Abstract
Nanobiotechnology is a promising field concerned with the using of engineered nanomaterials, which leads to the improvement of new human remedial against pathogenic bacteria modalities. In this work, silver nanoparticles (AgNPs) were prepared by an easy, cheap and low‐cost electro‐chemical method. The AgNPs were then loaded successfully on to multi‐walled carbon nanotubes (MWCNTs) using a modified chemical reaction process. The AgNPs on the MWCNTs were well spread and evenly distributed on the surfaces of the long nanotubes with well‐graphitised walls as examined by high‐resolution transmission electron microscopy. X‐ray diffraction and transmission electron microscopy were used for sample characterisation. Good dispersion of AgNPs was obtained on the surface of MWCNTs, resulting in an efficient reactivity of the carbon nanotubes surfaces. Finally, the antibacterial activity of AgNPs/MWCNTs hybrid was evaluated against two pathogenic bacteria Pseudomonas aeruginosa and Staphylococcus aureus exhibited excellent activity.
Inspec keywords: nanocomposites, X‐ray diffraction, nanofabrication, nanoparticles, transmission electron microscopy, toxicology, silver, antibacterial activity, microorganisms, nanomedicine, multi‐wall carbon nanotubes, electrochemistry
Other keywords: engineered nanomaterials, human remedial, pathogenic bacteria modalities, silver nanoparticles, multiwalled carbon nanotubes, modified chemical reaction process, well‐graphitised walls, high‐resolution transmission electron microscopy, cytotoxicity properties, functionalised carbon nanotubes, carbon nanotube surfaces, nanobiotechnology, low‐cost electrochemical method, AgNP‐MWCNT hybrid, X‐ray diffraction, antibacterial activity, Pseudomonas aeruginosa, Staphylococcus aureus, Ag‐C
1 Introduction
Nanotechnology is a new promising tool due to its common application in biology. It offers an eco‐friendly option for disease management and has many advantages over traditional chemical methods, which are related to environmental toxicity. [1]. Nanostructures such as single‐ and multi‐walled carbon nanotubes (SWCNTs and MWCNTs) are contributing widely to the water treatment processes. There are several advantages of nanotechnology in solving many problems related to the safety of the environment [2, 3].
The nanotechnology can adequately deal with many of the industrial applications by using various types of nanomaterials such as MWCNTs/SWCNTs metal oxide, Ag/TiO2 and MWCNTs [4]. Nanotechnology employs the materials of sizes <100 nm as linked with other fields such as engineering and chemistry [5, 6]. At this level, materials gain notably change in physical, chemical and biological features because of the greater surface area to volume ratio, sensing, and detection as well as pollution control [7, 8].
The silver nano‐antimicrobials are known as a promising candidate for medicine [9, 10]. They were found to exhibit excellent antimicrobial activity [11].
The size of AgNPs in aqueous suspension makes them accumulate and lead to a noticeable decrease in antibacterial activity in addition to using higher concentrations of AgNPs leads to a vastly toxic possibility to bacterial cells. The main reason of AgNPs cytotoxicity depends mainly on the generation of reactive oxygen species because of the release of silver ions [12]. Hence, the synthesis of new nanostructures by loading of AgNPs on secondary materials to develop antibacterial activity and decrease unwanted effects of silver is highly demanded.
Lately, improvement of MWCNTs/AgNP hybrid makes great progress for different technical applications such as sensor, biology medicine and as a catalyst. Carbon nanotubes (CNTs) including SWCNTs and MWCNTs drew large attention as a supportive material as a result of their exceptional features such as high surface area and chemical constancy after varying functionalisation methods [13]. Particularly, with the high surface area of MWCNTs, they could be worked as efficient patterns for loading the AgNPs on the MWCNTs surface with elevated constancy and excellent antimicrobial action [14]. Biological contaminants can be categorised into various groups, such as microorganisms, several adsorbents including activated carbon have practically excellent antimicrobial efficiencies and there are many factors that control this process [15]. The AgNPs loading MWCNTs were found to show growth inhibition for different Gram positive bacteria such as Staphylococcus aureus and Gram negative bacteria model such as Escherichia coli and Pseudomonas aeruginosa. Numerous efforts have been made to increase the antibacterial activity except toxicology of hybrid materials by combining the AgNPs and MWCNTs. However, the understanding of the action mechanism of Ag/MWCNTs composites on bacteria has not been satisfactory. Therefore, additional studies to further understand the interaction and bactericidal mechanisms of carbon–silver composites are actually needed [16, 17]. In this work, the silver ions were prepared and produced by an easy, cheap and low‐cost method; it was the electro‐chemical method that has a direct effect in killing or penetrating the walls of bacteria as compared to other methods of producing such as a physical or chemical method. Also, antimicrobial activity of the prepared Ag/MWCNTs hybrid was evaluated against two pathogenic bacteria including S. aureus and P. aeruginosa.
2 Literature review
Ag‐MWCNTs have shown improved biocide activity against E. coli, S. aureus, Salmonella typhi, and P. aeruginosa [18]. CNTs/silver nanocomposites exhibited antibacterial activity against Gram‐positive bacteria (S. aureus) and Gram‐negative bacteria (E. coli) [19]. CNTs have unique biocompatibility properties that improve the advantageous properties of the nanocomposite material such as inhibiting bacterial infectivity [20] and water purification processes [21]. The antibacterial rate of Ag‐MWCNTs increased to 92.9% against E. coli bacteria [22].
3 Vision of the study
This study was devoted to improving the antimicrobial activity of Ag/MWCNTs hybrid material on Gram‐negative and Gram‐positive bacteria depending on the concentration of AgNPs decorated F‐MWCNTs and their ability to damage the cell wall of bacteria for biomedical application.
4 Experimental work
4.1 Preparation of AgNPs/MWCNTs hybrid
The synthesis of silver loading on CNTs is accomplished by two steps:
(i) Synthesis of Ag0 is based on the eco‐friendly electrochemical techniques that did not need any addition of reducer agents that are usually toxic [23]. This process involves using two poles of pure Ag in bar form in distilled water that is applied to a battery voltage difference of 12–24 V and a reverse bias. The formation of nano silver colloidal in the electrochemical method is illustrated in Fig. 1. The time used for polarity exchanging was 4 min, before this time, the particles accumulation could cause gradual reduction in efficiency of the pole surface.
(ii) The F‐MWCNTs surface is produced by a combination of concentrated 95% H2 SO4 and 65% HNO3 (3:1) using ultrasonication technique for one hour.
Fig. 1.
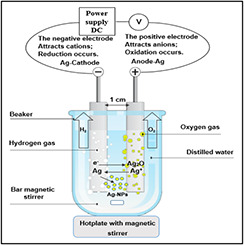
Simplified outline for electrochemical method
Then, 10 ml of Ag0 solution was dropped on a suitable amount of F‐MWCNTs that was functionalised using the step 2 process. Then, the mixture is reduced at 250°C to deposit Ag0 on to the functionalised CNTs surface by heat treatment for one hour. Finally, the resulting Ag/MWCNTs hybrid was collected and washed with deionised water then dried under vacuum at 100°C for 24 h. Then, the free‐standing nanocomposite was annealed at 400o C for one hour.
4.2 Characterisation of Ag/MWCNTs
An X‐ray diffractometer (6000‐Shimadzu) was used to prepare Cu‐Kα wavelength radiation (λ = 1.540600 Å) for the surface structure test. Transmission electron microscopy (TEM) (Philips EM208) and high‐resolution TEM (HRTEM) (JEOL, JEM‐2100) were used to determine the morphological and shape of AgNPs on the CNT surface.
4.3 Antibacterial activity
The very abundant pathogenic bacteria P. aeruginosa as a Gram‐negative bacteria model and S. aureus as a Gram‐positive model were kindly provided by the Biotechnology Department, the University of Technology, Baghdad for assessing the antibacterial action of Ag/MWCNTs hybrid. About 5×106 cells/ml of bacterial suspensions were seeded in nutrient broth at 37°C for 16–18 h. The reduction in viable cell number after interaction with AgNP/MWCNTs at different concentration (1, 3, 5) mg/ml was determined by the standard agar method. Moreover, bacterial viability is also studied at different incubation times (3, 5, and 10 h) [24].
5 Results and discussion
A schematic illustration of the formation mechanism of Ag/MWCNT nanocomposites shown in Fig. 2 is represented by functional groups introduced on F‐MWCNTs by chemo‐reduction.
Fig. 2.

Decoration schematic of AgNPs on the MWCNTs
The size of colloidal AgNPs is distributed uniformly with slight agglomeration, and the average diameter ranges from 12 to 70 nm with a different magnification as shown in Figs. 3 a and b. The electrochemical techniques are interesting because they allow us to obtain high pure particles by fast and easy techniques and control the particle size by adjusting the current density [25, 26].
Fig. 3.

TEM images of representative AgNPs formation using the electrochemical method at different magnifications
(a) Scale bar = 300 nm, (b) Scale bar = 80 nm
The raw MWCNTs tend to aggregate into a thick network of ropes due to their strong van der Waals attraction and high aspect ratio between the MWCNTs causing their poor solubility in many solvents [1]. Hence, their practical application is limited significantly. In order to expand their application, preparation of raw MWCNTs by the acid mixture of (H2 SO4 :HNO3) was adopted. The functional groups contain oxygen located on the surface, e.g. carboxyl (–COOH) and hydroxyl (–OH) groups that create a hydrogen bond with water molecules enhance the dispersity of the MWCNTs in aqueous solution. The oxygen‐containing functional groups on MWCNTs–COOH supplied nucleation sites for Ag ions and regularly distributed AgNPs [27]. The experimental result reveals that Ag/MWCNT nanocomposites have been successfully produced through the attachment of AgNPs onto the surface of acid‐treated MWCNTs. The AgNPs on the MWCNTs were well spread and evenly distributed on the surfaces of the long nanotubes with well‐graphitised walls as examined in HRTEM images, furthermore, bending tubes were observed in MWCNTs (Figs. 4 a and b). Large blocks and closely arranged MWCNTs reproduced in raw MWCNTs, which have become loosely and extremely dispersed after acid treatment, the same finding was noted by the authors of [28, 29]. The deposition of AgNPs outside and inside of F‐MWCNTs may result from long‐time heat treatment. Then, a narrow size distribution of AgNPs was observed with the absence of free particles in the background images of HRTEM and this proves all formed AgNPs were permanently attached to the CNTs.
Fig. 4.
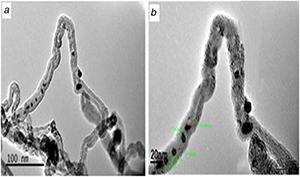
HRTEM images showing hybrid AgNPs deposited on MWCNTs at different magnifications
(a) Scale bar = 100 nm, (b) Scale bar = 20nm
Furthermore, structural confirmation of the silver and carbon nanomaterials was evaluated by investigation of Ag‐MWCNTs and MWCNTs‐COOH by X‐ray diffraction (XRD) patterns (Figs. 5 a –c). Two major diffraction peaks of F‐MWCNTs offered graphite after the functionalisation of MWCNTs, which correspond to the (002) and (100) at 2θ = 25.8° and 44.1°, respectively, illustrated in Fig. 5 a. The XRD pattern of Ag/MWCNTs shows three distinct diffraction peaks at 2θ = 38.1°, 44.3°, and 64.7°, which correspond to the (111), (200), and (220) crystalline planes of AgNPs (Fig. 5 b). The size of AgNPs to each peak was estimated from the full width at half maxima (FWHM) of the diffraction peaks of silver corresponding to AgNPs/MWCNTs as listed in Table 1.
Fig. 5.
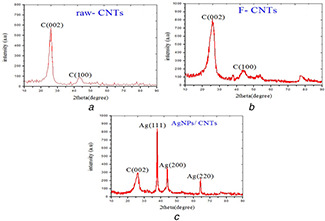
XRD patterns for
(a) Pristine, (b) Functionalised MWCNTs, (c) Ag/MWCNTs
Table 1.
Experimental X‐ray pattern peaks for the AgNPs
| Grain size (nm) | B (FWHM) (deg) | hkl | 2θ (deg) |
|---|---|---|---|
| 16.85 | 0.8371 | (111) | 38.1° |
| 19.11 | 0.4233 | (200) | 44.3° |
| 15.26 | 0.5919 | (220) | 64.7° |
The mean particle size is about 3.5 and 15 nm on the basis of Debye Scherrer's equation. The XRD pattern indicated that AgNPs consisted of pure phase, and no typical peaks were observed for other particles which actually confirm that the synthesised nanocomposites contain only AgNPs. Generally, HRTEM and XRD results confirm the synthesis of well dispersed and pure AgNPs on the entire surface of the MWCNTs.
The cytotoxicity of Ag/MWCNT hybrid was assessed at different concentrations (1, 3 and 5 mg/ml) by determining the capacity of the bacteria to create colonies, by calculating the colony forming units (CFU) number as illustrated in Figs. 6 a and b. It could be realised that the high concentrations of AgNPs/MWCNTs exhibit action activity resulting in the dropping of the number of CFUs against S. aureus than that of P. aeruginosa after 24 h of incubation; this result suggests that the bacterial growth inhibition of the hybrid sample might be due to the MWCNTs and AgNPs synergetic effect.
Fig. 6.
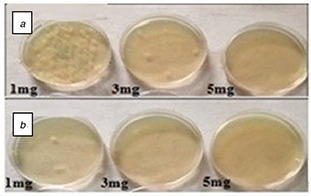
Antibacterial activity determination of Ag/MWCNTs hybrid at concentrations: 1, 3 and 5 mg/ml by the standard agar method after incubation with
(a) P. aeruginosa, (b) S. aureus
The Ag/MWCNT hybrid could kill the bacteria through physical contact to a bacterial cell by impairing the function of its membrane which may eventually lead to cell death.
The activity of Ag/MWCNT samples at various concentrations was more effective against S. aureus bacteria than that of P. aeruginosa bacteria, this may be from the weaker surfaces for Gram‐positive species as compared with the Gram‐negative ones [30].
The cell viability of the tested bacteria after 3, 5, and 10 h of incubation time on the Ag/MWCNT filters at different concentrations (1, 3 and 5 mg/ml) is summarised in Fig. 7. It can be realised that Ag/MWCNT samples were found to show considerable inhibitory influence against P. aeruginosa and S. aureus. The obtained results illustrated in Fig. 7 a exhibited that after 10 h of incubation at a concentration of 5 mg/ml the Ag/MWCNTs induced a serious decrease in S. aureus viability; in contrary, the Ag/MWCNT hybrid provokes a significant reduction in P. aeruginosa viability (Fig. 7 b). Some chemicals were added for MWCNT synthesis because of the ion exchange between –COOH on the surface of F‐MWCNTs and cell membrane of bacteria that worked as a barrier for pathogens growth. It also proposed that the increase of ionic strength of solution weakened the interactions between the F‐MWCNTs and bacteria due to the negative charge for the bacterial membrane and the surface of F‐MWCNTs, in that way increasing the adsorption efficiency of saline matter which led to bacteria death.
Fig. 7.
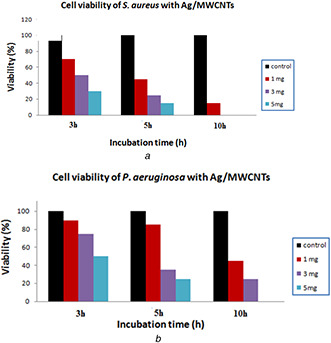
Antibacterial activity of Ag/MWCNT filters on
(a) S. aureus, (b) P. aeruginosa
Cell viability test was accomplished after 3, 5 and 10 h of incubation of each bacterial cell (5×103 CFU) with the different concentrations of Ag/MWCNT suspensions (1, 3, and 5 mg/ml) or phosphate buffered‐saline only (control) for 24 h. Cell survival was observed through a colony counting method that represented as a percentage with regard to control.
The scanning electron microscopy (SEM) images revealed that P. aeruginosa and S. aureus strictly stick to CNTs and entrapped into the MWCNTs network in vitro after 24 h of incubation with AgNPs/MWCNTs hybrid as illustrated in Fig. 8. The antimicrobial activity damages the cell membranes, which leads to the leakage of cell substances and eventually bacterial death after exposure to different concentrations of the hybrid. Bacteria have been shown to attach to carbons through van der Waals forces between the cell and the CNT; this process can clarify by what means CNTs stimulate arresting of cells [31].
Fig. 8.
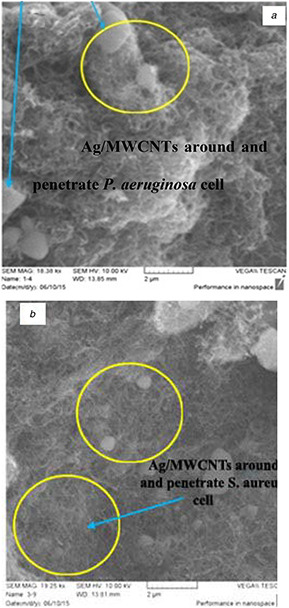
SEM images after incubation of AgNPs/MWCNTs for 24 h with
(a) P. aeruginosa, (b) S. aureus
It is interesting to note that the hybrid materials inactivate S. aureus more than P. aeruginosa. These results confirm that AgNPs/MWCNTs had potent toxicity to bacterial cells after long incubation time for both bacteria.
Moreover, it is found that the toxicity increases with the high concentration of hybrid materials, which is in agreement with those reported [32, 33]. However, our research establishes the in vitro direct attachment between Ag/MWCNTs and bacterial cells, so, in vivo analysis of our nanostructures would be advantageous in future work for diagnosing toxicity in clinical applications.
6 Conclusions
A modified chemical reaction method was introduced in this study for the preparation of AgNPs for loading on MWCNTs. Moreover, the resulted Ag/MWCNT hybrids are found to show an effective cytotoxicity in bacterial cells after long incubation time for both bacteria which depend on concentration and time, these results find the small sizes and fine dispersions of AgNPs loading on MWCNTs make it a promising tool for biomedical and industrial applications.
7 Acknowledgments
Authors gratefully acknowledge the nanotechnology and Advanced Material Research Centre staff at the University of Technology, Baghdad, Iraq for their support and conducting the SEM, XRD characterisations and to accomplish the antibacterial activity test. Besides, the authors are thankful to the University of Ioannina staff, Greece to accomplish TEM and HRTEM.
8 References
- 1. Athawale V. Paralikar P. Ingle A.P. et al.: ‘Biogenically engineered nanoparticles inhibit Fusarium oxysporum causing soft‐rot of ginger’, IET Nanobiotechnol., 2018, 12, (8), pp. 1084 –1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frimmel F.H. Reinhard N.: ‘Nanoparticles in water cycle’ (Springer‐Verlag, Berlin, 2010) [Google Scholar]
- 3. Haider A.J. AL‐Anbari R.H. Kadhim G.R. et al.: ‘Exploring potential environmental applications of TiO2 nanoparticles’, Energy Procedia, 2017, 119, pp. 332 –345 [Google Scholar]
- 4. Savage N. Diallo M.S.: ‘Nanomaterials and water purification: opportunities and challenges’, J. Nanoparticle Res., 2005, 7, (4–5), pp. 331 –342 [Google Scholar]
- 5. Kevin Dreher L.: ‘Health and environmental impact of nanotechnology: toxicological assessment of manufactured nanoparticles’, Toxicol. Sci., 2014, 77, pp. 3 –5 [DOI] [PubMed] [Google Scholar]
- 6. Shaurya P. Junghoon Y.: ‘Nanofluidics and microfluidics: systems and applications’ (Elsevier Inc., USA, 2014) [Google Scholar]
- 7. Qu X. Alvarez P.J.J. Li Q.: ‘Applications of nanotechnology in water and wastewater treatment’, Water Res., 2013, 47, (12), pp. 3931 –3946 [DOI] [PubMed] [Google Scholar]
- 8. Julia F. Samuel L.N. Charles R.T. et al.: ‘Silver nanoparticles: behavior and effects in the aquatic environment’, Environ. Int., 2011, 37, (2), pp. 517 –531 [DOI] [PubMed] [Google Scholar]
- 9. Tran Q.H. Nguyen V.Q. Le A.T.: ‘Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives’, Adv. Nat. Sci., Nanosci. Nanotechnol., 2013, 4, (3), p. 20 [Google Scholar]
- 10. Prucek R. Tuček J. Kilianová M. et al.: ‘The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles’, Biomaterials, 2011, 32, (21), pp. 4704 –4713 [DOI] [PubMed] [Google Scholar]
- 11. Duha A.S. Adawiya H.J. Mohammed R.M.: ‘Comparison of functionalization of multi‐walled carbon nanotubes treated by oil olive and nitric acid and their characterization’, Energy Procedia, 2013, 36, pp. 1111 –1118 [Google Scholar]
- 12. Kim J.D. Yun H. Kim G.C. et al.: ‘Antibacterial activity and reusability of CNT‐Ag and GO‐Ag nanocomposites’, Appl. Surf. Sci., 2013, 283, pp. 227 –233 [Google Scholar]
- 13. Seo Y. Hwang J. Kim J. et al.: ‘Antibacterial activity and cytotoxicity of multi‐walled carbon nanotubes decorated with silver nanoparticles’, Int. J. Nanomed., 2014, 9, pp. 4621 –4629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pendleton P. Schumann R. Wong S.H.: ‘Microcystin‐LR adsorption by activated carbon’, J. Colloid Interface Sci., 2001, 240, (1), pp. 1 –8 [DOI] [PubMed] [Google Scholar]
- 15. Lee D.K. Kim S.C. Cho I.C. et al.: ‘Photocatalytic oxidation of microcystin‐LR in a fluidized bed reactor having TiO2 ‐coated activated carbon’, Sep. Purif. Technol., 2004, 34, (1–3), pp. 59 –66 [Google Scholar]
- 16. Jameel Z.N. Haider A.J. Taha S.Y. et al.: ‘Evaluation of hybrid sol‐gel incorporated with nanoparticles as nano paint’. AIP Conf. Proc., USA, 2016, vol. 1758, (1), p. 020001 [Google Scholar]
- 17. Rodríguez‐S M.L. Rodríguez M.J. Blanco M.C. et al.: ‘Kinetics and mechanism of the formation of Ag nanoparticles by electrochemical techniques: a plasmon and cluster time‐resolved spectroscopic study’, J. Phys. Chem. B, 2005, 109, (3), pp. 1183 –1191 [DOI] [PubMed] [Google Scholar]
- 18. Yallappa S. Manjanna J. Dhananjaya B.L. et al.: ‘Phytochemically functionalized Cu and Ag nanoparticles embedded in MWCNTs for enhanced antimicrobial and anticancer properties’, Nano Micro Lett., 2016, 8, p. 120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dinh N.X. Chi D.T. Lan N.T. et al.: ‘Water‐dispersible silver nanoparticles‐decorated carbon nanomaterials: synthesis and enhanced antibacterial activity’, Appl. Phys. A, 2015, 119, p. 85 [Google Scholar]
- 20. Swe J.T. Chin W.L.: ‘Applications of nanocomposite materials in dentistry (Woodhead publishing series in biomaterials)’ (Woodhead Publishing, UK, 2019), pp. 93 –105 [Google Scholar]
- 21. Mohammad H.D. Abadi F.: ‘Nanoscale materials in water purification’, Micro Nano Technol., 2019, 1, pp. 87 –110 [Google Scholar]
- 22. Xu H. Deren W. Leyong H. et al.: ‘Preparation of a novel antibacterial coating precursor and its antibacterial mechanism’, Appl. Surf. Sci., 2019, 465, pp. 478 –485 [Google Scholar]
- 23. Wadkar M.M. Chaudhari V.R. Haram S.K.: ‘Synthesis and characterization of stable organosols of silver nanoparticles by electrochemical dissolution of silver in DMSO’, J. Phys. Chem. B, 2006, 110, (42), pp. 20889 –20894 [DOI] [PubMed] [Google Scholar]
- 24. Yu Y.Y. Chang S.S. Lee C.L. et al.: ‘Gold nanorods: electrochemical synthesis and optical properties’, J. Phys. Chem. B, 1997, 101, (34), pp. 6661 –6664 [Google Scholar]
- 25. Haider A.J. Thamir A.D. Ahmed D.S. et al.: ‘Deposition of silver nanoparticles on multiwalled carbon nanotubes by chemical reduction process and their antimicrobial effects’. AIP Conf. Proc., USA, 2016, 1758 [Google Scholar]
- 26. Ebbesen T.W. Hiura H. Bisher M.E. et al.: ‘Decoration of carbon nanotubes’, Adv. Mater., 1996, 8, (2), pp. 155 –157 [Google Scholar]
- 27. Marambio‐Jones C. Hoek E.M.V.: ‘A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment’, J. Nanoparticle Res., 2010, 12, (5), pp. 1531 –1551 [Google Scholar]
- 28. Suzuki S.: ‘Syntheses and applications of carbon nanotubes and their composites’ (IntechOpen, United Kingdom, 2013), ISBN 978‐953‐51‐1125‐2, 548 [Google Scholar]
- 29. Ma P.C. Tang B.Z. Kim J.: ‘Effect of CNT decoration with silver nanoparticles on electrical conductivity of CNT‐polymer composites’, Carbon, 2008, 46, (11), pp. 1497 –1505 [Google Scholar]
- 30. Liu S.B. Wei L. Hao L. et al.: ‘Sharper and faster ‘nano darts’ kill more bacteria: a study of antibacterial activity of individually dispersed pristine single‐walled carbon nanotube’, ACS Nano, 2009, 3, (12), pp. 3891 –3902 [DOI] [PubMed] [Google Scholar]
- 31. Busscher H.J. Dijkstra R.J.B. Langworthy D.E. et al.: ‘Interaction forces between waterborne bacteria and activated carbon particles’, J. Colloid Interface Sci., 2008, 322, (1), pp. 351 –357 [DOI] [PubMed] [Google Scholar]
- 32. Yan H. Gong A. He H. et al.: ‘Adsorption of microcystins by carbon nanotubes’, Chemosphere, 2006, 62, (1), pp. 142 –148 [DOI] [PubMed] [Google Scholar]
- 33. Tasis D. Tagmatarchis N. Bianco A. et al.: ‘Chemistry of carbon nanotubes’, Chem. Rev., 2006, 106, pp. 1105 –1136 [DOI] [PubMed] [Google Scholar]


