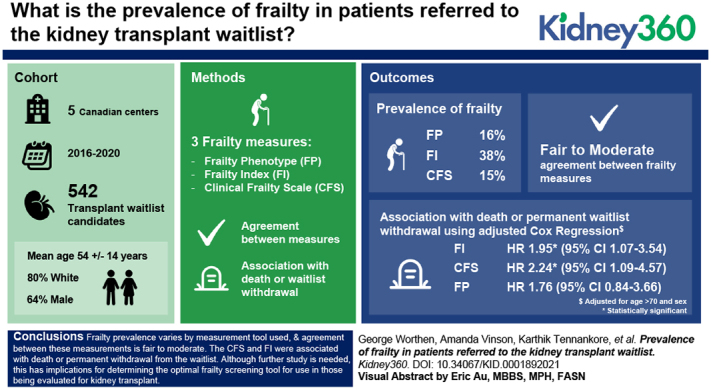
Visual Abstract
Keywords: transplantation, ESRD, frailty, geriatric nephrology, kidney, kidney transplantation, prevalence, waiting lists
Key Points
Frailty prevalence varies for the Frailty Phenotype, a frailty index, and the Clinical Frailty Scale in transplant candidates.
Agreement between these measures for determining frailty status was variable, suggesting they measure different aspects of frailty.
The frailty index and the Clinical Frailty Scale were associated with a shorter time to death or waitlist withdrawal in an unadjusted analysis.
Abstract
Background
Comparisons between frailty assessment tools for waitlist candidates are a recognized priority area for kidney transplantation. We compared the prevalence of frailty using three established tools in a cohort of waitlist candidates.
Methods
Waitlist candidates were prospectively enrolled from 2016 to 2020 across five centers. Frailty was measured using the Frailty Phenotype (FP), a 37-variable frailty index (FI), and the Clinical Frailty Scale (CFS). The FI and CFS were dichotomized using established cutoffs. Agreement was compared using κ coefficients. Area under the receiver operating characteristic (ROC) curves were generated to compare the FI and CFS (treated as continuous measures) with the FP. Unadjusted associations between each frailty measure and time to death or waitlist withdrawal were determined using an unadjusted Cox proportional hazards model.
Results
Of 542 enrolled patients, 64% were male, 80% were White, and the mean age was 54±14 years. The prevalence of frailty by the FP was 16%. The mean FI score was 0.23±0.14, and the prevalence of frailty was 38% (score of ≥0.25). The median CFS score was three (IQR, 2–3), and the prevalence was 15% (score of ≥4). The κ values comparing the FP with the FI (0.44) and CFS (0.27) showed fair to moderate agreement. The area under the ROC curves for the FP and FI/CFS were 0.86 (good) and 0.69 (poor), respectively. Frailty by the CFS (HR, 2.10; 95% CI, 1.04 to 4.24) and FI (HR, 1.79; 95% CI, 1.00 to 3.21) was associated with death or permanent withdrawal. The association between frailty by the FP and death/withdrawal was not statistically significant (HR, 1.78; 95% CI, 0.79 to 3.71).
Conclusion
Frailty prevalence varies by the measurement tool used, and agreement between these measurements is fair to moderate. This has implications for determining the optimal frailty screening tool for use in those being evaluated for kidney transplant.
Introduction
Frailty is a state of increased vulnerability due to degeneration in multiple systems, and has been shown to be highly predictive of morbidity and mortality in the elderly (1). Frailty can be defined by a Frailty Phenotype (FP), which is defined by physical characteristics, or as an accumulation of deficits across multiple domains, as in a frailty index (FI) (2).
Regardless of the definition used, frailty is highly prevalent in patients with kidney disease. Among those receiving dialysis, pooled prevalence using the FP has been reported at 37% (3). In those post–kidney transplantation, the prevalence has been estimated at almost half of that amount (20%) (4). Not only is frailty highly prevalent in patients with kidney disease, it is also associated with morbidity and mortality, similarly to those without kidney disease (5).
To date, few studies describe the prevalence of frailty in those being evaluated for kidney transplant, despite a recent multinational position paper identifying the need to include frailty in the waitlist evaluation as a priority area for transplantation (6). In a large cohort study measuring frailty in candidates for kidney transplant by the FP, 13% of the cohort was considered frail (7). Although informative, it is known that frailty status differs by the instrument used (8). It is uncertain which instruments yield the most clinically relevant measurement of frailty or how frail transplant candidates differ between measures. Gaining a better understanding of the differences in measured frailty using alternate tools in those being evaluated for kidney transplant has recently been identified as an important area of research (9).
We conducted a multicenter cohort study of Canadian kidney transplant waitlist candidates to (1) describe the prevalence of frailty using three commonly used assessment tools (the FP, the Clinical Frailty Scale [CFS], and an FI), (2) identify patient characteristics associated with frailty by each measure, (3) determine the level of agreement between measures, (4) assess the ability of the CFS and FI at predicting frailty status by the FP, and (5) determine the effect of frailty by each measure on time to death or permanent withdrawal from the waitlist.
Materials and Methods
Study Design and Participants
Five Canadian tertiary care centers that evaluate patients for waitlist eligibility (Nova Scotia, N=1; New Brunswick, N=1; Quebec, N=1; and Ontario, N=2) enrolled patients and collected data for this longitudinal cohort study (10). The aim of the primary study was to evaluate frailty among patients activated to the waitlist (on the basis of an assessment using all three measures conducted ±6 months from the waitlist date) and risk of death or permanent withdrawal from the waitlist. However, acknowledging differences in the process for waitlist activation at each site, in this study, we included patients whether or not their workup was complete. Patients unwilling to consent or unable to complete the frailty assessments without the help of a substitute decision maker were excluded. Only the first assessment of frailty with all three measures was used for participants with follow-up visits. In a prespecified sensitivity analysis, we restricted the cohort to participants with all three measures completed within 6 months of their activation date (Figure 1). This study was approved by the research ethics boards of all participating centers.
Figure 1.
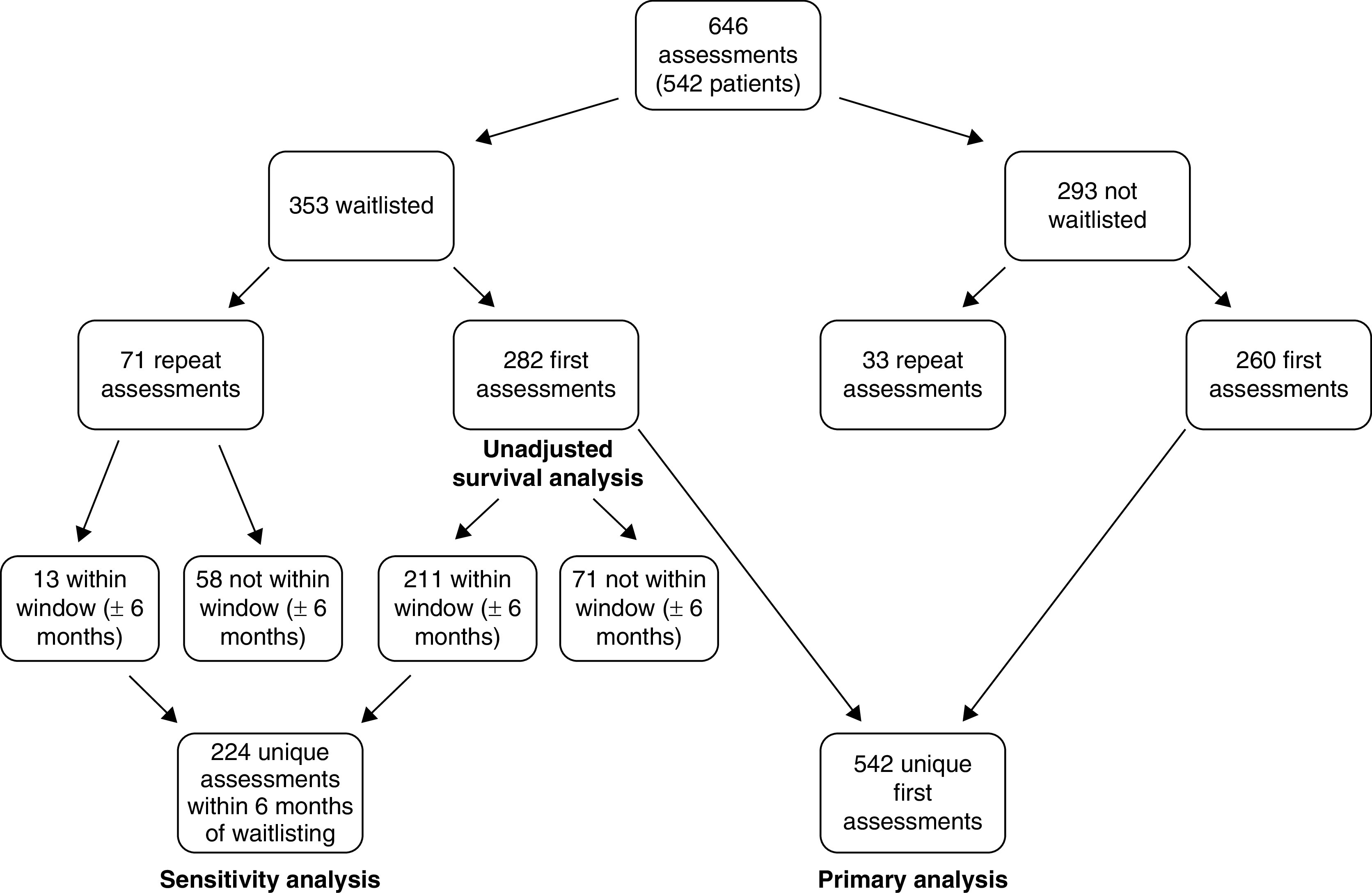
Cohort derivation.
Outcome Definitions
Frailty status was assessed using the FP, an FI, and the CFS. The FP comprises five components: shrinking (self-report of >5% unintentional weight loss over the preceding year on the basis of dry weight), exhaustion (self-report), weakness (average grip strength below an established cutoff), inactivity (estimated kilocalories per week below an established cutoff), and slowness (timed walk below an established cutoff) (11). This measure was chosen as the primary assessment tool due to the breadth of prior study validity (12–14). Both the Minnesota Leisure Time Activities (MLTA) scale and the International Physical Activity Questionnaire (IPAQ) were used to assess inactivity (15,16). The MLTA, being the scale used in the original FP, was used preferentially, and the IPAQ was only used in patients where the MLTA was not performed (n=41, 8%). To calculate a patient’s frailty score, each measure was graded as either present (one) or absent (zero), and then summed. A score of three or more classified a patient as frail, one or two classified a patient as prefrail, and zero classified a patient as fit (11).
An FI is a measure of deficit accumulation with characteristic properties that is cohort specific and contains ≥30 variables across multiple domains (17). The FI score is the ratio of deficits present in an individual to the total number of index deficits, with scores ranging from zero to one. The approach to creating an FI has been validated regardless of the items used, and FIs can use health record data and patient self-report items (18). We developed a 37-item transplant waitlist–specific FI using a standardized approach with an expert panel (a geriatrician, three transplant nephrologists, and a general nephrologist) for content validity. This index was tested in a cross-section of transplant candidates from Halifax, Nova Scotia (Supplemental Table 1). The mean index score was 0.15±0.10 (five out of 37 items) and the maximum score was 0.44 (16 items) (10). In prespecified sensitivity analyses, because the transplant-specific FI included the five components of the FP among its 37 deficits, we also analyzed agreement using a 32-item FI (without the FP components).
The CFS relies on clinical judgment to score a patient’s frailty severity on a scale from one (very fit) to eight (very severely frail). The CFS is highly reliable (r=0.84–0.95) for multiple raters in a dialysis population, and is correlated with the FI in the general (r=0.80) and dialysis population (r=0.57) (19).
At sites where waitlist eligibility is assessed in person by one nephrologist (N=3), site research coordinators conducted the tests and questionnaires to determine the FP and FI on this date. For the two remaining sites (in Nova Scotia and New Brunswick), acceptance to the waitlist is made by a committee along with an in-person assessment by the patients’ primary nephrologist. Eligible patients affiliated with these sites were identified by transplant recipient coordinators, and frailty assessments were performed by site research coordinators. For the FP and FI, all physicians assessing patients for waitlist eligibility were blinded to the results. In contrast, the CFS was assessed by the physician determining waitlist eligibility and/or the patient’s primary nephrologist.
Patients were followed for outcomes (death, transplant, permanent and temporary withdrawal) until March 2021. For the survival analysis, a composite outcome of death or permanent withdrawal from the waitlist was used. For the purposes of this analysis, patients who were withdrawn on a temporary basis for >90 days and who were not reactivated during the study period were also considered to be permanently withdrawn.
Potential Confounders and Additional Baseline Characteristics
Baseline data were collected prospectively on patients at each site by research coordinators, and this included demographic information (age, race, and sex), characteristics and comorbid conditions determined from chart documentation (body mass index, number of medications, diabetes, coronary artery disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, history of prior malignancy, chronic lung disease, chronic liver disease, prior failed kidney transplant, cause of ESKD, and dialysis information), and baseline laboratory investigations (hemoglobin, albumin, and parathyroid hormone).
Statistical Analyses
Descriptive statistics were reported as counts and percentages for categoric variables, means±SDs for normally distributed continuous variables, and medians and interquartile ranges for non-normally distributed continuous variables. The prevalence of frailty by the FP was reported using a cutoff of three or more. The prevalence of frailty by the FI and CFS were determined using established cutoffs (≥0.25 for the FI and four or more for the CFS) (20,21). These cutoffs were also used for determining diagnostic characteristics and calculating κ coefficients. To preserve the continuous nature of both the FI and CFS, area under the receiver operating characteristic curves (AUCs) were calculated, using the FP as the “gold standard.” Factors associated with frailty were determined using logistic regression and expressed as adjusted odds ratios (aORs).
κ Coefficients and AUCs were determined for unadjusted walk time as a comparator, given its close association with the FP (22). All analyses were repeated in a prespecified sensitivity analysis, restricted to those individuals with assessments 6 months before or after their date of waitlist activation.
Finally, a survival analysis was performed using both unadjusted and adjusted Cox proportional hazards model to determine the effect of frailty by each assessment tool on time to death or permanent withdrawal from the waitlist for those who were waitlisted.
Results
A total of 542 unique patients were enrolled between June 2016 and February 2020. Among them, the mean±SD age was 54±14 years, 36% were female, and 39% were obese (body mass index ≥30 kg/m2). In addition, 52% were receiving hemodialysis (hospital, satellite, and home based), 20% were receiving peritoneal dialysis, and 24% of the population had not yet been initiated on kidney replacement therapy (Supplemental Table 2).
Prevalence and Characteristics of Frailty
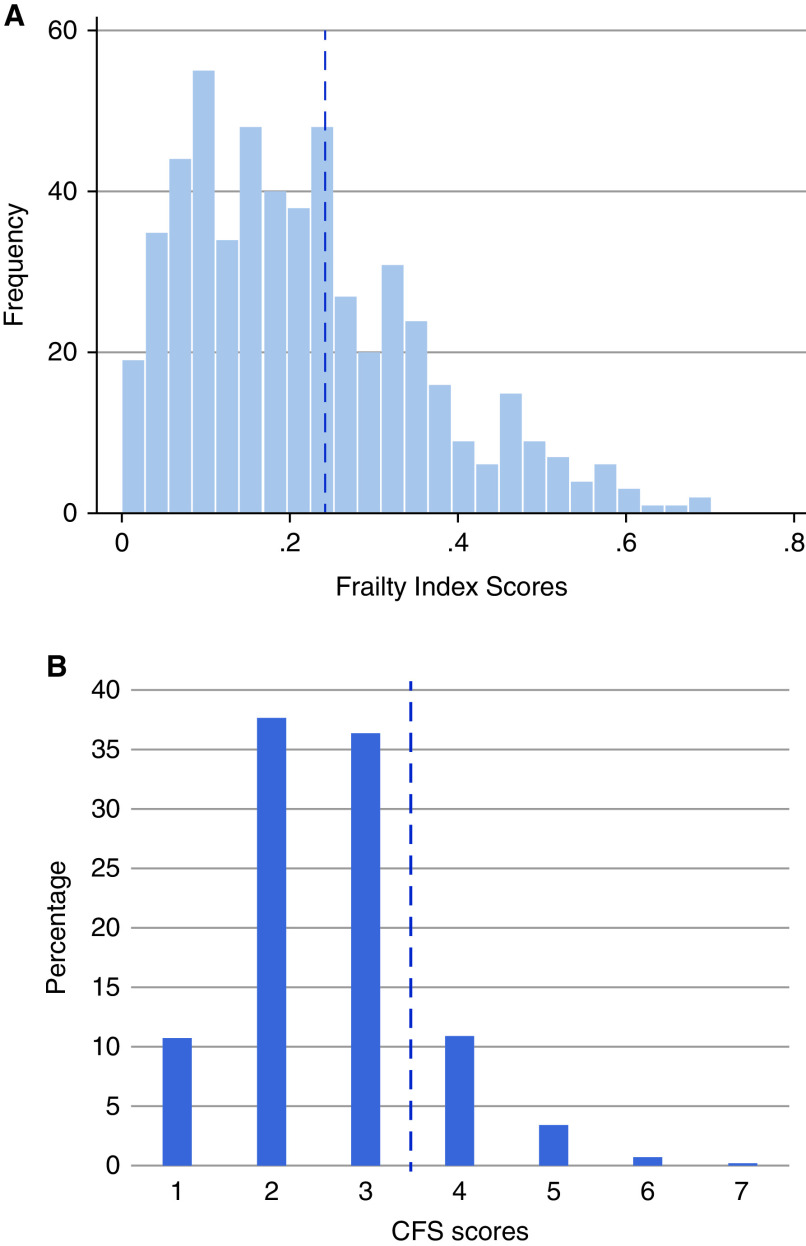
The characteristics of patients classified as frail by the FP, FI, and CFS are detailed in Table 1 and Supplemental Table 3. The prevalence of frailty was 16% by the FP, 34% by the FI, and 15% by the CFS. Impaired grip strength was the most common FP impairment (41%), whereas slow walk time was the least common (17%) (Supplemental Figure 1A). The average FI score was 0.22±0.14 (Supplemental Table 4), and scores ranged from zero to 0.70 (Figure 2A). The social/cognitive domain of the FI had the most significant impairment (Supplemental Figure 1B). According to the CFS, most patients were felt to be “managing well” (36%), corresponding to a score of three (Figure 2B).
Table 1.
Differences in patient characteristics among patients assessed as frail according to the FP, FI, and CFS
| Variable | Frailty Phenotype Score of ≥3 (n=88, 16%) | Frailty Index Score of ≥0.25 (n=183, 34%) | Clinical Frailty Scale Score of ≥4 (n=81, 15%) |
| Demographics | |||
| Age (yr), mean±SD | 58±14 | 57±13 | 46±12 |
| Age >65 yr, n (%) | 36 (41) | 56 (31) | 27 (34) |
| Male sex, n (%) | 51 (58) | 114 (62) | 46 (57) |
| White race, n (%) | 73 (83) | 145 (79) | 62 (77) |
| Site, n (%) | |||
| Atlantic Canada | 11 (13) | 32 (17) | 20 (25) |
| Hamilton | 14 (16) | 32 (17) | 26 (32) |
| London | 60 (68) | 115 (63) | 27 (33) |
| Montreal | 3 (3) | 4 (2) | 8 (10) |
| Cause of ESKD, n (%) | |||
| DM | 35 (40) | 74 (40) | 30 (37) |
| Vascular/HTN | 5 (6) | 11 (6) | 1 (1) |
| GN | 20 (23) | 40 (26) | 18 (22) |
| Congenital | 12 (14) | 21 (11) | 9 (11) |
| Structural | 5 (6) | 10 (5) | 7 (9) |
| Other | 11 (13) | 27 (15) | 16 (20) |
| Dialysis vintage yr, median (IQR) | 1.2 (0.4–2.2) | 1.2 (0.2–2.2) | 2.0 (1.2–2.9) |
| Dialysis type, n (%) | |||
| HD | 54 (61) | 107 (58) | 55 (68) |
| PD | 20 (23) | 46 (25) | 18 (22) |
| Pre-emptive | 13 (15) | 28 (15) | 5 (6) |
| Unknown | 1 (1) | 2 (1) | 3 (4) |
| Laboratory investigations, mean±SD | |||
| Albumin (g/L) | 37±6 | 37±5 | 36±6 |
| Hemoglobin (g/L) | 108±14 | 108±15 | 107±15 |
| Parathyroid hormone (pg/mL) | 51±53 | 50±48 | 57±54 |
| Comorbidities, n (%) | |||
| DM | 45 (51) | 99 (54) | 46 (57) |
| CAD | 25 (28) | 52 (29) | 22 (27) |
| CHF | 28 (32) | 72 (39) | 25 (31) |
| CVD | 12 (14) | 23 (13) | 12 (15) |
| PVD | 9 (10) | 20 (11) | 8 (10) |
| Chronic lung disease | 5 (6) | 15 (8) | 10 (12) |
| Chronic liver disease | 6 (7) | 11 (6) | 8 (10) |
| Previous malignancy | 14 (16) | 27 (15) | 13 (16) |
| Previous kidney transplant, n (%) | 6 (7) | 18 (10) | 8 (10) |
| BMI, n (%) | |||
| Underweight (<18.5 kg/m2) | 2 (2) | 7 (4) | 4 (5) |
| Normal (18.5–24.9 kg/m2) | 24 (27) | 39 (21) | 16 (20) |
| Overweight (25.0–29.9 kg/m2) | 26 (30) | 56 (31) | 21 (26) |
| Obese 1 (30.0–34.9 kg/m2) | 22 (25) | 53 (29) | 23 (28) |
| Obese 2 (35.0–39.9 kg/m2) | 12 (14) | 22 (12) | 10 (12) |
| Obese 3 (≥ 40.0 kg/m2) | 2 (2) | 6 (3) | 7 (9) |
| Karnofsky Performance Scale, n (%) | |||
| Fully active | 20 (23) | 55 (27) | 14 (17) |
| Ambulatory, light work | 50 (57) | 110 (54) | 39 (48) |
| Ambulatory, self-care | 9 (10) | 23 (11) | 16 (20) |
| Limited self-care, 50% of time in bed | 1 (1) | 3 (1) | 3 (4) |
| Disabled | 1 (1) | 2 (1) | 1 (1) |
| Unknown | 7 (8) | 12 (6) | 8 (10) |
| Activated to waitlist | 47 (53) | 107 (52) | 39 (48) |
FP, Frailty Phenotype; FI, frailty index; CFS, Clinical Frailty Scale; DM, diabetes mellitus; HTN, hypertension; IQR, interquartile range; HD, hemodialysis; PD, peritoneal dialysis; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cerebrovascular disease; PVD, peripheral vascular disease; BMI, body mass index.
Figure 2.
Distribution of (A) Clinical Frailty Scale (CFS) and (B) frailty index scores. Dotted lines represent cutoffs of four or more (CFS) and ≥0.25 (FI) used to determine frailty status.
Factors Associated with Frailty
Diabetes was associated with frailty by all three measures (Table 2, Supplemental Table 5). Being older than 65 years was associated with the FP (aOR, 2.44; 95% CI, 1.45 to 4.09), whereas the presence of coronary disease, heart failure, and cerebrovascular disease were only associated with frailty by the FI. The presence of chronic liver disease was associated with frailty by the CFS (aOR, 2.52; 95% CI, 1.01 to 6.28), as was having been on dialysis for over a year (aOR, 4.67; 95% CI, 2.56 to 8.49).
Table 2.
Clinical factors associated with frailty as measured by the Frailty Phenotype, frailty index and Clinical Frailty Scale score
| Variable | Adjusted Odds Ratio (95% CI) | ||
| FP (≥3) | FI (≥0.25) | CFS (≥4) | |
| Age >65 yr | 2.44 (1.45 to 4.09) | 1.31 (0.84 to 2.05) | 1.30 (0.74 to 2.31) |
| Male sex | 0.58 (0.35 to 0.95) | 0.74 (0.49 to 1.01) | 0.57 (0.33 to 0.96) |
| DM | 1.75 (1.07 to 2.86) | 2.35 (1.59 to 3.47) | 2.15 (1.26 to 3.67) |
| CAD | 1.57 (0.86 to 2.88) | 1.71 (1.03 to 2.85) | 1.21 (0.63 to 2.33) |
| CHF | 0.98 (0.58 to 1.68) | 1.96 (1.29 to 2.98) | 1.03 (0.58 to 1.83) |
| CVD | 1.91 (0.87 to 4.20) | 2.26 (1.10 to 4.64) | 1.78 (0.80 to 3.98) |
| PVD | 0.98 (0.41 to 2.37) | 1.36 (0.66 to 2.78) | 0.84 (0.32 to 2.18) |
| Chronic lung disease | 0.53 (0.19 to 1.48) | 0.87 (0.42 to 1.82) | 1.57 (0.68 to 3.61) |
| Chronic liver disease | 1.14 (0.44 to 2.95) | 1.06 (0.48 to 2.35) | 2.52 (1.01 to 6.28) |
| Dialysis vintage >1 year | 1.26 (0.76 to 2.08) | 1.32 (0.89 to 1.96) | 4.67 (2.56 to 8.49) |
FP, Frailty Phenotype; FI, frailty index; CFS, Clinical Frailty Score; DM, diabetes mellitus; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cerebrovascular disease; PVD, peripheral vascular disease.
Comparison of Frailty Assessment Tools
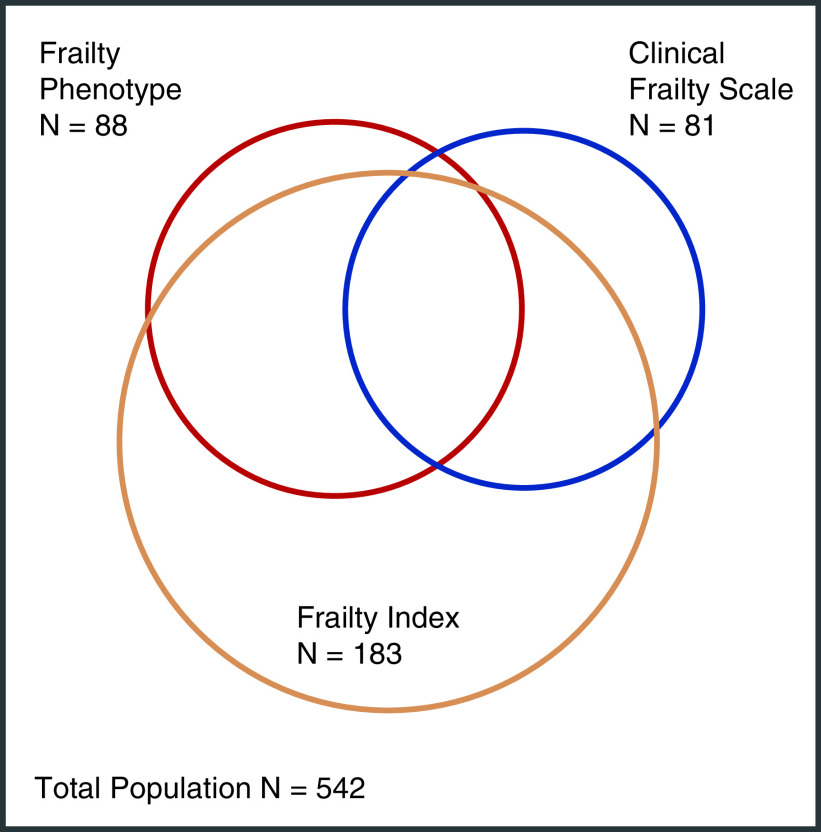
The κ agreement among the different frailty assessment tools is displayed in Table 3 and Supplemental Table 6 and ranged from 0.27 (FP and 32-item FI) to 0.44 (FP and 37-item FI). The greatest agreement occurred between unadjusted walk time and the FP (0.51). Simple agreement between the three frailty measures is represented visually in Figure 3.
Table 3.
Agreement between frailty assessment tools represented as κ coefficients
| Assessment Tools | κ Coefficients | Simple Agreement |
| FP versus FI | 0.44 | 0.78 |
| FP versus FI32 | 0.27 | 0.70 |
| FP versus CFS | 0.27 | 0.81 |
| FI37 versus CFS | 0.30 | 0.73 |
| FI32 versus CFS | 0.29 | 0.71 |
| WT versus FP | 0.51 | 0.86 |
Using predefined cutoffs of four or more for the CFS, ≥0.25 for the FI, and the FP definition for impaired walk time. FP, Frailty Phenotype; FI, frailty index; FI32, frailty index with Frailty Phenotype components removed; CFS, Clinical Frailty Scale; FI37, frailty index including only data able to be acquired remotely; WT, walk time.
Figure 3.
Proportional Venn diagram depicting overlap of frailty status as determined by the Frailty Phenotype, frailty index, and CFS.
Diagnostic characteristics of the different frailty assessment tools using the FP as the gold standard are represented in Table 4.
Table 4.
Diagnostic characteristics and survival analysis of frailty screening tools
| Assessment Tool Compared with the FP | Sensitivitya | Specificitya | PPVa | NPVa | AUC (95% CI) | N=282; Outcome=46 | |
| Unadjusted HR for Death or Permanent Withdrawal from the Waitlist (95% CI)a | Adjusted HR for Death or Permanent Withdrawal from the Waitlist (95% CI)a,b | ||||||
| FP | NA | NA | NA | NA | NA | 1.78 (0.86 to 3.71) | 1.76 (0.84 to 3.66) |
| FI | 0.864 | 0.764 | 0.415 | 0.967 | 0.863 (0.825 to 0.902) | 1.79 (1.00 to 3.21) | 1.95 (1.07 to 3.54) |
| FI32 | 0.716 | 0.696 | 0.313 | 0.927 | 0.788 (0.737 to 0.839) | 1.59 (0.89 to 2.88) | 1.73 (0.95 to 3.15) |
| CFS | 0.376 | 0.890 | 0.395 | 0.882 | 0.689 (0.631 to 0.747) | 2.10 (1.04 to 4.24) | 2.24 (1.09 to 4.57) |
| WT | 0.614 | 0.912 | 0.574 | 0.924 | 0.835 (0.784 to 0.885) | 1.18 (0.57 to 2.47) | 1.24 (0.59 to 2.62) |
FP, Frailty Phenotype; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the receiver operating characteristic curve; HR, hazard ratio; NA, not applicable; FI, frailty index; FI32, Frailty Index with FP components removed; CFS, Clinical Frailty Scale; WT, walk time.
Using predefined cutoffs of four or more for the CFS, ≥0.25 for the FI, and the FP definition for impaired walk time.
Adjusted for age >70 years and sex.
AUC values were highest for the 37-item FI (0.86); however, this decreased when the FP components were removed (0.79). The CFS resulted in an AUC value of 0.69 (Table 4, Supplemental Table 7).
Survival Analysis
A total of 282 patients were waitlisted during the study period, of which four died and 42 were withdrawn from the waitlist for a total of 46 outcomes. Unadjusted survival analysis of the waitlisted patients for the FP, FI, and CFS revealed hazard ratios of 1.78, 1.79, and 2.10, respectively, with only the CFS and FI achieving statistical significance. When this analysis was repeated with adjustment for age >70 and sex, the association remained (Figure 4,Table 4).
Figure 4.
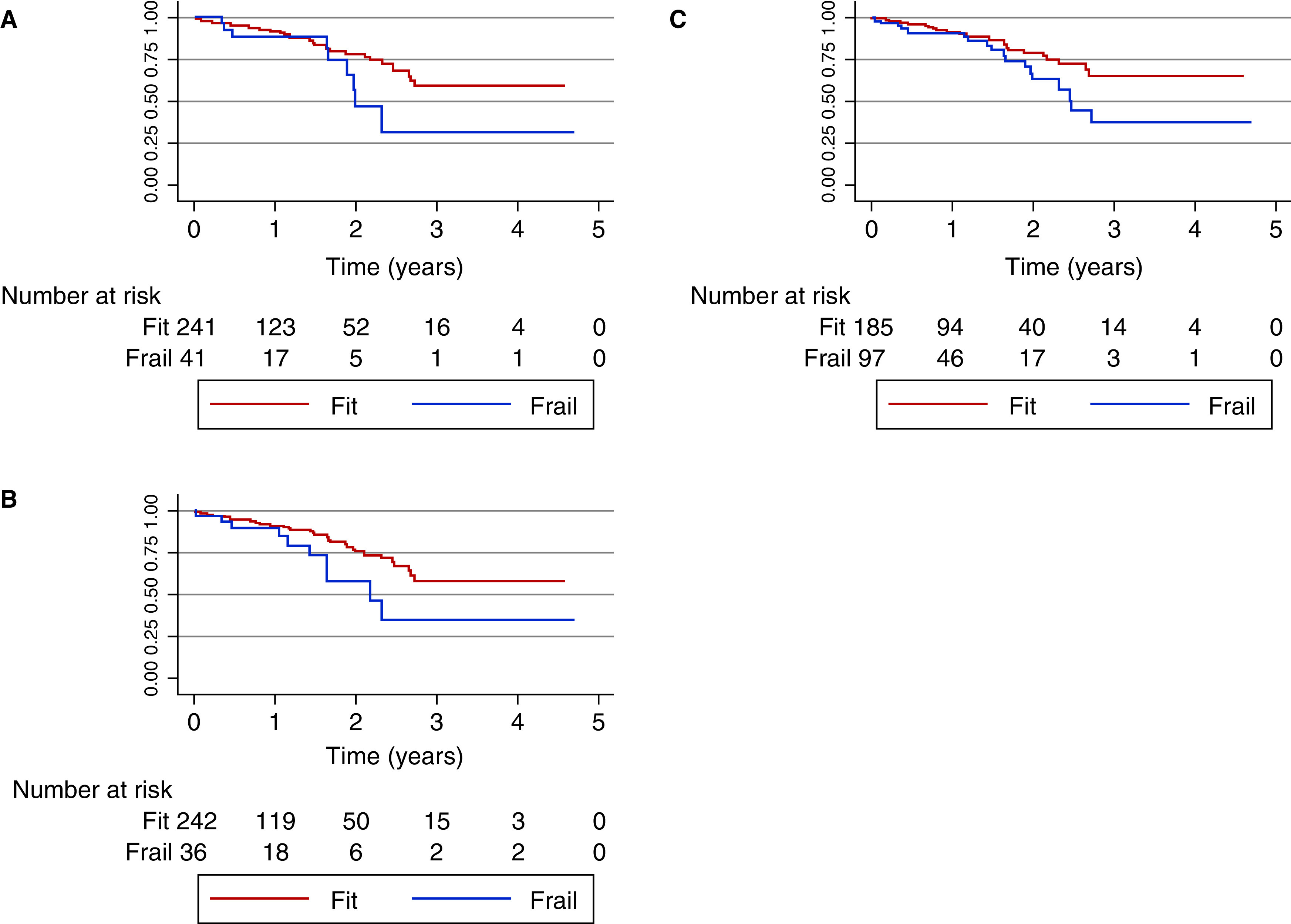
Kaplan–Meier curves for death or permanent withdrawal from the waitlist for frailty as defined by the (A) Frailty Phenotype, (B) frailty index, and (C) CFS.
Discussion
In this prospective cohort of kidney transplant waitlist candidates, we assessed three clinical assessment tools, two of which have not previously been studied in this population (7). We described the prevalence of frailty, identified characteristics associated with frailty, and assessed agreement between all three frailty definitions.
The prevalence of frailty in our cohort varied depending on which assessment tool was used. The prevalence of frailty by the FP (16%) in our cohort was in keeping with that reported in similar populations (7,23,24). Prevalence was higher using the FI (34%), likely due to the broader definition of frailty on which it is based. A previous study comparing the FP and FI in community-dwelling elderly patients found a similar disparity, with 18% of patients being frail by the FP and 48% by the FI (25).
The CFS yielded a comparable prevalence to the FP (15%). Previous studies using the CFS in those ≥65 years have reported a much higher prevalence, in the range of 40%–50% (26,27). There are multiple possible explanations for this finding. Firstly, patients in our cohort may have been “prescreened” before being referred for kidney transplantation. Because the CFS relies on clinical judgment and vignettes to assess cognitive and functional status, it is possible that patients who would be expected to score highly on the scale are not being referred for kidney transplantation. Additionally, tools that rely on clinical judgment may miss subclinical frailty identified by more objective measures, especially in cases where assessors are not familiar with the tool.
Although factors associated with frailty varied by assessment method, the presence of diabetes was associated with frailty status by all three measures. This is unsurprising, because the relationship between diabetes and frailty is well described (28). In addition to diabetes, the presence of coronary disease, heart failure, and cerebrovascular disease were associated with frailty by the FI. However, all of these comorbidities are also deficits included in the calculation of FI scores, which at least partially explains their association. Finally, dialysis vintage was associated with frailty only by the CFS. This may speak to the negative effect dialysis appears to have on functional status (29), or could represent bias; clinicians may expect that patients who have been on dialysis longer are more frail, and unconsciously score them higher.
κ Agreement and AUC values varied by assessment tool but were ultimately underwhelming. The κ agreement was generally fair, with values between 0.2 and 0.4, the only exceptions being those between the FP and the 37-item FI (which includes the five components of the FP) and walk time (one of the five FP components). In terms of AUC values, the FI predicted frailty status by the FP well; however, the predictive ability decreased when FP components were removed. The CFS predicted frailty status by the FP poorly. The low κ agreement and AUC values for the CFS and the FP are surprising; previous work in the general elderly population and in those on dialysis have shown κ agreement in the moderate to substantial range, and AUC values in the good to excellent range (22,30). These findings could be explained by the population studied; patients referred to the transplant waitlist are screened for eligibility and could reasonably be expected to be fitter than the general dialysis and elderly populations. Perhaps each frailty tool is capturing different aspects of vulnerability within our population, at a relatively early stage. If the tools are capturing different elements of vulnerability with a shared end state (severe frailty), this could explain the differing levels of agreement between our studies and those before it.
All assessment tools studied were found to have higher negative predictive values (NPVs) than positive predictive values. The NPVs for the FI (with and without the components of the FP included) were particularly strong, suggesting that a score <0.25 likely means that a patient will not be deemed frail by the FP. Having an impaired walk time also had a strong NPV, suggesting that patients with conserved gait speed are unlikely to be frail. In our cohort, impaired walk time was the least prevalent deficit measured by the FP (17%), a finding also demonstrated in patients post–kidney transplant (31). Taken together, this suggests that gait impairment is a late finding along the spectrum of frailty, and that, once present, a patient’s frailty severity can be expected to be significant.
In both an unadjusted and adjusted Cox survival analysis, the CFS and FI, but not the FP, predicted death or permanent withdrawal from the waitlist. Although this observation differs when compared with a similar analysis conducted in the United States using the FP (23), it is important to acknowledge that the point estimates of the association between frailty and death/withdrawal were comparable for both studies. It is possible that this preliminary analysis may be underpowered (10); therefore, further study is needed to recommend one frailty assessment more definitively over another in this population, and to truly ascertain the association between each measure and the outcome of death/withdrawal.
This study has several strengths. It is the first study to assess frailty in patients referred to the kidney transplant waitlist using a FI and the CFS (9), and the first to compare these tools with the FP. It is also one of the few studies to assess frailty in Canadian kidney transplant candidates, and the inclusion of subjects from multiple sites increased diversity within the population. The prospective nature of frailty assessments limited potential bias and improved the accuracy of data collection. The cohort was of sufficient size to allow for multivariate analysis and included subjects of all ages, not just older adults.
Despite these strengths, there are limitations. As stated above, the small sample size for outcomes at this early stage of the primary study likely means that our study was underpowered to make a definitive statement regarding the true effect of frailty status. Further study is necessary to make more definitive statements regarding which frailty screening tool is best for patients being evaluated for kidney transplant.
In summary, frailty is highly prevalent in patients referred for the transplant waitlist, and prevalence varies depending on the assessment tool used. Factors associated with frailty differed depending on which tool was used, and agreement between assessment tools was only fair to moderate, suggesting that each tool may be measuring a different aspect of vulnerability within this population. In an unadjusted analysis, the CFS and FI, but not the FP, increased the risk of death or permanent withdrawal from the waitlist. Further study is needed to make a more definitive statement on which frailty assessment should be favored in this population.
Disclosures
H. Cardinal reports receiving research funding from Astellas to the amount of $50,000 per year for the maintenance of the kidney transplant biobank at the Centre Hospitalier de l’Université de Montreal. This biobank is one of the biobanks affiliated with the Canadian Donation and Transplantation Research Program. N. Gogan reports serving on the board of directors for Atlantic Branch of Kidney Foundation of Canada. L. Gunaratnam reports receiving research funding from the Canadian Donation and Transplantation Program, Canadian Institutes of Health Research, and Kidney Foundation of Canada; and having consultancy agreements with, and honoraria from, Novartis and Paladin Labs Inc. T. Keough-Ryan reports receiving honoraria from Alexion Pharma Canada, Doctors Nova Scotia, and Novartis Pharma Canada Inc. R. S. Suri reports receiving honoraria from Amgen, Janssen, and Otskuka; and serving as a scientific advisor for, or member of, the Canadian Institutes of Health Research Institute of Cardiorespiratory Health and the Canadian Society of Nephrology. N. Tangri reports receiving honoraria from AstraZeneca Inc., Bayer, BI-Lilly, Janssen, Otsuka Pharmaceuticals, and Pfizer; receiving research funding from AstraZeneca Inc., Bayer, BI-Lilly, Janssen, Otsuka, and Tricida Inc.; having ownership interest in Clinpredict Ltd., Healthlogic, Mesentech Inc., PulseData Inc., Renibus, and Tricida Inc.; having consultancy agreements with Healthlogic, Mesentech Inc., PulseData Inc., Renibus, and Tricida Inc.; serving as a scientific advisor for, or member of, Mesentech, Pulsedata Inc., Renibus, and Tricida Inc.; and having other interests in/relationships with the National Kidney Foundation. K. Tennankore reports receiving research funding from Astellas Canada and Otsuka Canada; receiving honoraria from, and serving on a speakers bureau for, AstraZeneca, Bayer, and Otsuka; and having consultancy agreements with AstraZeneca, Baxter, Janssen, and Otsuka. A. Vinson reports having consultancy agreements with, and receiving research funding from, Paladin Labs Inc. K. West reports having consultancy agreements with, and receiving honoraria from, Envarsus Canada. All remaining authors have nothing to disclose.
Funding
This work was supported by Astellas Pharma Canada; Gouvernement du Canada, Canadian Institutes of Health Research (grant 407165), and the Kidney Foundation of Canada.
Author Contributions
H. Cardinal, S. Doucette, N. Gogan, L. Gunaratnam, T. Keough-Ryan, B. A. Kiberd, B. Prasad, K. Rockwood, R. S. Suri, N. Tangri, K. Tennankore, A. Vinson, M. Walsh, K. West, G. Worthen, and S. Yohanna reviewed and edited the manuscript; S. Doucette, L. Sills, K. Tennankore, and A. Vinson were responsible for methodology; K. Rockwood, K. Tennankore, A. Vinson, and G. Worthen conceptualized the study; L. Sills and K. Tennankore were responsible for resources and provided supervision; L. Sills, K. Tennankore, and G. Worthen were responsible for data curation and project administration; K. Tennankore was responsible for funding acquisition; K. Tennankore and G. Worthen were responsible for formal analysis, investigation, and validation; and G. Worthen was responsible for software and visualization and wrote the original draft.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0001892021/-/DCSupplemental.
Prevalence of individual components of the Frailty Phenotype (A) as well as mean FI scores by domain (B). Download Supplemental Figure 1, PDF file, 282 KB (853KB, pdf)
Prevalence of frailty index components in a cross section of transplant candidates from Halifax, NS. Download Supplemental Table 1, PDF file, 282 KB (853KB, pdf)
Baseline characteristics. Download Supplemental Table 2, PDF file, 282 KB (853KB, pdf)
Differences in demographics among patients by frailty status according to the Frailty Phenotype. Download Supplemental Table 3, PDF file, 282 KB (853KB, pdf)
Frailty index and Clinical frailty scale scores for the overall population and across subgroups. Download Supplemental Table 4, PDF file, 282 KB (853KB, pdf)
Clinical factors associated with frailty as measured by the FI, and CFS treated as continuous variables. Download Supplemental Table 5, PDF file, 282 KB (853KB, pdf)
Agreement between frailty assessment tools represented as kappa coefficients for the sensitivity analysis population. Download Supplemental Table 6, PDF file, 282 KB (853KB, pdf)
Diagnostic characteristics of frailty screening tools for the sensitivity analysis population. Download Supplemental Table 7, PDF file, 282 KB (853KB, pdf)
References
- 1.Kojima G, Iliffe S, Walters K: Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 47: 193–200, 2018. 10.1093/ageing/afx162 [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J: Frailty consensus: A call to action. J Am Med Dir Assoc 14: 392–397, 2013. 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima G: Prevalence of frailty in end-stage renal disease: A systematic review and meta-analysis. Int Urol Nephrol 49: 1989–1997, 2017. 10.1007/s11255-017-1547-5 [DOI] [PubMed] [Google Scholar]
- 4.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, Chow E, Alachkar N, Desai N, Varadhan R, Walston J, Segev DL: Frailty and mortality in kidney transplant recipients. Am J Transplant 15: 149–154, 2015. 10.1111/ajt.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury R, Peel NM, Krosch M, Hubbard RE: Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr 68: 135–142, 2017. 10.1016/j.archger.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Segall L, Nistor I, Pascual J, Mucsi I, Guirado L, Higgins R, Van Laecke S, Oberbauer R, Van Biesen W, Abramowicz D, Gavrilovici C, Farrington K, Covic A: Criteria for and appropriateness of renal transplantation in elderly patients with end-stage renal disease: A literature review and position statement on behalf of the European Renal Association-European Dialysis and Transplant Association Descartes Working Group and European Renal Best Practice. Transplantation 100: e55–e65, 2016. 10.1097/TP.0000000000001367 [DOI] [PubMed] [Google Scholar]
- 7.Haugen CE, Thomas AG, Chu NM, Shaffer AA, Norman SP, Bingaman AW, Segev DL, McAdams-DeMarco M: Prevalence of frailty among kidney transplant candidates and recipients in the United States: Estimates from a National Registry and Multicenter Cohort Study. Am J Transplant 20: 1170–1180, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S, Afilalo J, Lipsitz LA, Popma JJ, Khabbaz KR, Laham RJ, Guibone K, Grodstein F, Lux E, Kim DH: Frailty phenotype and deficit accumulation frailty index in predicting recovery after transcatheter and surgical aortic valve replacement. J Gerontol A Biol Sci Med Sci 74: 1249–1256, 2019 10.1093/gerona/gly196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harhay MN, Rao MK, Woodside KJ, Johansen KL, Lentine KL, Tullius SG, Parsons RF, Alhamad T, Berger J, Cheng XS, Lappin J, Lynch R, Parajuli S, Tan JC, Segev DL, Kaplan B, Kobashigawa J, Dadhania DM, McAdams-DeMarco MA: An overview of frailty in kidney transplantation: Measurement, management and future considerations. Nephrol Dial Transplant 35: 1099–1112, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tennankore KK, Gunaratnam L, Suri RS, Yohanna S, Walsh M, Tangri N, Prasad B, Gogan N, Rockwood K, Doucette S, Sills L, Kiberd B, Keough-Ryan T, West K, Vinson A: Frailty and the kidney transplant wait list: Protocol for a multicenter prospective study. Can J Kidney Health Dis 7: 2054358120957430, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group: Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 12.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, Walston JD, Segev DL: Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 61: 896–901, 2013. 10.1111/jgs.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen KL, Dalrymple LS, Glidden D, Delgado C, Kaysen GA, Grimes B, Chertow GM: Association of performance-based and self–reported function–based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol 11: 626–632, 2016. 10.2215/CJN.03710415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas A, Shaffer A, Ying H, Norman S, Segev D, McAdams-DeMarco M: Prevalence of frailty among kidney transplant recipients in the United States. Presented at the 2018 American Transplant Congress, Seattle, WA, June 2–6, 2018
- 15.Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G: A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31: 741–755, 1978. 10.1016/0021-9681(78)90058-9 [DOI] [PubMed] [Google Scholar]
- 16.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P: International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35: 1381–1395, 2003. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 17.Mitnitski AB, Mogilner AJ, Rockwood K: Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 1: 323–336, 2001. 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K: A standard procedure for creating a frailty index. BMC Geriatr 8: 24, 2008 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark DA, Khan U, Kiberd BA, Turner CC, Dixon A, Landry D, Moffatt HC, Moorhouse PA, Tennankore KK: Frailty in end-stage renal disease: Comparing patient, caregiver, and clinician perspectives. BMC Nephrol 18: 148, 2017. 10.1186/s12882-017-0558-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockwood K, Theou O: Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J 23: 210–215, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockwood K, Andrew M, Mitnitski A: Unconventional views of frailty a comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 62: 738–743, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Nixon AC, Bampouras TM, Pendleton N, Mitra S, Dhaygude AP: Diagnostic accuracy of frailty screening methods in advanced chronic kidney disease. Nephron 141: 147–155, 2019. 10.1159/000494223 [DOI] [PubMed] [Google Scholar]
- 23.McAdams-DeMarco MA, Ying H, Thomas AG, Warsame F, Shaffer AA, Haugen CE, Garonzik-Wang JM, Desai NM, Varadhan R, Walston J, Norman SP, Segev DL: Frailty, inflammatory markers, and waitlist mortality among patients with end-stage renal disease in a prospective cohort study. Transplantation 102: 1740–1746, 2018. 10.1097/TP.0000000000002213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández MP, Miguel PM, Ying H, Haugen CE, Chu NM, Rodríguez Puyol DM, Rodríguez-Mañas L, Norman SP, Walston JD, Segev DL, McAdams-DeMarco MA: Comorbidity, frailty, and waitlist mortality among kidney transplant candidates of all ages. Am J Nephrol 49: 103–110, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson MQ, Theou O, Yu S, Adams RJ, Tucker GR, Visvanathan R: Frailty prevalence and factors associated with the Frailty Phenotype and Frailty Index: Findings from the North West Adelaide Health Study. Australas J Ageing 37: 120–126, 2018. 10.1111/ajag.12487 [DOI] [PubMed] [Google Scholar]
- 26.Gregorevic KJ, Hubbard RE, Lim WK, Katz B: The clinical frailty scale predicts functional decline and mortality when used by junior medical staff: A prospective cohort study. BMC Geriatr 16: 117, 2016. 10.1186/s12877-016-0292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly S, O’Brien I, Smuts K, O’Sullivan M, Warters A: Prevalence of frailty among community dwelling older adults in receipt of low level home support: A cross-sectional analysis of the North Dublin Cohort. BMC Geriatr 17: 121, 2017. 10.1186/s12877-017-0508-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinclair AJ, Rodriguez-Mañas L: Diabetes and frailty: Two converging Conditions? Can J Diabetes 40: 77–83, 2016. 10.1016/j.jcjd.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 29.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Seth Landefeld C, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin KA, Lee W, Moskowitz D, Kobashi KC, Lucioni A, Reed MJ, Nash M, Lee UJ: A rapid method to preoperatively assess frailty for older patients with pelvic floor conditions. J Urol 203: 1172–1177, 2020. 10.1097/JU.0000000000000739 [DOI] [PubMed] [Google Scholar]
- 31.McAdams-DeMarco MA, Ying H, Olorundare I, King EA, Haugen C, Buta B, Gross AL, Kalyani R, Desai NM, Dagher NN, Lonze BE, Montgomery RA, Bandeen-Roche K, Walston JD, Segev DL: Individual frailty components and mortality in kidney transplant recipients. Transplantation 101: 2126–2132, 2017. 10.1097/TP.0000000000001546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prevalence of individual components of the Frailty Phenotype (A) as well as mean FI scores by domain (B). Download Supplemental Figure 1, PDF file, 282 KB (853KB, pdf)
Prevalence of frailty index components in a cross section of transplant candidates from Halifax, NS. Download Supplemental Table 1, PDF file, 282 KB (853KB, pdf)
Baseline characteristics. Download Supplemental Table 2, PDF file, 282 KB (853KB, pdf)
Differences in demographics among patients by frailty status according to the Frailty Phenotype. Download Supplemental Table 3, PDF file, 282 KB (853KB, pdf)
Frailty index and Clinical frailty scale scores for the overall population and across subgroups. Download Supplemental Table 4, PDF file, 282 KB (853KB, pdf)
Clinical factors associated with frailty as measured by the FI, and CFS treated as continuous variables. Download Supplemental Table 5, PDF file, 282 KB (853KB, pdf)
Agreement between frailty assessment tools represented as kappa coefficients for the sensitivity analysis population. Download Supplemental Table 6, PDF file, 282 KB (853KB, pdf)
Diagnostic characteristics of frailty screening tools for the sensitivity analysis population. Download Supplemental Table 7, PDF file, 282 KB (853KB, pdf)