Summary
Background
Long-term care facilities (LTCFs) have reported high SARS-CoV-2 infection rates and related mortality, but the proportion of infected people among those who have survived, and duration of the antibody response to natural infection, is unknown. We determined the prevalence and stability of nucleocapsid antibodies (the standard assay for detection of previous infection) in staff and residents in LTCFs in England.
Methods
This was a prospective cohort study of residents 65 years or older and of staff 65 years or younger in 201 LTCFs in England between March 1, 2020, and May 7, 2021. Participants were linked to a unique pseudo-identifier based on their UK National Health Service identification number. Serial blood samples were tested for IgG antibodies against SARS-CoV-2 nucleocapsid protein using the Abbott ARCHITECT i-system (Abbott, Maidenhead, UK) immunoassay. Primary endpoints were prevalence and cumulative incidence of antibody positivity, which were weighted to the LTCF population. Incidence rate of loss of antibodies (seroreversion) was estimated from Kaplan-Meier curves.
Findings
9488 samples were included, 8636 (91·0%) of which could be individually linked to 1434 residents and 3288 staff members. The cumulative incidence of nucleocapsid seropositivity was 34·6% (29·6–40·0) in residents and 26·1% (23·0–29·5) in staff over 11 months. 239 (38·6%) residents and 503 women (81·3%) were included in the antibody-waning analysis, and median follow-up was 149 days (IQR 107–169). The incidence rate of seroreversion was 2·1 per 1000 person-days at risk, and median time to reversion was 242·5 days.
Interpretation
At least a quarter of staff and a third of surviving residents were infected with SAR-CoV-2 during the first two waves of the pandemic in England. Nucleocapsid-specific antibodies often become undetectable within the first year following infection, which is likely to lead to marked underestimation of the true proportion of people with previous infection. Given that natural infection might act to boost vaccine responses, better assays to identify natural infection should be developed.
Funding
UK Government Department of Health and Social Care.
Introduction
Older people in long-term care facilities (LTCFs) have the greatest mortality burden from infection with SARS-CoV-2. Contributing factors to this mortality burden include immunosenescence, age-mediated inflammatory immune responses that increase susceptibility to severe infection, and close proximity to infected individuals within enclosed LTCFs.1 Approximately 410 000 older people live in 11 000 LTCFs in England2 but, partly due to sparse PCR testing at the start of the pandemic, the true burden of SARS-CoV-2 infection in this population is unknown. Antibodies against viral proteins, including nucleocapsid and spike, are produced in response to SARS-CoV-2 infection3 and probably correlate with immunity against reinfection.4 There is also growing evidence that previously infected individuals develop greater antibody responses to SARS-CoV-2 vaccination than people who have not been infected.5, 6, 7 Robust antibody production has been shown in older LTCF residents who have survived SARS-CoV-2 infection;8, 9, 10 however, there have been few large-scale sero-surveys to describe the extent of past exposure and immunity in this population.
There have been three waves of infection since the start of the COVID-19 pandemic in the UK (March to May, 2020; September, 2020, to April, 2021; and May 2021 to ongoing at the time of publication), with most deaths of LTCF residents occurring in the first wave.11, 12 To curb the effect of the pandemic in vulnerable populations, widespread vaccination was rolled out in LTCFs from Dec 8, 2020, resulting in a decline in infections and deaths.12, 13 As the spike protein is the only immunogen present within most current vaccines, detection of antibodies against viral nucleocapsid can be used as an indicator of previous natural infection.14, 15
Research in context.
Evidence before this study
A search of Ovid MEDLINE and MedRxiv was done on July 21, 2021, to identify studies done in long-term care facilities (LTCFs) that described seroprevalence using the terms “COVID-19” or “SARS-CoV-2” and “nursing home” or “care home” or “residential” or “long term care facility” and “antibody” or “serology” without date, language, or article type restrictions. One meta-analysis was identified, published before the introduction of SARS-CoV-2 vaccination, that included two studies with a combined sample size of 291 which estimated seroprevalence as 59% in LTCF residents. There were 28 seroprevalence surveys of natural-acquired SARS-CoV-2 antibodies in LTCFs; 16 were conducted in response to outbreaks and 12 done in care homes without known outbreaks. 16 studies included more than one LTCF and all were done in autumn, 2020, after the first wave of infection but before subsequent peaks. Seroprevalence studies done following an LTCF outbreak were biased towards positivity as the included population was known to have been previously infected. In the 12 studies that were done outside of known outbreaks, seroprevalence varied significantly according to local prevalence of infection. The largest of these studies was a cross-sectional study done in more than 9000 residents and 10 000 staff from 362 LTCFs in Madrid, Spain, that estimated seroprevalence as 31·5% in staff and 55·4% in residents. However, as this study was performed in one city, it may not be generalisable to the whole of Spain, and sequential sampling was not performed. Of the 28 studies, nine undertook longitudinal sampling for a maximum of 4 months although three of these reported from the same cohort of LTCFs in London, UK. None of the studies reported on antibody waning among the whole resident population.
Added value of this study
We estimated the proportion of care home staff and residents with evidence of SARS-CoV-2 natural infection using data from over 3000 staff and 1500 residents in 201 geographically dispersed LTCFs in England. Population selection was independent of outbreak history and the sample is therefore more reflective of the population who reside and work in LTCFs. Our estimates of the proportion of residents with previous natural infection are substantially higher than estimates based on population-wide PCR testing, due to limited testing coverage at the start of the pandemic. 1361 individuals had at least one positive antibody test and participants were followed up for up to 11 months, which allowed modelling of the time to loss of antibody in over 600 individuals in whom the date of primary infection could be reliably estimated. This is the longest reported serological follow-up in a population of LTCF residents, a group who are known to be most at risk of severe outcomes following infection with SARS-CoV-2, and provides important evidence on the duration that nucleocapsid antibodies remained detectable over the first and second waves of the pandemic.
Implications of all the available evidence
A substantial proportion of the LTCF population will have some level of natural immunity to infection as a result of past infection. Immunological studies have highlighted greater antibody responses to vaccination in seropositive individuals, so vaccine efficacy in this population might be affected by the large proportion of individuals who have survived past infection. In addition, although the presence of nucleocapsid-specific antibodies is generally considered as the standard marker for previous infection, we find that antibody waning is such that up to 50% of people will lose detectable antibody responses within 8 months; therefore estimates of the proportion of the LTCF population with previous infection based on antibody testing are likely to be falsely low. Individual previous natural infection history is critical to assess the impact of factors such as vaccine response or protection against reinfection. These findings may have implications for duration of immunity following natural infection and indicate that alternative assays for previous infection should be developed.
Waning antibody titres in the months following natural infection have been observed across different populations.16, 17 Nucleocapsid-specific antibodies decline more rapidly than spike antibodies, although the clinical significance of this is still unknown.18, 19 This nucleocapsid antibody waning is a potential concern as many research and surveillance studies use antibodies to nucleocapsid to differentiate patients with and without previous natural infection in order to estimate vaccine effectiveness. As such, definitive information on the prevalence and stability of nucleocapsid-specific antibodies within specific populations is required. We did a prospective seroprevalence survey in 201 LTCFs in England to estimate the proportion of surviving staff and residents in LTCFs with antibodies to nucleocapsid protein and the duration of antibody-positivity in this group.
Methods
Study design and participants
This prospective cohort study (VIVALDI) has been recruiting staff and residents from 201 geographically dispersed LTCFs in England that provide care to adults older than 65 years since June 11, 2020.20 LTCFs vary in size and include a mix of for-profit and not-for-profit providers. In brief, eligible LTCFs were identified by the provider or the National Institute for Health Research Clinical Research Network. Residents and staff within selected LTCFs were offered participation in the study. We excluded staff older than 65 years and residents younger than 65 years. An age threshold of 65 was used to differentiate between staff and residents in cases in which the role of the participant was unknown (n=672). Sequential rounds of blood sampling were undertaken at 8-week intervals for the first two to three rounds of sampling, and subsequent rounds were set to follow first and second dose vaccine administration. Individuals donated a maximum of four samples between June 11, 2020, and May 7, 2021, and were able to join the study at any round. LTCF staff and residents underwent regular screening (weekly in staff, monthly in residents) to detect SARS-CoV-2 viral RNA using PCR testing of nasopharyngeal swabs, and additional testing as part of outbreak investigations.21 Written informed consent was obtained from all participants and if individuals were assessed as lacking capacity to consent, a nominated or personal consultee acted on their behalf. Ethical approval for this study was obtained from the South Central - Hampshire B Research Ethics Committee (20/SC/0238).
Procedures
Participants were linked to a unique pseudo-identifier based on their UK National Health Service (NHS) identification number. Antibody results were submitted to NHS England (NHSE) and linked to PCR test results using the unique pseudo-identifier in the NHS COVID-19 datastore, a secure online data repository established in response to the pandemic. Every care facility in the UK is allocated a unique identifier by the Care Quality Commission, the national social care regulatory body. Individual-level records on diagnostic International Classification of Diseases 10th edition admission codes and dates of hospitalisation were retrieved from the Hospital Episode Statistics database which is maintained by NHSE and linked within the COVID-19 datastore by pseudo-identifier. Maximum monthly bed occupancy and staffing numbers were retrieved from the Capacity Tracker dataset, a tool completed by LTCFs that collects operational data. Data linkage covered the period of March 1, 2020, to May 7, 2021.
All serum samples were tested for IgG to nucleocapsid protein using the semi-quantitative Abbott ARCHITECT i-system (Abbott, Maidenhead, UK) immunoassay. An index value of 0·8 or higher was defined as positive to maximise test sensitivity while maintaining specificity.22, 23 Quantitative IgG titres were obtained using the V-PLEX COVID-19 respiratory panel 2 kit (96-well, ten spot plate coated with SARS CoV-2 antigens [spike protein, spike receptor-binding domain]; Meso Scale Diagnostics, Rockville, MD, USA). The positivity cutoffs were determined by the testing laboratory.24 RT-PCR testing of nasopharyngeal swabs was done in a national network of laboratories, established as part of the pandemic response and carried out as part of pillar 1 and 2 testing within the national testing programme.25
Seroprevalence was weighted to the LTCF population. The weighted analysis is reported by sample number and included all samples from eligible LTCFs including those that could not be linked to a pseudo-identifier.
Time to antibody loss was estimated only in individuals who could be linked to a pseudo-identifier that enabled identification of sequential samples and had at least two eligible samples separated by more than 4 weeks. It was not possible to ascertain the precise date of infection for most participants because of limited PCR testing. As such, individuals were included in the analysis if the date of infection, and therefore seroconversion, could be inferred through: a positive PCR test before the first positive antibody test (seroconversion was assumed to occur 14 days following the PCR date [A]); hospitalisation with confirmed or suspected COVID-19 before the first positive antibody test (seroconversion was the admission date [B]); a negative antibody test that preceded a positive antibody test (seroconversion date was taken to be the midpoint between tests [C]); or if the first positive antibody test was before August 1, 2020, therefore primary infection occurred during the first pandemic wave (seroconversion was recorded as May 5, 2020, [14 days after the peak of cases nationally in the first wave;12 D]). If it was possible to estimate seroconversion dates based on more than one criterion, these were prioritised in the following order: PCR (A), hospitalisation (B), and seroconversion (C, D). Individuals in whom the estimated date of seroconversion was more than 120 days before the first antibody test were excluded to improve the representativeness of our sample, as it is likely there will be additional individuals in this group who seroreverted before their first antibody test. Samples from individuals who seroreverted based on the Abbott assay result underwent additional testing to detect antibodies against spike and receptor-binding domain proteins.
Statistical analysis
Sample size for the VIVALDI study was originally based on the precision of estimates for antibody prevalence. However, the time horizon and number of included LTCFs have since expanded to address rapidly changing policy priorities. Therefore, no sample size calculations were done for this Article.20 Unweighted estimates of seropositivity were calculated for all samples according to region, type of care provider, LTCF size, and testing interval. We also estimated the proportion of individuals with at least one positive sample over the sampling period. LTCF-level estimates of seroprevalence were calculated by testing round. Weighted seroprevalence estimates according to samples were calculated separately for staff and residents and stratified by testing interval (June 1–July 31, 2020; Aug 1–Sept 30, 2020; Oct 1–Nov 30, 2020; Dec 1, 2020 to Jan 31, 2021; Feb 1–28, 2021; and March 1–April 30, 2021). These testing intervals were selected to be representative of the study population, as they reflected the time period that it took for most of the LTCFs in the study to undergo one round of testing. To account for LTCF-level testing rate, weights were calculated separately for staff and residents per sampling round using N/n, where N is the maximum bed occupancy or maximum total staffing for that month and n is the number of residents or staff tested. An individual-level cumulative incidence, defined as the proportion of individuals tested in the entire study period with at least one positive antibody result, was calculated overall and separately for staff and residents and weighted using N/n for the whole testing period in each LTCF. This analysis excluded samples that could not be linked to a pseudo-identifier as it was not possible to link multiple samples to the same individual.
We estimated the cumulative incidence of nucleocapsid-antibody waning in staff and residents separately by fitting Kaplan-Meier curves and compared them using the log-rank test. The event of interest was a negative antibody test (seroreversion) following a positive antibody test. Time at risk was taken in days from the estimated date of seroconversion, defined earlier. The date of seroreversion was the midpoint between last positive antibody test and first negative antibody test. Individuals who did not serorevert were censored at the date of their last antibody test. A sensitivity analysis that estimated cumulative incidence of antibody waning in participants from groups A, B, and C only, for whom seroconversion date estimates were the most reliable, was done separately. A subgroup analysis estimated cumulative incidence of seroreversion according to severity of primary infection. In the absence of detailed clinical data, history of COVID-19 hospitalisation (confirmed or suspected) was used as a proxy for severe illness. In the seroreverted cohort, quantitative antibody titres were compared between staff and residents at three points: at first positive antibody test, at day 30, and at day 60–90 following estimated seroreversion. Date of seroreversion followed the definitions outlined earlier, based on the Abbott assay. A p value <0·05 was considered to indicate statistical significance. All statistical analyses were done in STATA version 16.0, with weighting and clustering acknowledged using the complex survey functions.
Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Data linkage began on March 1, 2020, and 9488 samples were collected between June 11, 2020, and May 7, 2021, and included in the analysis, of which 8636 (91·0%) could be linked to a pseudo-identifier (appendix p 1). These 8636 samples were derived from 1434 LTCF residents who contributed 2833 samples (32·8%) and 3288 staff who contributed 5803 samples (67·2%); table 1). Of the 201 LTCFs included, 176 recruited at least one resident. A mean of 1·98 (SD 0·88) samples were collected from residents in 176 LTCFs and 1·76 (0·79) samples from staff in 201 LTCFs. The median age of staff was 48 years (IQR 35–56) and of residents was 87 years (81–92). Across all LTCFs the mean proportion of residents sampled in at least one round of testing was 31·8% (SD 19·5%) and of staff was 42·1% (SD 20·7%).
Table 1.
Baseline characteristics
| Proportion of samples that were antibody-positive* | Proportion of individuals that were antibody-positive at any time* | ||
|---|---|---|---|
| Total | 2516/9488 (26·5%) | 1361/4722 (28·8%) | |
| Sex | |||
| Female | 1895/7089 (26·7%) | 1124/3892 (28·9%) | |
| Male | 424/1545 (27·4%) | 237/828 (28·6%) | |
| Unknown | 197/854 (23·1%) | 0/2 | |
| LTCF role | |||
| Resident | 965/3016 (32·0%) | 484/1434 (33·8%) | |
| Staff member | 1551/6472 (24·0%) | 877/3288 (26·7%) | |
| Region of England | |||
| London | 291/809 (36·0%) | 155/350 (44·3%) | |
| South East | 473/1683 (28·1%) | 259/861 (30·1%) | |
| East | 95/574 (16·6%) | 47/261 (18·0%) | |
| East Midlands | 164/911 (18·0%) | 96/521 (18·4%) | |
| West Midlands | 124/607 (20·4%) | 73/318 (23·0%) | |
| South West | 328/1616 (20·3%) | 184/873 (21·1%) | |
| North West | 297/1042 (28·5%) | 168/538 (31·2%) | |
| North East | 590/1571 (37·6%) | 290/654 (44·3%) | |
| Yorkshire and the Humber | 154/675 (22·8%) | 89/346 (25·7%) | |
| LTCF type | |||
| For-profit chains | 1860/6503 (28·6%) | 966/2989 (32·3%) | |
| Not-for-profit chains | 563/2429 (23·2%) | 320/1326 (24·1%) | |
| Independent | 93/506 (18·4%) | 75/407 (18·4%) | |
| LTCF size | |||
| Small (<50 beds) | 916/3843 (23·8%) | 492/1939 (25·4%) | |
| Medium (50–99 beds) | 1549/5491 (28·2%) | 839/2688 (31·2%) | |
| Large (≥100 beds) | 51/154 (33·1%) | 30/95 (31·6%) | |
| Interval | |||
| June 1–July 31, 2020 | 694/2225 (31·2%) | NA | |
| Aug 1–Sept 30, 2020 | 495/1794 (27·6%) | NA | |
| Oct 1–Nov 30, 2020 | 360/1349 (26·7%) | NA | |
| Dec 1, 2020, to Jan 31, 2021 | 200/1136 (17·6%) | NA | |
| Feb 1–28, 2021 | 221/920 (24·0%) | NA | |
| March 1–April 30, 2021 | 546/2064 (26·5%) | NA | |
LTCF=long-term care facility. NA=not applicable.
Based on nucleocapsid antibody detected using Abbott assay.
Antibodies to nucleocapsid proteins were detected in 2516 (26·5%) of 9488 samples (table 1). Antibody positivity varied by region and was greatest in London and England and lowest in the east of England and the East Midlands. The proportion of people who were antibody positive was highest in for-profit LTCFs and lowest in independent LTCFs and varied over time with the lowest seroprevalence seen from Dec 1, 2020, to Jan 31, 2021. The proportion of antibody-positive individuals was lowest in LTCFs with less than 50 beds and highest in large LTCFs, although only 154 samples were included from LTCFs with at least 100 beds. The LTCF characteristics and the changes in LTCF seroprevalence are outlined in the appendix (pp 2, 5).
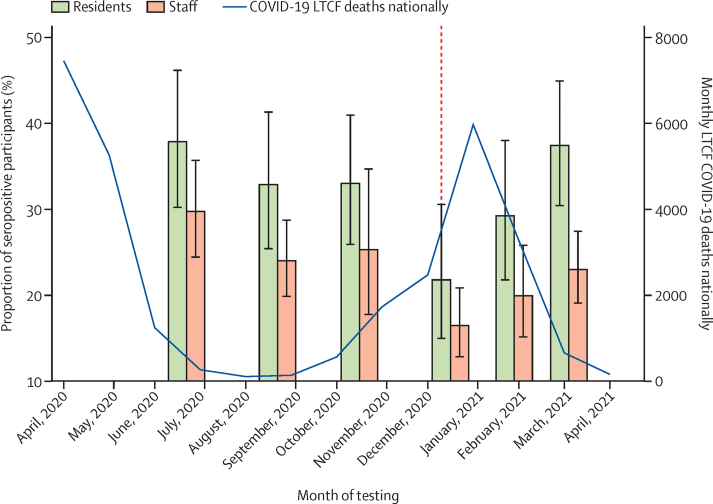
Cumulative incidence of antibody positivity in the whole population was 28·2% (95% CI 25·0–31·7) and was significantly higher in residents (34·6% [29·6–40·0]) than in staff (26·1% [23·0–29·5; p<0·0001]). Weighted seroprevalence was highest in both staff and residents in the first two months of testing (June 1–July 31, 2020), just after the first wave of the pandemic, compared with subsequent time intervals. Seroprevalence declined over the following 6 months, reaching a minimum of 21·8% (95% CI 15·0–30·6) in residents and 16·5% (12·8–20·9) in staff from Dec 1, 2020, to Jan 31, 2021, before rising again from March 1 to April 30, 2021, to 37·4% (30·5–44·9) in residents and 23·0% (19·1–27·5) in staff (figure 1; table 2).
Figure 1.
Weighted seroprevalence with 95% CIs stratified by staff or resident and interval of testing compared with monthly COVID-19 associated deaths
COVID-19 associated deaths are defined as deaths occurring within 28 days of a COVID-19 diagnosis in LTCFs in England according to the Care Quality Commission reporting.26 In view of limited PCR testing coverage in the first wave of the pandemic, data on COVID-19 deaths were considered a more accurate measure of the disease burden in LTCFs over the pandemic. The red dashed line represents the start date of the UK vaccination programme (Dec 8, 2020). LTCFs=long-term care facilities.
Table 2.
Weighted seroprevalence stratified by time interval and resident or staff role
| All participants (n=9488) | Residents (n=3016) | Staff (n=6472) | |
|---|---|---|---|
| June 1–July 31, 2020 | 30·6 (25·4–36·4) | 37·9 (30·2–46·2) | 29·8 (24·5–35·7) |
| Aug 1–Sept 30, 2020 | 26·4 (22·0–31·5) | 32·9 (25·4–41·3) | 24·0 (19·9–28·8) |
| Oct 1–Nov 30, 2020 | 26·5 (21·5–32·2) | 33·0 (25·9–41·0) | 25·3 (17·8–34·7) |
| Dec 1, 2020, to Jan 31, 2021 | 17·4 (13·6–22·0) | 21·8 (15·0–30·6) | 16·5 (12·8–20·9) |
| Feb 1–28, 2021 | 22·0 (17·2–27·6) | 29·3 (21·8–38·0) | 20·0 (15·1–25·8) |
| March 1–April 30, 2021 | 26·1 (21·9–30·7) | 37·4 (30·5–44·9) | 23·0 (19·1–27·5) |
Data are % (95% CI). Data are weighted for LTCF-level testing rate in staff and residents.
A total of 619 individuals were eligible for inclusion in the antibody-waning analysis (appendix p 5). 108 individuals had a positive PCR test before the first positive antibody test (seroconversion was assumed to occur 14 days following the PCR date; group A); five had hospitalisation with confirmed or suspected COVID-19 before the first positive antibody test (seroconversion was the admission date; group B); three had a negative antibody test that preceded a positive antibody test (seroconversion date was taken to be the midpoint between tests; group C); and 503 had a first positive antibody test before August 1, 2020, with the assumption that primary infection occurred during the first pandemic wave (seroconversion was recorded as May 5, 2020, [14 days after the peak of cases nationally in the first wave; group D]). Of the 619 individuals who were included in the antibody-waning analysis, 239 (38·6%) were residents and 230 (61·3%) were staff, with 503 women (81·3%) and 116 (18·7%) men. The median age was 59 years (IQR 45–82) and the median follow-up was 149 days (IQR 107–169). Further details on the included samples and antibody testing results by round are available in the appendix (pp 5–6). 377 (99%) of 380 staff in this analysis had a positive baseline antibody sample and at least one subsequent sample (appendix p 6). Of these 380 staff (including three people who had a baseline negative sample and a subsequent positive sample), 218 (57%) were sampled in at least two subsequent testing rounds and 137 (64%) of the 215 baseline antibody-positive staff remained antibody-positive. 239 residents had a positive baseline antibody sample, of whom 154 (64%) had a third blood sample and 119 (77%) of these 154 residents remained seropositive. There were three cases of seroconversion during follow-up and all occurred in staff.
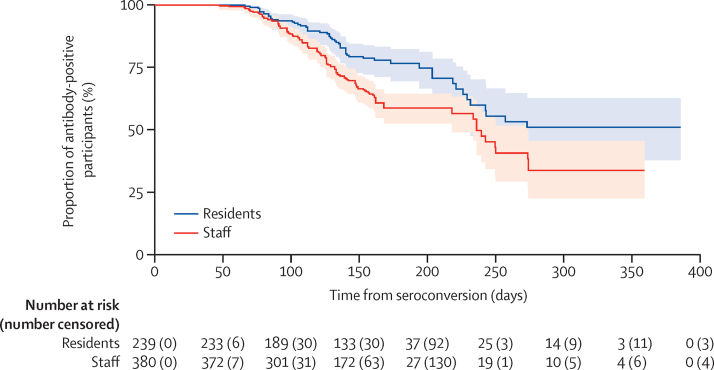
Seroreversion, defined as loss of a detectable nucleocapsid-specific antibody response, occurred in 55 (23%) of 239 residents and 133 (35%) of 380 staff. In the majority of cases (129 [69%] of 188), seroreversion occurred 90–180 days following the estimated date of seroconversion (appendix p 6). Kaplan-Meier plots were used to estimate the median time to seroreversion (figure 2). The overall incidence rate of seroreversion was 2·1 per 1000 person-days at risk and the estimated median time to seroreversion was 242·5 days. The incidence of seroreversion was greater in staff than residents (2·4 vs 1·5 per 1000 person-days at risk; p=0·00034; figure 2; appendix p 6).
Figure 2.
Kaplan-Meier plot of time to antibody loss from estimated date of seroconversion in staff and residents
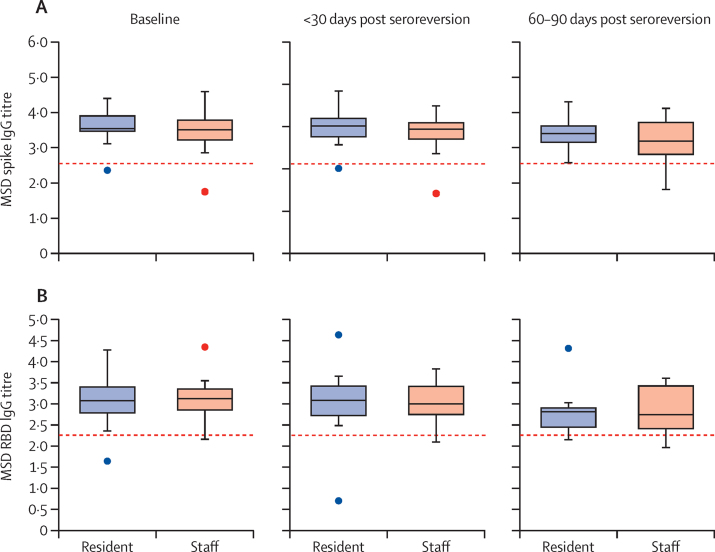
20 individuals were admitted to hospital with confirmed or suspected COVID-19 before their first positive antibody test, therefore are likely to have experienced more severe primary infection than the non-hospitalised cohort. Subgroup analysis estimated no significant difference in the incidence rate of antibody loss between hospitalised and non-hospitalised individuals (1·1 vs 2·1 per 1000 person-days at risk; p=0·15; appendix pp 3, 6). A sensitivity analysis was done in 116 individuals (73 residents and 43 staff) who belonged to the PCR-confirmed, hospitalised, or seroconverted groups (groups A, B, and C). The median follow-up in these individuals was 89·5 days (IQR 66·3–159·5), and the incidence rate of seroreversion remained higher in staff than residents (1·5 vs 0·9 per 1000 person-days; p=0·0096; appendix pp 4, 6). Quantitative antibody titres to spike and receptor-binding domain proteins were plotted over time in a subset of 41 individuals (18 residents and 23 staff) who had seroreverted. Samples were available at 60–90 days following seroreversion for 16 individuals (7 staff and 9 residents). For all non-nucleocapsid antibodies tested, the median antibody titre did not recede below the threshold for positivity (figure 3).
Figure 3.
Quantitative antibody titres on a logarithmic scale over 90 days following nucleocapsid antibody seroreversion for spike antibody (A) and RBD antibody (B)
Titres are presented at date of first positive antibody (baseline; n=41), 0–30 days (n=41), and 60–90 days (n=16) after estimated date of seroreversion. Titres are reported from MSD assay according to a logarithmic scale. The red dashed line denotes cutoff for test positivity (spike protein=350 AU/mL, RBD=180 AU/mL). AU=arbitrary units. MSD=Meso Scale Diagnostics. RBD=receptor-binding domain.
Discussion
In the first two waves of the pandemic at least one-third of residents and one-quarter of staff in this study were infected with SARS-CoV-2. Seroprevalence was significantly greater in residents than in staff and declined in the winter months between pandemic waves, which suggests significant waning of antibody concentrations. Indeed, around half of the antibody-positive population seroreverted within 8 months based on antibodies to nucleocapsid proteins, although spike antibody titres remained elevated for at least an additional 90 days. This rapid waning suggests that serological surveys from LTCFs might have underestimated the true proportion of individuals with previous infection. A potential implication of these findings is that vaccine effectiveness in LTCF staff and residents in the context of high background prevalence of natural infection might differ from estimates of vaccine effectiveness in infection-naive populations. This theory is proposed because immunological studies suggest that natural infection augments vaccine-induced cellular and humoral immune responses in care home residents and staff.5, 6, 7
In common with other serological studies, our study highlights the limitations of estimating the prevalence of natural infection based on antibody titres against nucleocapsid protein.27 Direct comparison of seroprevalence estimates between studies and countries is challenging due to variation in the timing of pandemic waves and waning immunity. The largest published prevalence study to date included 9332 LTCF residents from 362 LTCFs in Madrid, Spain and reported that 5172 (55·4%) residents had detectable anti-nucleocapsid antibodies between July 7 and Oct 23, 2020, following the first wave of the pandemic; however, as these estimates were unweighted and the study was limited to one region, these findings might not be generalisable to the national prevalence of infection.28 In England, national surveillance data from June 14 to July 11, 2021, report a weighted nucleocapsid antibody prevalence of 16·6% in the general population, lowest in those aged 70–84 years.12 These estimates are based on samples from blood donors in the community in whom infection rates were likely to be lower than individuals in LTCFs, particularly over the first pandemic wave.2 Our study also found lower seroprevalence in independent LTCFs when compared with for-profit chains, and in larger versus smaller LTCFs; large, for-profit LTCFs have been widely described as risk factors for SARS-CoV-2 transmission.1, 29
Our study makes a valuable contribution to a growing body of evidence of rapid waning of nucleocapsid antibody titres following natural infection across age groups, which presents challenges for measuring natural infection.19, 27 Although serological follow-up has been reported from LTCFs for up to 4 months,9 we present antibody titres over 11 months and report that around half of individuals will have undetectable nucleocapsid antibodies 8 months from initial seroconversion. Antibodies waned more rapidly in staff than residents, which might be because older residents are more likely than younger staff to have experienced severe primary infection, which has been associated with slower seroreversion in the literature.30 Although we could not confirm this theory with clinical data, the subgroup analysis examined the effect of disease severity on the rate of seroreversion using hospitalisation with COVID-19 as a proxy for severe disease and showed no difference between hospitalised and non-hospitalised participants, which might reflect the relatively small number of hospitalisations in the cohort. One key factor relates to the sensitivity of the nucleocapsid-specific antibody assay. The Abbott assay is used widely and has consistently been found to have sufficient diagnostic accuracy in head-to-head comparisons with other available assays.22, 31 Furthermore, to improve sensitivity while maintaining specificity, we implemented a lower threshold for positivity (index value 0·8).22, 23 As most individuals included in the seroreversion analysis had their primary infection before circulation of viral variants (such as the alpha and delta variants) in England,12 it is unlikely that these variants would have influenced our results, although variation in waning according to viral strain should be explored in future analyses.
This study is one of the largest longitudinal serosurveys undertaken in frail older adults in LTCFs, who are often excluded from research studies. Participants were recruited from a range of small and large LTCFs including for-profit and not-for-profit providers from all regions in England, and longitudinal samples were obtained from more than 1500 frail residents. As we sampled a range of care providers and geographical regions in England, our results are likely to be generalisable to the wider LTCF population in this country. However, despite our efforts to use consultees for residents lacking capacity to consent, it is possible that this population are under-represented in our cohort due to the additional complexities that this posed for the LTCFs in enrolling these residents.
Despite a large sample, 25 (12%) of 201 LTCFs did not sample any residents, highlighting the challenges of recruiting residents into research studies. We attempted to account for the unrecruited residents in these LTCFs by stratifying our estimates by time interval and subject type and weighting for the LTCF size. Additionally, age was used to infer subject type for 672 (7%) 9488 of participants for whom these data were missing, which might have introduced bias due to misclassification. Due to limited PCR and antibody testing nationally over the first wave of the pandemic, in some cases we estimated the date of primary infection and subsequent seroconversion using available data, which might have affected precision. Although subgroup analysis in participants with a more reliable estimate of seroconversion date showed a lower rate of seroreversion, follow-up in this cohort was significantly shorter than in the main cohort. Shorter follow-up would be expected to lead to a lower calculated rate of seroreversion because seroreversion is very rare in the initial period after seroconversion (ie, in the first 40 days). It is likely that duration of follow-up was shorter because regular PCR testing, which enabled estimation of most seroconversion dates for individuals included in the sensitivity analysis (108 [93%] of 116 individuals), only became widely available from June, 2020, when whole care home testing was introduced.21 It was not possible to ascertain how many discrete infection episodes participants experienced; however, as protection against reinfection has been described for up to 10 months in this population,10 recurrent infections were probably relatively rare. If reinfections did occur, then the rate of seroreversion might have been slightly higher than we have reported. Finally, we only sampled individuals who survived SARS-CoV-2 infection, which means we cannot infer the proportion of all care home residents (survivors and those who died) who were infected over successive waves of the pandemic.
Although LTCFs were severely affected in the first wave of the pandemic, national infection rates have dropped significantly since the roll-out of vaccination.12 Immunological studies have shown that previous natural infection with SARS-CoV-2 elicits a humoral immune response to COVID-19 vaccination that has a greater magnitude than that seen in infection-naive people.5, 6 A large proportion of people in LTCFs have had past infection, which might have led to an overestimation of the protective effect of vaccination in care home staff and residents. The median duration of residency in an LTCF is 462 days32 and there is considerable staff turnover. As such, it may be possible that lower vaccine effectiveness will be observed in LTCFs over time as the proportion of naturally infected individuals declines.
One third of LTCF residents and one quarter of staff in England showed evidence of having a SARS-CoV-2 infection over the first two waves of the pandemic. However, antibody titres against nucleocapsid protein wane significantly over the first 8 months following infection. As this assay is the current gold standard for assessment of previous natural infection, it is likely that many people with past natural infection will be falsely labelled as infection-naive, with resultant underestimation of past infection in this population. Previous natural infection status is an important determinant of both the vaccine response and risk of reinfection and, as such, novel serological assays to determine previous infection status should be developed.
Data sharing
De-identified test results and limited metadata will be made available for use by researchers in future studies, subject to appropriate research ethical approvals once the VIVALDI study cohort has been finalised. These datasets will be accessible via the Health Data Research UK Gateway.
Declaration of interests
LS and TP report grants from the Department of Health and Social Care during the conduct of the study and LS is a member of the Social Care Working Group, which reports to the Scientific Advisory Group for Emergencies. AI-S is employed by the Department of Health and Social Care who funded the study. AH reports funding from the Covid Core Studies Programme and is a member of the New and Emerging Respiratory Virus Threats Advisory Group at the Department of Health and Environmental Modelling Group of the Scientific Advisory Group for Emergencies. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the staff and residents in the LTCFs that participated in this study, Rachel Bruton who project managed the study at the University of Birmingham, and Mark Marshall at NHS England who pseudonymised the electronic health records. This report is independent research funded by the Department of Health and Social Care (COVID-19 surveillance studies). MK is funded by a Wellcome Trust Clinical PhD Fellowship (222907/Z/21/Z). LS is funded by a National Institute for Health Research Clinician Scientist Award (CS-2016–007). AH is supported by Health Data Research UK (LOND1), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and Wellcome Trust. The views expressed in this publication are those of the authors and not necessarily those of the NHS, UK Health Security Agency, or the Department of Health and Social Care.
Contributors
LS, AH, AC, TP, and MK conceptualised the study. MK, TP, LS, and AC developed the statistical analysis plan. MK did the formal statistical analysis. MK, CF, BA, RG, MS, and AI-S were involved with project administration. MK did the literature review. LS and AH obtained research funding. GT, NK, PS, TL, and PM did laboratory investigations. MK wrote the first draft of the manuscript. All authors revised and edited the manuscript. MK and TP accessed and verified the data. All authors had full access to all the data reported in the study. LS, AC, and MK shared the final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Burton JK, Bayne G, Evans C, et al. Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK. Lancet Healthy Longev. 2020;1:e21–e31. doi: 10.1016/S2666-7568(20)30012-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell D, Comas-Herrera A, Henderson D, et al. COVID-19 mortality and long-term care: a UK comparison. Aug 29, 2020. https://ltccovid.org/wp-content/uploads/2020/08/COVID-19-mortality-in-long-term-care-final-Sat-29-v1.pdf
- 3.Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 4.Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11 doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tut G, Lancaster T, Krutikov M, et al. Profile of humoral and cellular immune responses to single doses of BNT162b2 or ChAdOx1 nCoV-19 vaccines in residents and staff within residential care homes (VIVALDI): an observational study. Lancet Healthy Longev. 2021;2:e544–e553. doi: 10.1016/S2666-7568(21)00168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeffery-Smith A, Dun-Campbell K, Janarthanan R, et al. Infection and transmission of SARS-CoV-2 in London care homes reporting no cases or outbreaks of COVID-19: prospective observational cohort study, England 2020. Lancet Reg Health Eur. 2021;3 doi: 10.1016/j.lanepe.2021.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeffery-Smith A, Iyanger N, Williams SV, et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.5.2100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krutikov M, Palmer T, Tut G, et al. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): a prospective cohort study. Lancet Healthy Longev. 2021;2:e362–e370. doi: 10.1016/S2666-7568(21)00093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Office for National Statistics Coronavirus (COVID-19) infection survey technical article. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsurveytechnicalarticle/wavesandlagsofcovid19inenglandjune2021
- 12.Public Health England Weekly national influenza and COVID-19 surveillance report executive summary - week 29 2021. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1005056/Weekly_Flu_and_COVID-19_report_w29.pdf
- 13.GOV.UK; Vaccine update: issue 315, December 2020, COVID-19 special edition - GOV.UK. December, 2020. https://www.gov.uk/government/publications/vaccine-update-issue-315-december-2020-covid-19-special-edition/vaccine-update-issue-315-december-2020-covid-19-special-edition#covid-19-vaccination-programme
- 14.Blain H, Tuaillon E, Gamon L, et al. Spike antibody levels of nursing home residents with or without prior COVID-19 3 weeks after a single BNT162b2 vaccine dose. JAMA. 2021;325:1898–1899. doi: 10.1001/jama.2021.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houlihan CF, Beale R. The complexities of SARS-CoV-2 serology. Lancet Infect Dis. 2020;20:1350–1351. doi: 10.1016/S1473-3099(20)30699-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Elslande J, Oyaert M, Ailliet S, et al. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol. 2021;136 doi: 10.1016/j.jcv.2021.104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfego D, Sullivan A, Poirier B, Williams J, Adcock D, Letovsky S. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krutikov M, Palmer T, Donaldson A, et al. Study protocol: understanding SARS-Cov-2 infection, immunity and its duration in care home residents and staff in England (VIVALDI) Wellcome Open Res. 2021;5:232. doi: 10.12688/wellcomeopenres.16193.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GOV.UK; Coronavirus (COVID-19) testing in adult care homes. 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-testing-in-adult-care-homes
- 22.Ainsworth M, Andersson M, Auckland K, et al. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020;20:1390–1400. doi: 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58:e00941–e00980. doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowell AC, Butler MS, Jinks E, et al. Children develop strong and sustained cross-reactive immune responses against Spike protein following SARS-CoV-2 infection, with enhanced recognition of variants of concern. medRxiv. 2021 doi: 10.1101/2021.04.12.21255275. published online April 29. (preprint). [DOI] [Google Scholar]
- 25.Gov.UK; Coronavirus (COVID-19): scaling up testing programmes. April 4, 2020. https://www.gov.uk/government/publications/coronavirus-covid-19-scaling-up-testing-programmes
- 26.Office for National Statistics Number of deaths in care homes notified to the Care Quality Commission, England. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/dataets/numberofdeathsincarehomesnotifiedtothecarequalitycommissionengland
- 27.Ward H, Cooke GS, Atchison C, et al. Prevalence of antibody positivity to SARS-CoV-2 following the first peak of infection in England: serial cross-sectional studies of 365,000 adults. Lancet Reg Health Eur. 2021;4 doi: 10.1016/j.lanepe.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Candel FJ, Barreiro P, San Román J, et al. The demography and characteristics of SARS-CoV-2 seropositive residents and staff of nursing homes for older adults in the community of Madrid: the SeroSOS study. Age Ageing. 2021;50:1038–1047. doi: 10.1093/ageing/afab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stall NM, Jones A, Brown KA, Rochon PA, Costa AP. For-profit long-term care homes and the risk of COVID-19 outbreaks and resident deaths. CMAJ. 2020;192:E946–E955. doi: 10.1503/cmaj.201197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59:e02257–e02260. doi: 10.1128/JCM.02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forder J, Fernandez J-L. Length of stay in care homes. 2011. https://eprints.lse.ac.uk/33895/1/dp2769.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified test results and limited metadata will be made available for use by researchers in future studies, subject to appropriate research ethical approvals once the VIVALDI study cohort has been finalised. These datasets will be accessible via the Health Data Research UK Gateway.