 16, (10), p. 23446 [DOI] [PubMed] [Google Scholar]
- 69. Hatchett D.W. White H.S.: ‘Electrochemistry of sulfur adlayers on the low‐index faces of silver’, J. Phys. Chem., 1996, 100, (23), pp. 9854 –9859 [Google Scholar]
- 70. Patil S.V. Borase H.P. Patil C.D. et al.: ‘Biosynthesis of silver nanoparticles using latex from few euphorbian plants and their antimicrobial potential’, Appl. Biochem. Biotechnol., 2012, 167, (4), pp. 776 –790 [DOI] [PubMed] [Google Scholar]
- 71. Prabhu S. Poulose E.K.: ‘Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical application, and toxicity effects’, Int. Nano Lett., 2012, 2, (1), pp. 1 –10 [Google Scholar]
- 72. Chen S.F. Li J.P. Qian K. et al.: ‘Large scale photochemical synthesis of M@TiO2 nanocomposites (M = Ag, Pd, Au, Pt) and their optical properties, CO oxidation performance, and antibacterial effect’, Nano Res., 2010, 3, (4), pp. 244 –255 [Google Scholar]
- 73. Chudasama B. Vala A.K. Andhariya N. et al.: ‘Enhanced antibacterial activity of bifunctional Fe3 O4 ‐Ag core‐shell nanostructures’, Nano Res., 2009, 2, (12), pp. 955 –965 [Google Scholar]
- 74. Kuo P.C. Damu A.G. Lee K.H. et al.: ‘Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia ’, Biorg. Med. Chem., 2004, 12, pp. 537 –544 [DOI] [PubMed] [Google Scholar]
- 75. Tee T.T. Cheah Y.H. Hawariah L.P.A.: ‘F16, a fraction from Eurycoma longifolia jack extract, induces apoptosis via a caspase‐9‐independent manner in MCF‐7 cells’, Anticancer Res., 2007, 27, (5A), pp. 3425 –3430 [PubMed] [Google Scholar]
- 76. Al‐Salahi O.S.A. Ji D. Majid A.M.S.A. et al.: ‘Anti‐tumor activity of Eurycoma longifolia root extracts against K‐562 cell line: in vitro and in vivo study’, PLoS One, 2014, 9, (1), p. e83818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hajjouli S. Chateauvieux S. Teiten M.H. et al.: ‘Eurycomanone and eurycomanol from Eurycoma longifolia Jack as regulators of signaling pathways involved in proliferation, cell death and inflammation’, Molecules, 2014, 19, (9), pp. 14649 –14666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zakaria Y. Rahmat A. Pihie A.H.H.L. et al.: ‘Eurycomanone induce apoptosis in HepG2 cells via up‐regulation of p53’, Cancer cell Int., 2009, 9, (1), p. 16 [DOI] [PMC free article] [PubMed] [Google Scholar]


