Abstract
Background
Dysregulated inflammation is associated with poor outcomes in COVID-19. We aimed to assess the efficacy of namilumab (a granulocyte-macrophage colony stimulating factor inhibitor) and infliximab (a tumour necrosis factor inhibitor) in hospitalised patients with COVID-19, to prioritise agents for phase 3 trials.
Methods
In this randomised, multicentre, multi-arm, multistage, parallel-group, open-label, adaptive, phase 2, proof-of-concept trial (CATALYST), we recruited patients (aged ≥16 years) admitted to hospital with COVID-19 pneumonia and C-reactive protein (CRP) concentrations of 40 mg/L or greater, at nine hospitals in the UK. Participants were randomly assigned with equal probability to usual care or usual care plus a single intravenous dose of namilumab (150 mg) or infliximab (5 mg/kg). Randomisation was stratified by care location within the hospital (ward vs intensive care unit [ICU]). Patients and investigators were not masked to treatment allocation. The primary endpoint was improvement in inflammation, measured by CRP concentration over time, analysed using Bayesian multilevel models. This trial is now complete and is registered with ISRCTN, 40580903.
Findings
Between June 15, 2020, and Feb 18, 2021, we screened 299 patients and 146 were enrolled and randomly assigned to usual care (n=54), namilumab (n=57), or infliximab (n=35). For the primary outcome, 45 patients in the usual care group were compared with 52 in the namilumab group, and 29 in the usual care group were compared with 28 in the infliximab group. The probabilities that the interventions were superior to usual care alone in reducing CRP concentration over time were 97% for namilumab and 15% for infliximab; the point estimates for treatment–time interactions were –0·09 (95% CI –0·19 to 0·00) for namilumab and 0·06 (–0·05 to 0·17) for infliximab. 134 adverse events occurred in 30 (55%) of 55 patients in the namilumab group compared with 145 in 29 (54%) of 54 in the usual care group. 102 adverse events occurred in 20 (69%) of 29 patients in the infliximab group compared with 112 in 17 (50%) of 34 in the usual care group. Death occurred in six (11%) patients in the namilumab group compared with ten (19%) in the usual care group, and in four (14%) in the infliximab group compared with five (15%) in the usual care group.
Interpretation
Namilumab, but not infliximab, showed proof-of-concept evidence for reduction in inflammation—as measured by CRP concentration—in hospitalised patients with COVID-19 pneumonia. Namilumab should be prioritised for further investigation in COVID-19.
Funding
Medical Research Council.
Introduction
Severe COVID-19 is associated with high mortality and disability in survivors. An excessive and dysregulated immune response contributes to these poor outcomes, as evidenced by the ability of corticosteroids and IL-6 receptor blockade to reduce mortality in hospitalised patients requiring oxygen.1, 2
Inflammatory monocytes and macrophages appear central to this dysregulated response,3 resulting in disruption of pulmonary endothelial barrier integrity, microvascular thrombosis,4 and lung tissue damage.5 A genome-wide association study has identified the monocyte and macrophage chemotactic protein CCR2 as being associated with severe COVID-19.6 Transcriptomic analysis of blood, lung, and bronchoalveolar fluid has revealed a predominance of activated inflammatory monocytes and macrophages within the lung, alongside expression of procoagulant genes within alveolar macrophages.7 Notably, the aberrant expression of proliferation markers in blood monocytes correlates with severe disease,8 and is likely to reflect a pathological early release of monocytes from the bone marrow.9 Inflammatory monocytes and macrophages might be further activated and polarised to an inflammatory phenotype in severe disease by interaction with immune complexes containing hypoglycosylated anti-spike protein antibodies.10
Research in context.
Evidence before this study
We searched PubMed and medRxiv on May 10, 2021, from database inception, with no language restrictions, using the terms “(“randomised” OR “trial”)” AND “(“anti-GM-CSF” OR “namilumab” OR “mavrilimumab” OR “otilimab” OR “lenzilumab” OR “gimsilumab” OR “TJ003234” OR “anti-TNF” OR “infliximab” OR “adalimumab” OR “etanercept” OR “golimumab” OR “certolizumab”)” AND “(“COVID*” OR “SARS-CoV-2” OR “SARS-CoV”)”. Two small non-randomised studies with drugs targeting granulocyte-macrophage colony stimulating factor (GM-CSF) or its receptor (lenzilumab and mavrilimumab) and one study with a tumour necrosis factor (TNF) inhibitor (infliximab) have suggested potential efficacy of these drugs but have had substantial limitations, such as small sample size, use of historical controls, and being conducted before the routine use of corticosteroids. One randomised controlled trial with mavrilimumab was small and inconclusive. Two larger trials with other GM-CSF inhibitors have also been published. Otilimab showed a benefit compared with usual care for the primary endpoint of respiratory failure-free survival at day 28 in a predefined subgroup of patients aged 70 years or older. Lenzilumab, given as a course of three doses, in non-ventilated hospitalised patients showed a benefit over standard care in the primary outcome of survival without ventilation, an effect that seemed more pronounced in patients aged 85 years or younger and with C-reactive protein (CRP) concentration of less than 150 mg/L. We identified no published randomised trials of TNF inhibitors in COVID-19.
Added value of this study
To our knowledge, this is the first randomised trial of namilumab and infliximab in COVID-19. We found that both drugs were safe and that namilumab, but not infliximab, showed proof-of-concept evidence of reduction in inflammation—as measured by CRP concentration—in hospitalised patients with COVID-19 pneumonia. Secondary clinical outcomes were concordant with the primary outcome, with trends to improvement in patients recruited from both wards and intensive care units, although the study was not formally powered to assess these outcomes.
Implications of all the available evidence
Consistent with emerging evidence implicating GM-CSF and inflammatory monocytes and macrophages in the pathogenesis of severe COVID-19, namilumab improved inflammation as measured by CRP in hospitalised patients with COVID-19 pneumonia. Namilumab should be prioritised for further study in COVID-19.
Inflammatory monocytes and macrophages or their activity can be targeted therapeutically in several different ways. Given that trials with clinical outcomes require large numbers of patients to show effects, we designed a multi-arm proof-of-concept trial with a biomarker primary outcome to expedite decision making on potentially effective therapeutic options for COVID-19. The aim was to provide early biological signals of efficacy to efficiently prioritise agents with the highest likelihood of success for study in established phase 3 platform trials.11 The first two agents studied were namilumab and infliximab.
Namilumab is an anti-granulocyte-macrophage colony stimulating factor (GM-CSF) monoclonal antibody with a good safety profile up to phase 2 that has been studied in inflammatory conditions such as rheumatoid arthritis. GM-CSF is a multifunctional cytokine that is a growth factor for granulocytes and monocytes and has an important role in immune responses. In particular, it drives the activation, maturation, survival, and trafficking of monocyte-derived macrophages, and their polarisation towards a more inflammatory phenotype. Increased GM-CSF levels are closely associated with disease severity in COVID-19,12 and GM-CSF-expressing T cells are clonally expanded in the lungs in severe disease.13 Notably, GM-CSF might also enhance the procoagulant activities of macrophages,14 and blood clots are a recognised side-effect of recombinant GM-CSF (sargramostim), which suggests that dysregulated GM-CSF expression might predispose to the microvascular thrombosis characteristic of COVID-19.4
Infliximab is a widely used anti-tumour necrosis factor (TNF) monoclonal antibody. TNF is an important proinflammatory cytokine and its inhibition has shown efficacy in many chronic immune-mediated inflammatory diseases. TNF inhibition has been shown to reduce mortality and disease severity in several mouse models of viral respiratory infection.15, 16 An inflammatory monocyte and macrophage subset associated with severe COVID-19 shares transcriptional similarities to macrophages stimulated with both TNF and interferon gamma (IFNγ).17 Some data have suggested that patients with immune-mediated inflammatory diseases who contract COVID-19 while on treatment with TNF inhibitors have better outcomes.18
We aimed to provide early proof-of-concept signals of the effect of namilumab and infliximab on inflammation in patients with COVID-19 compared with usual care, to efficiently prioritise these approaches for subsequent testing in larger trials powered for clinical outcomes.
Methods
Study design
We conducted a randomised, multicentre, multi-arm, multistage, parallel-group, open-label, adaptive, phase 2, proof-of-concept trial (CATALYST),11 across nine hospitals in the UK (Queen Elizabeth Hospital, Birmingham; Heartlands Hospital, Birmingham; John Radcliffe Hospital, Oxford; Royal Bolton Hospital, Bolton; Imperial St Mary's Hospital, London; Royal Hallamshire Hospital, Sheffield; University Hospital of Wales, Cardiff; Good Hope Hospital, Birmingham; and University College Hospital, London). A placebo control was not included due to the operational difficulties imposed by the COVID-19 pandemic and the proposed multigroup design, and following advice from patient and public involvement. This trial was approved by the East Midlands Nottingham 2 Research Ethics Committee (20/EM/0115).
Participants
Eligible patients were aged 16 years or older, admitted to hospital with a clinical picture strongly suggestive of SARS-CoV-2 pneumonia (confirmed by chest x-ray or CT scan, with or without a positive RT-PCR assay), and with a C-reactive protein (CRP) concentration of 40 mg/L or greater. The requirement for raised CRP concentration replaced an inclusion criterion for low oxygenation status (oxygen saturation ≤94% while breathing ambient air or a ratio of partial pressure of oxygen to the fraction of inspired oxygen of ≤300 mmHg) early in the course of recruitment, following a change in primary outcome (protocol changes are summarised in the appendix pp 7–8). Exclusion criteria are listed in the appendix (p 4).
Written informed consent was obtained from all patients with capacity. If the patient was deemed to not have capacity, due to severity of illness for example, informed consent was obtained from the patient's personal legal representative or, if unavailable, a professional legal representative according to the requirements of the UK Health Research Authority. Patients with representative consent were re-consented as soon as possible after regaining capacity.
Randomisation and masking
Randomisation was performed by an automated minimisation procedure that attempted to allocate participants in a balanced manner between treatment groups available at the site, allowing for the sole stratification variable (care location within the hospital; ward or intensive care unit [ICU]) and with a 20% random component (appendix p 4). At one site (Royal Bolton Hospital), infliximab was unavailable as an intervention. Patients were not masked to treatment allocation. Although clinical staff were aware of treatment allocation, aggregate outcomes were not provided to them, the trial management committee, or the trial steering committee.
Procedures
Participants assigned to namilumab received a single intravenous dose of 150 mg given over 1 h on day 1. Participants assigned to infliximab received a single intravenous dose of 5 mg/kg given over 2 h on day 1. Participants were followed-up for 28 days. Blood tests for the primary and secondary outcomes and safety were taken on days 1, 3, 5, 7, 9, and 14, or until truncated by discharge or death. Physiological measures were collected until day 14, discharge, or death, and included the ratio of the oxygen saturation to fractional inspired oxygen concentration (SpO2:FiO2). Most measures were taken daily, but the oxygen concentration measures were taken twice daily.
The WHO clinical progression scale was assessed daily for 28 days, which provided a score on a scale of 1–10 (appendix p 8), where 1 is asymptomatic, 4 is hospitalised without oxygen, 6 is hospitalised with non-invasive ventilation or high-flow nasal oxygen, 7 is hospitalised with mechanical ventilation, and 10 is death; data for level 0 (no viral load detected) were not collected.19 If a patient was discharged earlier than day 28, this outcome was collected by means of a diary and scheduled telephone calls.
Outcomes
The primary outcome measure was CRP concentration, collected over time (on days 1, 3, 5, 7, 9, and 14 at a minimum) until day 14. Published data indicate that CRP concentrations and trajectory in COVID-19 pneumonia are strongly associated with clinical outcomes, including respiratory failure and death, as well as with lung changes observed on CT.11 As our objective was to have a rapid, biologically driven efficacy signal using continuous, readily available data and a small sample size, we had initially chosen SpO2:FiO2 ratio as the primary outcome. However, subsequent modelling of data from a large cohort of patients hospitalised with COVID-19 in the first wave indicated that the SpO2:FiO2 ratio might not be a viable outcome measure of sickness. This finding led to an early change in the primary outcome to CRP concentration, before any analysis of trial data, as previously described.11
Clinical secondary outcomes were the WHO clinical progression scale as a principal clinical efficacy measure, as well as hospital survival status and hospital-free days, all assessed up to day 28. Hospital-free days were defined as the number of days between date of hospital discharge and day 28, with patients who died or who were alive and in hospital on day 28 being counted as having 0 hospital-free days. Other secondary outcomes were length of hospital stay, proportion of patients discharged at day 28, and destination of discharge. Physiological outcomes measured up to day 14 or hospital discharge or death, if earlier, were the SpO2:FiO2 ratio, body temperature, and respiratory rate. Lymphocyte and neutrophil counts, neutrophil–lymphocyte ratios, and ferritin, d-dimers, and lactate dehydrogenase were also measured; these outcomes will be presented alongside exploratory biological outcomes in a future publication.
Safety outcome measures were survival status and adverse events defined by the Common Terminology Criteria for Adverse Events version 4.03, which fulfilled one of the following criteria: grade 3 or worse, secondary infection, or allergic reaction. Data on harms were collected until day 28, utilising telephone follow-up if participants were discharged earlier, and were submitted on case report forms by site investigators. Attribution for serious adverse events was made by site investigators and reviewed by namilumab or infliximab group leads or the chief investigator. Given the known safety profile of infliximab, infection and allergic reaction were anticipated adverse events. Low neutrophil count was an anticipated adverse event with namilumab.
Statistical analysis
The data were analysed according to a prespecified statistical analysis plan (appendix pp 19–28). Each intervention group was compared with the control group independently, including only control patients for whom that intervention was a randomisation option; ie, patients in the usual care group who were randomly assigned after the infliximab group closed or at the single study site where infliximab was not a randomisation option were not included in the infliximab comparison. For the primary endpoint of CRP concentration, Bayesian multilevel regression models20 were used, allowing for nesting of the repeated measures data within patient and non-linear responses, implemented within Stan using the Bayesian regression models (brms) package.21 Default priors as chosen by brms were utilised in all models and updated at any analysis point; these are chosen to be very weakly informative, the default covariance structure was implemented. The full details on how these are decided upon are provided in the package documentation.22
Posterior probabilities for the treatment–time interaction covariates were used to conduct decision making at interim analyses, specifically the probability that the covariate was less than 0, which indicates a positive treatment effect in the direction of the intervention as per the model formulation. The fitted models incorporated population-level effects for both the intercept and time, random effects for the intercept and time for patient, and fixed effects for age, care location (ward or ICU), a main treatment effect, a treatment–time interaction, a treatment–location interaction, and a higher-order time term.
For the WHO scale, Bayesian longitudinal ordinal regression models were used, implemented using brms,21 including in the model formulation fixed effects for location, age, a main treatment effect, and a treatment–time interaction, and random effects for both the intercepts and time for patient. For consistency with other trials, time to a 2-point improvement for this outcome was also calculated. Kaplan-Meier curves were produced for time to improvement and the Greenwood method was utilised in calculating CIs. Results for other outcome measures were not modelled; the results are summarised graphically or tabulated. The full outline for the statistical analysis of all secondary endpoints for the study is provided in the appendix (pp 26–27).
Conditional probability plots—for the aforementioned models—are presented, which show the mean predicted values of the natural logarithm of CRP concentration and, for the WHO scale, the predicted probability of being in each of the WHO outcome categories, conditioned on model parameter values. These plots provide an easy to interpret visualisation of effect of treatment on these outcomes over time.
Where relevant, estimates of uncertainty for any point estimates at the stated confidence or probability level, typically 95%, have been included.
Interim analyses were planned after every 20 evaluable participants per group were enrolled up to 60 participants, and CRP data was considered by the data monitoring committee in the context of the emerging safety data to make a recommendation (appendix pp 4, 26). No form of bias adjustment was applied.
Success was defined as a 90% probability of an intervention treatment being better than usual care in reducing CRP concentration as per the posterior probability for the treatment–time interaction covariate, whereas futility was defined as a probability of less than 50%. The operating characteristics, based on a simpler analysis of area under the curve for sequential CRP data, have been previously published,11 and are presented in the appendix (pp 4–5). These indicated a mean total sample size of 43–70 patients per comparison would be required dependent on the assumptions.
Preplanned subgroup analyses were conducted to ascertain the effect of treatment on the primary outcome measure in participants recruited from wards versus ICUs, and with non-severe disease versus severe disease at baseline, with severe defined as requiring non-invasive or invasive ventilation. The effect of age was also studied.12 Post-hoc analyses were conducted to exclude participants without a positive SARS-CoV-2 PCR test, and to assess the effect of baseline remdesivir use, smoking status, and frailty.
The primary outcome was analysed on a modified intention-to-treat population, which included all participants who received trial treatment and had a baseline CRP measurement and at least one measurement after treatment.
The modified intention-to-treat population for secondary outcomes included all patients who received any trial treatment and who had available data for the respective outcome. The safety population included all patients in the usual care group and all patients who had received a trial intervention in the intervention groups. Data on all reported harms, as well as for those meeting the prespecified criteria, were summarised descriptively.
An independent data monitoring committee reviewed unblinded data at interim analyses to advise the trial steering committee on whether the trial data (and results from other relevant research), justified the continuing recruitment of further patients. The data monitoring committee operated in accordance with a trial-specific charter based on the template created by the Damocles Group. Statistical analyses were done in Stata 16 and R version 4.0.3. This trial is registered with ISRCTN, 40580903.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
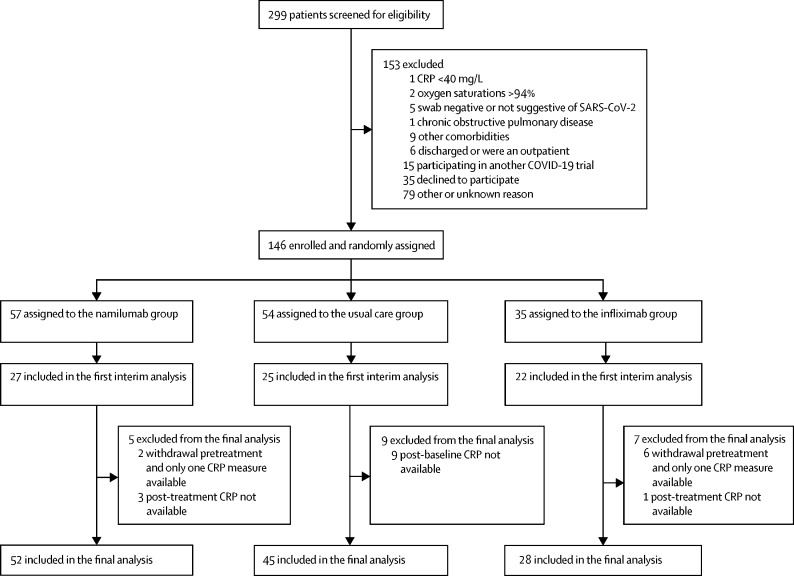
Between June 15, 2020, and Feb 18, 2021, 299 patients were screened for eligibility and 146 were enrolled and randomly assigned to usual care (n=54), namilumab (n=57), or infliximab (n=35; figure 1 ). Data from the COVID-19 genomics UK consortium have shown that the main circulating strains of SARS-CoV-2 in the UK within this time period were the original B lineage, the B.1.177 lineage, and the B.1.1.7 lineage (alpha variant). Following a data monitoring committee review on Jan 21, 2021, where recommendations on both groups were made on the basis of the primary outcome analysis, the trial steering committee advised to stop the infliximab group for futility (probability of benefit 21%) but to continue to recruit to usual care and namilumab, which met the criteria for success (probability of benefit 99%), to collect further secondary outcome clinical data. A subsequent data monitoring committee meeting on Feb 23, 2021, advised closing the remaining groups as the trial was close to maximal recruitment and recent changes to standard of care with routine use of tocilizumab would affect the conduct of the trial. In total, nine patients withdrew after randomisation but before receiving treatment and were not included in the analysis: five at their own or a relative's request (one in the namilumab group and four in the infliximab group), one at the request of the treating physician in the usual care group, one who was reassessed as not having COVID-19 in the infliximab group, one due to initial non-disclosure of information that met an exclusion criterion in the namilumab group, and one who was withdrawn before treatment when the data monitoring committee recommended to stop enrolment in the infliximab group.
Figure 1.
Trial profile
CRP=C-reactive protein.
Overall, groups were evenly matched for baseline characteristics, although fewer patients in the infliximab group had remdesivir at enrolment than in the namilumab or usual care groups. Most participants had a positive PCR assay for SARS-CoV2 (table 1 ). Overall, 53 (98%) of 54 patients in the usual care group, 55 (97%) of 57 in the namilumab group, and 33 (94%) of 35 in the infliximab group received oxygen at baseline. For the usual care and namilumab comparison, 16 (30%) of 54 in the usual care group and 21 (37%) of 57 in the namilumab group received high-flow nasal oxygen or continuous positive airway pressure, and 11 (20%) and 11 (19%), respectively, were intubated and mechanically ventilated. Almost all patients received dexamethasone as part of usual care at enrolment, and around half received remdesivir. Subsequent to enrolment, all patients except one in the namilumab group received dexamethasone, 36 (68%) of 53 in the usual care group and 37 (67%) of 55 in the namilumab group received remdesivir, and three (6%) and five (9%) received tocilizumab, respectively. For the infliximab versus usual care comparison, all patients received dexamethasone, 26 (79%) of 33 in the usual care group and 16 (53%) of 30 in the infliximab group received remdesivir before or following randomisation, and two (6%) and one (3%) received tocilizumab, respectively.
Table 1.
Baseline characteristics of all randomised patients
|
Namilumab vs usual care |
Infliximab vs usual care |
||||
|---|---|---|---|---|---|
| Usual care group (n=54) | Namilumab group (n=57) | Usual care group (n=34) | Infliximab group (n=35) | ||
| Sex | |||||
| Female | 17 (31%) | 23 (40%) | 13 (38%) | 16 (46%) | |
| Male | 37 (69%) | 34 (60%) | 21 (62%) | 19 (54%) | |
| Age, years | 62·8 (51·9–70·5) | 56·2 (47·6–63·3) | 64·5 (51·9–71·9) | 55·4 (46·1–70·5) | |
| Ethnicity | |||||
| White | 33 (61%) | 34 (60%) | 23 (68%) | 17 (49%) | |
| Black | 1 (2%) | 1 (2%) | 0 | 2 (6%) | |
| South Asian | 7 (13%) | 8 (14%) | 3 (9%) | 4 (11%) | |
| Other | 11 (20%) | 14 (25%) | 7 (21%) | 9 (26%) | |
| Not known | 2 (4%) | 0 | 1 (3%) | 3 (9%) | |
| Clinical frailty score level 4–8* | 7 (13%) | 4 (7%) | 5 (15%) | 5 (14%) | |
| Any smoking history | 22 (41%) | 15 (26%) | 11 (32%) | 12 (34%) | |
| Body-mass index, kg/m2 | 29·5 (25·4–34·7) | 30·5 (27·1–35·4) | 30·7 (25·2–34·3) | 32·3 (26·9–35·9) | |
| Background respiratory disease† | 13 (24%) | 13 (23%) | 10 (29%) | 8 (23%) | |
| Background diabetes | 22 (41%) | 17 (30%) | 12 (35%) | 11 (31%) | |
| Care location | |||||
| Ward | 33 (61%) | 33 (58%) | 22 (65%) | 22 (63%) | |
| ICU | 21 (39%) | 24 (42%) | 12 (35%) | 13 (37%) | |
| SARS-CoV-2 PCR result | |||||
| Positive | 50 (93%) | 54 (95%) | 30 (88%) | 29 (83%) | |
| Negative | 3 (6%) | 2 (4%) | 3 (9%) | 6 (17%) | |
| Previous COVID-19 treatment at baseline | |||||
| Corticosteroids | 49 (91%) | 53 (93%) | 29 (85%) | 33 (94%) | |
| Remdesivir | 29 (54%) | 32 (56%) | 21 (62%) | 10 (29%) | |
| Antibiotics | 46 (85%) | 48 (84%) | 28 (82%) | 31 (89%) | |
| Time to enrolment, days‡ | 1 (1–3) | 1 (1–2) | 2 (1–3) | 1 (1–2) | |
| CRP concentration, mg/L | 108·0 (60·0–160·0) | 94·6 (55·4–171·0) | 88·0 (48·8–142·0) | 99·0 (46·0–173·0) | |
| Lymphocyte count, 109/L | 0·8 (0·6–1·2) | 0·9 (0·6–1·1) | 0·9 (0·6–1·3) | 0·9 (0·6–1·0) | |
| Neutrophil count, 109/L | 7·2 (5·4–10·0) | 7·5 (5·0–10·1) | 7·2 (5·5–11·0) | 6·8 (4·5–9·5) | |
| Ferritin, μg/L | 750 (490–1685) | 791 (433–1621) | 676 (506–1022) | 642 (435–1114) | |
| D-dimers, ng/mL | 787 (376–1822) | 592 (227–1418) | 739 (414–1184) | 398 (235–805) | |
Data are n (%) or median (IQR). ICU=intensive care unit. CRP=C-reactive protein.
Vulnerable, mildly frail, moderately frail, or severely frail.
The number of patients that had at least one lung disease comorbidity of chronic obstructive pulmonary disease, asthma, or interstitial lung disease.
Time from date of hospital admission to date of randomisation.
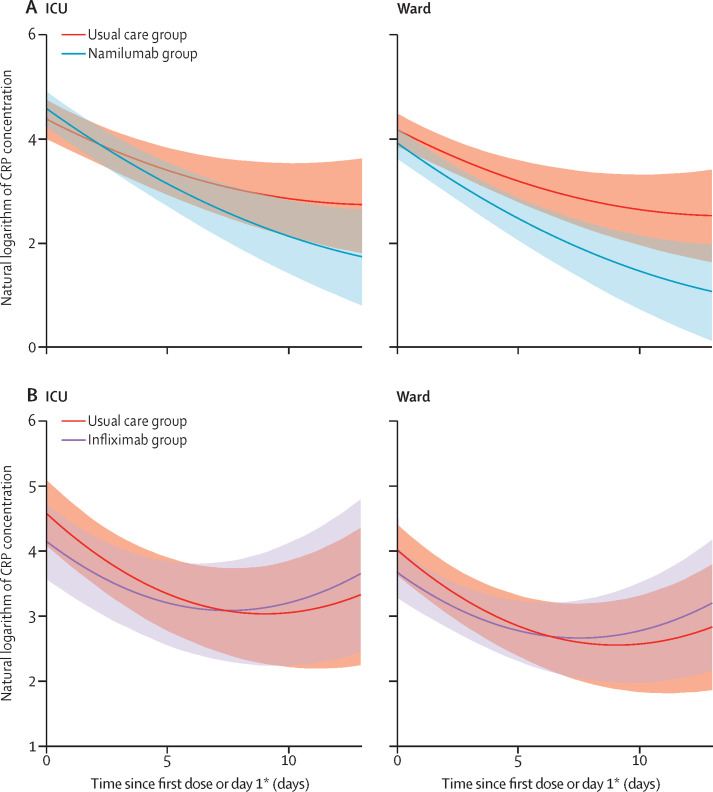
45 patients in the usual care group versus 52 in the namilumab group and 29 in the usual care group versus 28 in the infliximab group were evaluable for the primary outcome. Analysis of the primary outcome showed a 97% probability that namilumab plus usual care was superior to usual care alone in reducing CRP concentration over time, with a point estimate for the treatment–time interaction of –0·09 (95% CI –0·19 to 0·00; figure 2 ). Model fitted values were in good agreement with raw data. This effect was consistent in ward and ICU groups based on care location at randomisation, as visualised in the conditional effects plots (figure 2), and in patients with severe and non-severe disease at baseline (appendix p 14). The effect of namilumab on CRP concentration was independent of age (appendix p 15). The probability of infliximab being superior to usual care alone was 15%, with a point estimate for the treatment–time interaction of 0·06 (95% CI –0·05 to 0·17). This finding was consistent across ward and ICU groups (figure 2), and in patients with severe and non-severe disease at baseline (appendix p 14).
Figure 2.
Conditional effects plots of the natural logarithm of CRP concentration modelled over time in patients by care location
(A) Namilumab versus usual care. (B) Infliximab versus usual care. Shaded areas are 95% CIs. CRP=C-reactive protein. ICU=intensive care unit. *Day 1 in usual care group.
At the whole population level, and consistent with our previous findings and published data, CRP concentration over time was related to the outcomes of discharge, death, and continued hospitalisation at day 28 (appendix p 13).
In the post-hoc sensitivity analyses to assess the effect of baseline remdesivir use, smoking status, and frailty, the inference for both drugs remained unchanged (data not shown). Likewise, excluding patients without a positive SARS-CoV-2 PCR test did not change the inference for either drug (data not shown). Effects of namilumab and infliximab on CRP concentration were also consistent with an area under the curve analysis (data not shown). Point estimates for natural logarithm of CRP predicted values with associated credible intervals at baseline, day 7, and day 14 for both ward and ICU patients are shown in the appendix (p 9).
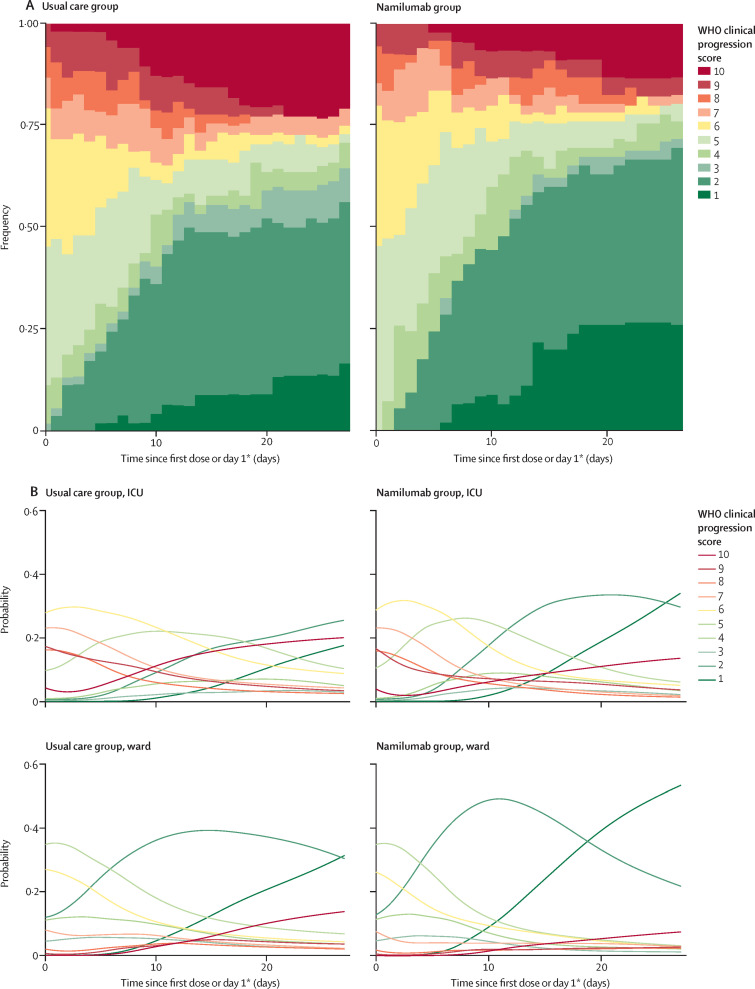
Among the secondary endpoints, the principal efficacy outcome was the WHO clinical progression scale. For the modified intention-to-treat comparisons, data were available for 53 patients in the usual care group and 55 in the namilumab group. In the namilumab group, for patients recruited from both wards and ICUs, the probability of having lower WHO clinical progression scale scores was consistently increased over time compared with usual care alone (figure 3 ). For example, the groups were similar at baseline but by day 28, the probability of discharge (WHO levels 1–3 combined) was 47% for ICU patients and 64% for ward patients in the usual care group versus 66% and 77%, respectively, in the namilumab group (appendix p 10). At day 14, the probability of an ICU patient still needing non-invasive ventilation, invasive ventilation, or to have died (WHO level ≥6) was 54% in the usual care group versus 36% in the namilumab group. Time to a 2-point improvement was also seen to be shorter in the namilumab group than in the usual care group (table 2 , appendix p 16). Similar improvements on the WHO scale outcome compared with usual care were not observed with infliximab (appendix pp 11, 17). The median number of hospital-free days was 17 (IQR 0–23) in the usual care group versus 20 (3–23) in the namilumab group, and 17 (0–23) in the usual care group versus 17 (3–23) in the infliximab group. Data on respiratory rate, body temperature, and destination of discharge were non-informative and therefore these data are not shown.
Figure 3.
WHO clinical progression score over 28 days for usual care versus namilumab
(A) Stacked bar chart of raw data for whole population eligible for comparison. (B) Conditional effects plots of WHO score modelled over time, showing the probability of being at each level on each day for patients by care location. ICU=intensive care unit. *Day 1 in usual care group.
Table 2.
Time to a 2-point improvement in the WHO clinical progression scale, for the overall population and by care location, in the modified intention-to-treat population
|
Namilumab vs usual care |
Infliximab vs usual care |
|||
|---|---|---|---|---|
| Usual care group | Namilumab group | Usual care group | Infliximab group | |
| Overall | ||||
| Participants | 53 | 55 | 33 | 29 |
| Time, days | 10 (7–12) | 8 (6–9) | 10 (6–14) | 15 (6–21) |
| Ward | ||||
| Participants | 33 | 33 | 22 | 20 |
| Time, days | 9 (6–12) | 8 (5–10) | 9 (5–12) | 15 (5–NR) |
| ICU | ||||
| Participants | 20 | 22 | 11 | 9 |
| Time, days | 14 (5–NR) | 8 (6–11) | 14 (4–NR) | 19 (6–28) |
Data are n or median (95% CI). ICU=intensive care unit. NR=not recordable.
Median length of hospital stay was 10 days (range 1–28) in the usual care group and 8 days (2–28) in the namilumab group. For usual care versus infliximab, the median length of hospital stay was 10 days (1–28) in the usual care group and 11 days (2–28) in the infliximab group.
By day 28, there were fewer deaths and more discharges in the namilumab group than in the usual care alone group; 43 (78%) of 55 patients were discharged, six (11%) were still in hospital, and six (11%) had died in the namilumab group compared with 33 (61%) of 54, 11 (20%), and ten (19%) in the usual care alone group (table 3 ). Despite the challenges we described in modelling the SpO2:FiO2 ratio, trends to improvement in oxygenation status were observed in the namilumab group (appendix p 18).
Table 3.
Hospital discharge status at day 28
|
Namilumab vs usual care |
Infliximab vs usual care |
|||||
|---|---|---|---|---|---|---|
| Usual care group | Namilumab group | Difference in proportions (95% CI) | Usual care group | Infliximab group | Difference in proportions (95% CI) | |
| Overall | ||||||
| Participants | 54 | 55 | .. | 34 | 29 | .. |
| Discharged | 33 (61%) | 43 (78%) | −0·17 (−0·34 to −0·00) | 22 (65%) | 22 (76%) | −0·11 (−0·34 to 0·11) |
| In hospital | 11 (20%) | 6 (11%) | 0·09 (−0·04 to 0·23) | 7 (21%) | 3 (10%) | 0·10 (−0·07 to 0·28) |
| Death | 10 (19%) | 6 (11%) | 0·08 (−0·06 to 0·21) | 5 (15%) | 4 (14%) | 0·01 (−0·16 to 0·18) |
| Ward | ||||||
| Participants | 33 | 33 | .. | 22 | 20 | .. |
| Discharged | 28 (85%) | 29 (88%) | −0·03 (−0·20 to 0·14) | 19 (86%) | 16 (80%) | 0·06 (−0·16 to 0·29) |
| In hospital | 4 (12%) | 2 (6%) | 0·06 (−0·08 to 0·20) | 2 (9%) | 1 (5%) | 0·04 (−0·11 to 0·19) |
| Death | 1 (3%) | 2 (6%) | −0·03 (−0·13 to 0·07) | 1 (5%) | 3 (15%) | −0·10 (−0·28 to 0·07) |
| ICU | ||||||
| Participants | 21 | 22 | .. | 12 | 9 | .. |
| Discharged | 5 (24%) | 14 (64%) | −0·40 (−0·67 to −0·13) | 3 (25%) | 6 (67%) | −0·42 (−0·81 to −0·02) |
| In hospital | 7 (33%) | 4 (18%) | 0·15 (−0·11 to 0·41) | 5 (42%) | 2 (22%) | 0·19 (−0·19 to 0·58) |
| Death | 9 (43%) | 4 (18%) | 0·25 (−0·02 to 0·51) | 4 (33%) | 1 (11%) | 0·22 (−0·11 to 0·56) |
Data are n (%) unless otherwise stated. Data were available on all patients (in the modified intention-to-treat population). ICU=intensive care unit.
For the namilumab versus usual care comparison, a total of 279 adverse events were reported in 59 (54%) of 109 patients in the safety population (134 events in 30 [55%] of 55 patients in the namilumab group and 145 in 29 [54%] of 54 in the usual care group). Of these, 103 (77%) events in the namilumab group and 131 (90%) in the usual care group were grade 3 or worse (appendix p 12). Infections were more common in the namilumab group (20 events in eight patients) than in the usual care group (ten events in seven patients). There were ten serious adverse events in each of the usual care and namilumab groups, respectively. All except one of the serious adverse events in the namilumab group were considered unrelated to treatment; the related event was a re-admission with bacterial pneumonia 26 days after receiving namilumab, on a background of a prolonged admission for social reasons and known chronic obstructive pulmonary disease.
For the infliximab versus usual care comparison, a total of 214 adverse events were reported in 37 (60%) of 63 patients in the safety population; (102 events in 20 [69%] of 29 patients in the infliximab group and 112 in 17 [50%] of 34 in the usual care group). Of these, 78 (77%) events in the infliximab group and 101 (90%) in the usual care group were grade 3 or worse (appendix p 12). There were four infection events in the infliximab group and seven in the usual care group. There were six serious adverse events in the infliximab group and five in the usual care group; all were considered unrelated to treatment. Death occurred in four (14%) patients in the infliximab group compared with five (15%) in the usual care group. There were no additional deaths in the safety population outside of the modified intention-to-treat population.
Discussion
Our trial showed that the addition of namilumab, but not infliximab, to usual care reduced inflammation as measured by CRP concentration in hospitalised patients with COVID-19, compared with usual care alone. The secondary clinical outcomes were consistent and shared the same directionality as the primary outcome for both interventions, despite not being formally powered to assess for such differences. Our proof-of-concept findings with GM-CSF inhibition are consistent with our hypothesis that recruitment and activation of inflammatory monocytes and macrophages are important in the pathogenesis of severe COVID-19. These findings are also consistent with published findings from small, non-randomised trials,23, 24 and large, randomised trials of other GM-CSF inhibitors in COVID-19; otilimab showed a benefit for the primary endpoint of being alive and free of respiratory failure at day 28 in a predefined subgroup of patients aged 70 years or older.25 Lenzilumab, given as a three-dose course in non-ventilated hospitalised patients, showed a benefit over standard care in the primary outcome of survival without ventilation, an effect that seemed more pronounced in patients aged 85 years or younger and with CRP concentration of less than 150 mg/L.26 Our data suggest the effect of a single dose of namilumab on CRP concentration and WHO clinical progression score is independent of age, although this requires confirmation in larger studies. Although it is not possible to directly compare these studies given the differences in sample sizes, inclusion criteria, and study designs, the overall randomised controlled trial data suggest a benefit of GM-CSF inhibition in COVID-19. For example, we observed mortality in the namilumab group of 11% compared with 19% in the usual care alone group. In the lenzilumab and otilimab phase 3 trials, mortality was 10% in the intervention group versus 14% in the standard care group (day 28), and 23% versus 24% (day 60), respectively. In two phase 2 mavrilimumab trials, mortality was 8% in the intervention group versus 21% in the usual care group (day 29),27 and 5% versus 16% (day 28).28 Benefit has also been observed with IL-6 inhibition, with a meta-analysis showing a day 28 mortality of 22% in the intervention groups compared with 25% in the usual care or placebo groups.29
In the absence of large treatment effects, small trials using traditional clinical outcomes might give inconclusive or contrary findings in COVID-19, as exemplified by earlier studies of tocilizumab. The CATALYST trial was designed to use a repeatedly collected continuous measure of CRP concentration with a Bayesian adaptive approach that we predicted would require a smaller sample size to show evidence of efficacy or futility. CRP concentrations, including the rate of decline, have been associated with clinical outcome in COVID-19,11 and we hypothesised that an immunomodulatory agent unable to alter CRP concentration would be a less promising candidate to take forward into phase 3 trials. With many options for repurposing immunomodulatory therapies in COVID-19, we contend that such a prioritisation approach will make the most efficient use of phase 3 resources and accelerate development of effective treatments.
By contrast with the observed effect of namilumab, we did not find a similar benefit on CRP concentration with infliximab, and the group was stopped for futility. TNF is an important proinflammatory cytokine produced by macrophages as well as other cell types, with context-dependent pleiotropic effects including further activation of inflammatory monocytes and macrophages and upregulation of inflammatory mediators, such as IL-6. A previous non-randomised study of infliximab suggested potential efficacy, albeit with substantial limitations including small sample size, use of historical controls, and being conducted before the routine use of corticosteroids.30 This previous finding, together with circumstantial data, justified our inclusion of infliximab.18 However, although TNF inhibitors are widely used in inflammatory diseases, not all immune-mediated inflammatory diseases are responsive, and TNF itself might suppress specific proinflammatory factors that might be relevant to COVID-19, such as type 1 interferon expression and Th17 cell differentiation.31 Inhibition of such cross-regulatory effects might underlie our negative findings, or simply indicate that TNF is not on an essential path to driving inflammatory responses as measured by CRP in patients hospitalised with COVID-19. GM-CSF inhibition might also have an additional benefit in retarding neutrophil recruitment and activation that could be of importance in the pathogenesis of severe COVID-19 and acute respiratory distress syndrome.32 Our safety data suggest that the absence of an effect with infliximab is not due to an increase in secondary infections. We cannot exclude the possibility of benefit with infliximab being seen in a specific subgroup of patients, in larger studies, or with a dose higher than the standard dose we employed (although this dose was in large molar excess relative to published concentrations of circulating TNF in COVID-19). It should also be noted that remdesivir use at baseline was lower in the infliximab group than in the usual care group, although the negative SOLIDARITY trial for remdesivir suggests this difference might not have unduly influenced our results,33 and results of our post-hoc sensitivity analyses were consistent. The clear divergence in primary outcome is broadly reflected in the secondary clinical findings and justifies the prioritisation of GM-CSF inhibition over TNF inhibition at this dose for further study in hospitalised patients with COVID-19.
GM-CSF has an important role in the differentiation of alveolar macrophages, and consequently in surfactant clearance, as well as being an important survival factor for lung epithelial cells. Absence of GM-CSF signalling, through genetic defect in the receptor or very high levels of polyclonal autoantibodies to GM-CSF, has been associated with pulmonary alveolar proteinosis (PAP). PAP has been an adverse event of special interest in previous clinical trials of GM-CSF inhibitors but, to our knowledge, has never been observed. It is important to note that therapeutic monoclonal antibodies will not completely inhibit GM-CSF signalling, which appears to be a requirement for PAP,34 but rather will downregulate excessive pathway activation; absence of GM-CSF does not prevent macrophage uptake of surfactant as much as its catabolism, therefore the effect of short-term inhibition is likely to be less pronounced on surfactant clearance when compared with long-term inhibition; and downregulation of monocyte activation, which is the aim of GM-CSF inhibition, should itself lead to a reduction in alveolar epithelial cell damage in COVID-19. However, it is also important to note an opposing view that administration of GM-CSF might have therapeutic benefits, and the results of clinical trials of inhaled and intravenous sargramostim are awaited.35
Our study has some limitations. Like many other trials in COVID-19, we did not use a placebo control. However, the discordant results of the two intervention groups compared with usual care alone, as well as the objective nature of CRP data, suggest this absence of a placebo does not explain the positive findings we observed with namilumab. Our sample size was too small for a definitive assessment of clinical outcomes and further studies are required for this, as well as to better understand the population that might benefit most. Our results might not generalise to hospitalised patients without evidence of pneumonia or raised CRP, or patients not requiring hospitalisation. Harms data are difficult to interpret given the small number of participants, absence of masking, the severity of the background illness, and that data were being collected during a pandemic. Overall, the number of total adverse events did not differ between namilumab and usual care groups. However, our data do highlight the need to monitor secondary infections in future COVID-19 trials, particularly given the use of combination immune-modulating treatments.
Despite the advances of dexamethasone and tocilizumab in COVID-19, mortality among patients with severe disease remains high.2 Therefore, considerable unmet medical need remains, and data pointing to the role of both inflammatory monocytes and macrophages and GM-CSF in severe COVID-19, together with our findings reported here, strongly suggest that targeted GM-CSF inhibitors such as namilumab should be further investigated in hospitalised patients with COVID-19.
Data sharing
Participant data and the associated supporting documentation will be available within 6 months after the publication of this manuscript. Details of our data request process are available online from the Cancer Research UK Clinical Trials Unit (CRCTU) at https://www.birmingham.ac.uk/research/crctu/data-sharing-policy.aspx. Only scientifically sound proposals from appropriately qualified research groups will be considered for data sharing. The decision to release data will be made by the CRCTU Director's Committee, who will consider the scientific validity of the request, the qualifications and resources of the research group, the views of the Chief Investigator and the Trial Steering Committee, consent arrangements, the practicality of anonymising the requested data, and contractual obligations. A data sharing agreement will cover the terms and conditions of the release of trial data and will include publication requirements, authorship and acknowledgements, and obligations for the responsible use of data. An anonymised encrypted dataset will be transferred directly using a secure method and in accordance with the University of Birmingham's information technology guidance on encryption of datasets.
Declaration of interests
BAF reports consultancy for Novartis, Bristol Myers Squibb, Servier, Galapagos, and Janssen and research funding from Servier and Galapagos. MR is currently undertaking a Senior Clinical Fellowship financed by Roche. PK reports consultancy for Bristol Myers Squibb and AstraZeneca, and research funding from Bayer and Pfizer. DR is a former employee of GlaxoSmithKline. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This trial was supported by the Medical Research Council, grant number MC_PC_20007. SG was supported by a Senior Investigator Award from the National Institute of Health Research (NIHR). Staff at the CRCTU are supported by core funding grants from Cancer Research UK (C22436/A25354), the NIHR Biomedical Research Centre (BRC-1215-20009), The Kennedy Trust for Rheumatology Research as part of the Arthritis Trials Acceleration Programme (KENN161704), and Innovate UK as part of the Midlands Wales Advanced Therapy Treatment Centres (104232). This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centres at Birmingham, Oxford, Imperial College London, and University College London. GC is supported by a NIHR Research Professorship. The views expressed in this paper are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care. Namilumab was provided free of charge by Izana Bioscience (Oxford, UK; now part of Roivant). Infliximab was provided free of charge by Celltrion (Incheon, South Korea).
Contributors
BAF, TV, MR, TW, DP, AR, RS, DRT, JB, SG, DR, and PK conceived the study. BAF, TV, DS, MR, TW, DP, AR, RS, DRT, JB, HM, L-PH, PNN, SG, DR, and PK designed the clinical trial. BAF and DP were group leads for namilumab. MR and DR were group leads for infliximab. TV, TW, JS, DP, MSB, GC, NM, ZG, MPW, JP, and AR recruited patients or collected data, or both. DS, CG, and SG conducted the statistical analysis and accessed and verified the data. BAF wrote the first draft of the manuscript. All authors revised and approved the manuscript for submission. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
CATALYST investigators:
Bryan Williams, Rebecca Turner, Vincenzo Libri, Francis Mussai, Gary Middleton, Sarah Bowden, Mansoor Bangash, Fang Gao-Smith, Jaimin Patel, Elizabeth Sapey, Mark Thomas, Mark Coles, Peter Watkinson, Naj Rahman, Brian Angus, Alexander J. Mentzer, Alex Novak, Marc Feldman, Alex Richter, Sian Faustini, Camilla Bathurst, Joseph Van de Wiel, Susie Mee, Karen James, Bushra Rahman, Karen Turner, Adam Hill, Anthony Gordon, Christina Yap, Michael Matthay, Danny McAuley, Andrew Hall, Paul Dark, and Andrew McMichael
Supplementary Material
References
- 1.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filbin MR, Mehta A, Schneider AM, et al. Plasma proteomics reveals tissue-specific cell death and mediators of cell–cell interactions in severe COVID-19 patients. bioRxiv. 2020 doi: 10.1101/2020.11.02.365536. published online Nov 3. (preprint). [DOI] [Google Scholar]
- 6.Pairo-Castineira E, Clohisey S, Klaric L, et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 7.Daamen AR, Bachali P, Owen KA, et al. Comprehensive transcriptomic analysis of COVID-19 blood, lung, and airway. SciRep. 2021;11 doi: 10.1038/s41598-021-86002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann ER, Menon M, Knight SB, et al. Longitudinal immune profiling reveals distinct features of COVID-19 pathogenesis. medRxiv. 2020 doi: 10.1101/2020.06.13.20127605. published online June 16. (preprint). [DOI] [Google Scholar]
- 9.Schulte-Schrepping J, Reusch N, Paclik D, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoepel W, Chen H-J, Allahverdiyeva S, et al. Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammatory responses. bioRxiv. 2020 doi: 10.1101/2020.07.13.190140. published online July 13. (preprint). [DOI] [Google Scholar]
- 11.Veenith T, Fisher BA, Slade D, et al. CATALYST trial protocol: a multicentre, open-label, phase II, multiarm trial for an early and accelerated evaluation of the potential treatments for COVID-19 in hospitalised adults. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abg9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Kilian C, Turner JE, et al. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abf6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams MA, White SA, Miller JJ, et al. Granulocyte-macrophage colony-stimulating factor induces activation and restores respiratory burst activity in monocytes from septic patients. J Infect Dis. 1998;177:107–115. doi: 10.1086/513802. [DOI] [PubMed] [Google Scholar]
- 15.Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Mears JR, Shakib L, et al. IFN-γ and TNF-α drive a CXCL10+ CCL2+ macrophage phenotype expanded in severe COVID-19 and other diseases with tissue inflammation. bioRxiv. 2020 doi: 10.1101/2020.08.05.238360. published online Aug 5. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson PC, Liew DFL, Liew JW, et al. The potential for repurposing anti-TNF as a therapy for the treatment of COVID-19. Med (N Y) 2020;1:90–102. doi: 10.1016/j.medj.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelman A, Hill J, Vehtari A. Cambridge University Press; Cambridge: 2020. Regression and other stories. [Google Scholar]
- 21.Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. J Stat Softw. 2017;80:1–28. [Google Scholar]
- 22.Bürkner P-C, Gabry J, Weber S, et al. brms: Bayesian regression models using ‘Stan’. 2021. https://CRAN.R-project.org/package=brms
- 23.Temesgen Z, Assi M, Shweta FNU, et al. GM-CSF neutralization with lenzilumab in severe COVID-19 pneumonia: a case-cohort study. Mayo Clin Proc. 2020;95:2382–2394. doi: 10.1016/j.mayocp.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Luca G, Cavalli G, Campochiaro C, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020;2:e465–e473. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel J, Beishuizen A, Ruiz XB, et al. A randomized trial of otilimab in severe COVID-19 pneumonia (OSCAR) medRxiv. 2021 doi: 10.1101/2021.04.14.21255475. published online April 17. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temesgen Z, Burger CD, Baker J, et al. Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00494-X. published online Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pupim L, Wang TS, Hudock K, et al. LB0001 mavrilimumab improves outcomes in phase 2 trial in non-mechanically-ventilated patients with severe COVID-19 pneumonia and systemic hyperinflammation. Ann Rheum Dis. 2021;80(suppl 1):198–199. [Google Scholar]
- 28.Cremer PC, Abbate A, Hudock K, et al. Mavrilimumab in patients with severe COVID-19 pneumonia and systemic hyperinflammation (MASH-COVID): an investigator initiated, multicentre, double-blind, randomised, placebo-controlled trial. Lancet Rheumatol. 2021;3:e410–e418. doi: 10.1016/S2665-9913(21)00070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stallmach A, Kortgen A, Gonnert F, Coldewey SM, Reuken P, Bauer M. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure-a cautionary case series. Crit Care. 2020;24:444. doi: 10.1186/s13054-020-03158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Notley CA, Inglis JJ, Alzabin S, McCann FE, McNamee KE, Williams RO. Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for Th1 and Th17 cells. J Exp Med. 2008;205:2491–2497. doi: 10.1084/jem.20072707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masso-Silva JA, Moshensky A, Lam MTY, et al. Increased peripheral blood neutrophil activation phenotypes and NETosis in critically ill COVID-19 patients: a case series and review of the literature. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab437. published online May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for COVID-19—interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piccoli L, Campo I, Fregni CS, et al. Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis. Nat Commun. 2015;6 doi: 10.1038/ncomms8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta P, Porter JC, Manson JJ, et al. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respir Med. 2020;8:822–830. doi: 10.1016/S2213-2600(20)30267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Participant data and the associated supporting documentation will be available within 6 months after the publication of this manuscript. Details of our data request process are available online from the Cancer Research UK Clinical Trials Unit (CRCTU) at https://www.birmingham.ac.uk/research/crctu/data-sharing-policy.aspx. Only scientifically sound proposals from appropriately qualified research groups will be considered for data sharing. The decision to release data will be made by the CRCTU Director's Committee, who will consider the scientific validity of the request, the qualifications and resources of the research group, the views of the Chief Investigator and the Trial Steering Committee, consent arrangements, the practicality of anonymising the requested data, and contractual obligations. A data sharing agreement will cover the terms and conditions of the release of trial data and will include publication requirements, authorship and acknowledgements, and obligations for the responsible use of data. An anonymised encrypted dataset will be transferred directly using a secure method and in accordance with the University of Birmingham's information technology guidance on encryption of datasets.