Abstract
The authors prepared surface modified (with polyelectrolyte layers), tea polyphenols (TPP) encapsulated, gelatin nanoparticles (TPP‐GNP) and characterised them. The size of the spherical nanoparticles was ∼50 nm. Number of polyelectrolyte layers and incubation time influenced the encapsulation efficiency (EE); highest EE was noted in nanoparticles with six polyelectrolyte layers (TPP‐GNP‐6L) incubated for 4 h. TPP released from TPP‐GNP‐6L in simulated biological fluids indicated protection and controlled release of TPP due to encapsulation. Mathematical modelling indicated anomalous type as a predominant mode of TPP release. TPP‐GNP‐6L exhibited enhanced pharmacokinetics in rabbit model compared with free TPP. The area under the concentration‐time curve and mean residence time were significantly higher in TPP‐GNP‐6L compared with free TPP which provide an evidence of higher bioavailability of TPP due to encapsulation. The authors demonstrated that encapsulation of TPP into GNPs favoured slow and sustained release of TPP with improved pharmacokinetics and bioavailability thereby can prolong the action of TPP.
Inspec keywords: gelatin, nanoparticles, encapsulation, biomedical materials, nanomedicine, particle size, polymer electrolytes, polymer films, nanofabrication
Other keywords: bioavailability, pharmacokinetics, gelatin nanoparticles, surface modified tea polyphenols, polyelectrolyte layers, spherical nanoparticle size, incubation time, encapsulation efficiency, TPP‐GNP‐6L, simulated biological fluids, mathematical modelling, TPP release, rabbit model, concentration‐time curve, mean residence time, time 4 h
1 Introduction
In spite of impressive data on several health benefits, the bottle‐neck to develop tea polyphenols (TPP) as a therapeutic agent or food supplement is low bioavailability at the target site. This low bioavailability of TPP is due to its poor absorption, instability, and metabolic transformation under physiological conditions [1]. TPP, particularly catechins, are unstable in solution and get quickly oxidised [2]. Solution pH plays important role in its stability [3]; close to 80% of EGCG degrades after 1 h incubation in simulated intestinal fluid pH [4] leaving only 0.1–1.1% of the orally administered catechins available in the systemic circulation in human [5]. TPP undergoes bio‐transformation such as methylation, glucuronidation, and sulphation in the small intestine, which also contributes in its low bioavailability at the target site [6]. Low oral bioavailability of TPP results in 5 to 50 time lower plasma concentration shown to produce biological activities in in vitro condition [7, 8]. Therefore, improving the bioavailability of TPP is crucial to its success as therapeutic agent or food supplement.
TPP loaded protein or polysaccharide‐based nanoparticles can be an alternative to using of free TPP and improve its bioavailability and half‐life. Gelatin could be an excellent material in this regard as it is biocompatible, biodegradable, has high accumulation at the tumour site, less antigenicity and prolonged in vivo circulation time [9]. Unique protein structure and accessible functional groups in gelatin increase opportunities for its use in targeted drug delivery [10]. Recently gelatin based drug delivery systems are developed for the treatment of bacterial vaginosis [11]. In addition, the surface of gelatin nanoparticles (GNP) is positively charged [12], therefore, amenable to a coating of polyelectrolyte over it. Coating of polyelectrolyte has also the advantage of high drug loading capacity by providing more sites for drug adsorption, controlling drug loading/release characteristics, providing a template for attaching tumour‐targeting agents and increasing colloidal stability [13]. Recently polyelectrolyte complex formation between oppositely charged polyelectrolyte with polymer‐bound (non) reactive polyelectrolyte was studied extensively in the presence of NaCl and CaCl2 as a function of ionic strength [14].
To our knowledge, there is only limited work done on surface modified GNP for delivery of TPP while no information available on the mechanism of TPP release and pharmacokinetics of TPP loaded GNPs (TPP‐GNPs). Shutava et al. [12] encased EGCG into gelatin‐based nanoparticles with surrounding layer‐by‐layer (LbL) coating of polyelectrolyte. They reported loading/release of EGCG from these nanoparticles and retention of biological activity of released EGCG under in vitro conditions. To further develop the TPP delivery system using GNP, we studied the mechanism of TPP release from GNP by mathematical modelling and pharmacokinetics of TPP in a rabbit model.
2 Material and methods
Gelatin A (Bloom number 300) and B (Bloom number 225), glutaraldehyde, polystyrene sulphonate (PSS), poly‐L‐glutamic acid (PGA), polyallylamine hydrochloride (PAH), poly‐L‐lysine (PLL), protamine sulphate (ProtS), dextran sulphate (DexS), DPPH (2,2‐diphenyl‐1‐picrylhydrazyl) and TPP were purchased from Sigma‐Aldrich Co., USA. Other chemicals were purchased from Hi‐Media, India.
2.1 Preparation of GNPs
GNPs were prepared by double desolvation method as described by Coester et al. [15] with minor modification. In brief, gelatin A/B (500 mg) was dissolved in water (10 ml) at 40°C and acetone (10 ml) was added. The supernatant was discarded, water (10 ml) was added at 40°C and pH was adjusted to 3. Finally, 40 ml of acetone and 100 µl of glutaraldehyde (25%) were added and stirred overnight. GNPs were separated by centrifugation (7000 rpm for 30 min at 10°C). The pellet was mixed with 1 ml acetone (75%) and centrifuged. Morphology, polydispersity, and size of GNPs were analysed by atomic force microscope (AFM), scanning electron microscope (SEM) and dynamic light scattering (DLS). The surface of GNPs was modified by LbL assembly of six polyelectrolyte layers over GNPs. Briefly, 3 mg of polyelectrolyte – PSS, PAH, PGA, PLL, sulphate (ProtS) and DexSwas sequentially added to 25 mg of GNPs, incubated for 30 min. After addition of one polyelectrolyte, nanoparticles were separated by centrifugation at 7000 rpm for 20 min at 10°C before adding the next polyelectrolyte (Fig. 1). The surface charge of LbL assembled GNPs was monitored by ζ potential analyser.
Fig. 1.
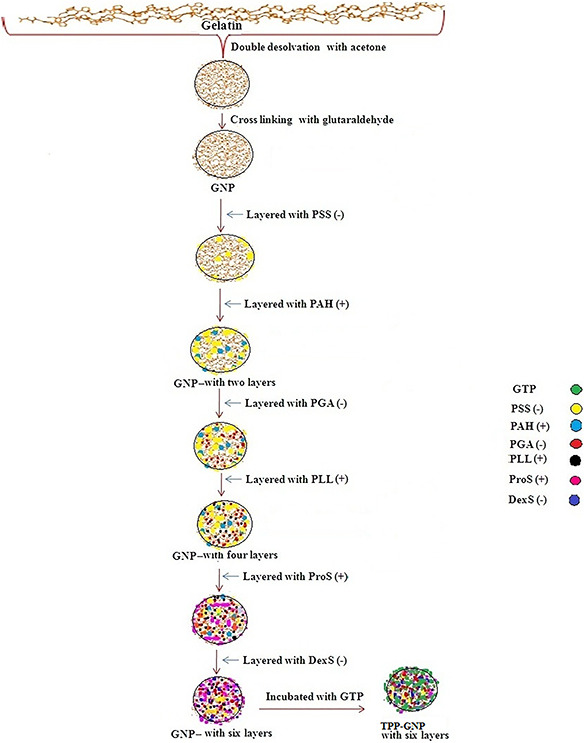
Schematic diagrams of the synthesis process of six polyelectrolyte layers modified GNPs and TPP‐GNPs
2.2 Encapsulation efficiency (EE) of GNPs
1 ml of TPP solution (containing 5 mg TPP) was added to 25 mg GNPs (without and with layers) and incubated for different times. TPP loaded GNPs (TPP‐GNPs) were separated by centrifugation at 7000 rpm for 20 min at 10°C and unloaded TPP was estimated from the supernatant by Folin–Ciocalteu assay of Swain and Hillis [16]. EE was calculated using the following formula:
2.3 In vitro release of TPP from TPP‐GNPs
TPP‐GNPs (25 mg) were suspended in 10 ml phosphate buffer saline (PBS, release medium) and incubated at room temperature under continuous shaking (100 rpm). Samples were collected at different time intervals and replenished with an equal volume of fresh release medium. TPP was estimated in the collected samples by Folin–Ciocalteu assay of Swain and Hillis [16] and release of TPP was calculated using the following formula:
2.4 In vitro drug release in simulated biological fluids
In vitro drug release was carried out in simulated saliva fluid (SSF, pH 6.5), gastric fluid (SGF, pH 1.2 containing pepsin), intestinal fluid in fasted condition (SIF, pH 6.5), intestinal fluid in fed condition (SIF, pH 5.0) and colon fluid (SCF, pH 7) according to Marques et al. [17]. TPP‐GNPs (25 mg) were suspended in 10 ml simulated biological fluid and incubated at room temperature under continuous shaking (100 rpm). Samples were collected at different time intervals and replenished with an equal volume of fresh release medium. TPP was estimated in the collected samples and release of TPP was calculated as described in the previous Section 2.3.
2.5 Mathematical modelling, release kinetics and mechanism
To study the mechanism of drug release, the in vitro release data was fitted to various mathematical models such as zero order, first order, Higuchi, Hixson–Crowell and Korsemeyer–Peppas models. The correlation coefficient (R 2) was used as an indicator for the best fit for each of the models while release exponent (n) was used to determine the mechanism of release [18].
2.6 Pharmacokinetics of TPP‐GNPs
Healthy New Zealand rabbits weighing ∼1.0 to 1.5 kg were selected for the study. The study protocol was approved by the institutional animal ethical committee for this experiment. Free TPP and TPP‐GNPs with six polyelectrolyte layers (TPP‐GNP‐6L) were orally administered as a single dose of 50 mg/kg body weight. Blood samples (0.5 ml each) were collected from the marginal ear vein using a butterfly infusion needle at time intervals of 0.5, 2, 4, 8 and 12 h after the drug administration. Plasma TPP concentration was determined by Folin–Ciocalteu assay of Swain and Hillis [16] and pharmacokinetics were determined. Pharmacokinetic parameters such as C max (peak plasma concentration), T max (time to peak plasma concentration), t 1/2 (half‐life), and AUC0‐∞ (area under plasma concentration‐time curve) were calculated from plasma concentration of TPP versus time data by open one compartment model. The mean residence time (MRT), corresponding to the drug transit time was calculated following Attia et al. [19].
2.7 Data analysis
The results were expressed as mean values with ±S.E. and means were separated by Duncan's MRT (DMRT) at P ≤ 0.05.
3 Results and discussion
3.1 Preparation of GNPs
The appearance of white colour after addition of acetone during second desolvation stage indicated GNP formation which was confirmed by SEM and AFM observations (Fig. 2). Spherical GNPs with a diameter of ∼50 nm was observed under SEM while AFM images were slightly ovoid shaped. The size of the nanoparticles markedly influences EE. EE decreased with increased size that might be due to decreased surface area [20]. All GNPs showed smooth surface. Polydispersity index (PDI) of GNPs was measured as 0.469 ± 0.05 by DLS. PDI of <0.5 confirmed narrow size distribution of particles. GNPs are safe with enhanced circulation time under in vivo condition [9]. The polyelectrolyte layering over GNPs was monitored by examining ζ potential of the GNPs. GNPs without polyelectrolyte layers showed positively charged surface which was sequentially changed to negative and positive due to adsorption of polyanions and polycations, respectively. At pH 7.4, the surface charge (ζ potential) of the unloaded GNPs with six layers was negative with surface charge of around −25 mV (Figs. 3 A and B). Since, GNPs without layers are positively charged [21], the absorption of Dex S results in change of surface charge into negative one that confirms the assembly of poly electrolytes. The surface charge of the TPP loaded GNPs with six polyelectrolyte layers was around −15 and −10 mV for TPP‐GNP‐A and TPP‐GNP‐B, respectively. There was slight shift in the surface charge of the nanoparticles after loading with TPP (Figs. 3 C and D) which might be due to lesser exposure of negatively charged surface due to TPP binding. Similar pattern of shift in surface charge of the GNPs was also reported by others [22, 23].
Fig. 2.
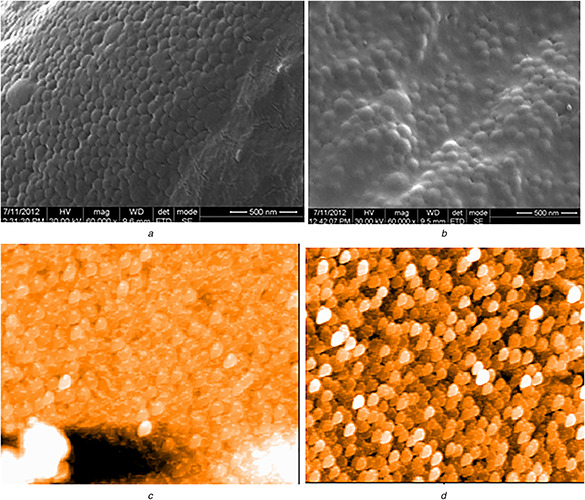
SEM (A, B) and AFM (C, D) images of GNPs prepared from gelatin A and B, respectively
Fig. 3.
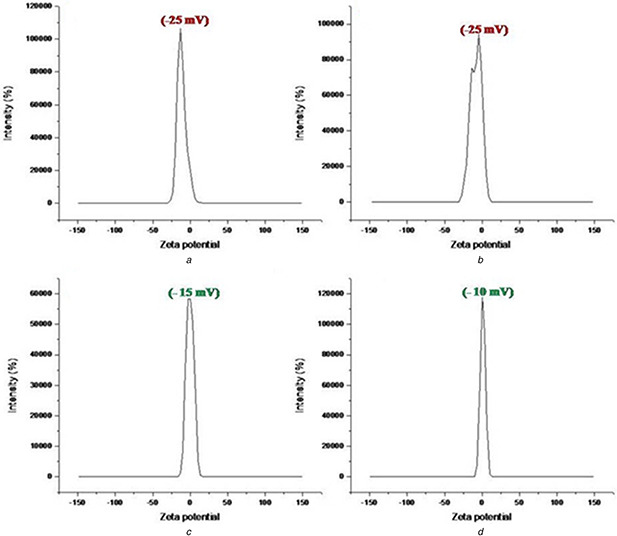
ζ potential of unloaded (A, B) and TPP loaded TPP‐GNP (C, D) prepared from gelatin A and B, respectively
3.2 EE of GNPs
The quantity of GNPs and polyelectrolyte showed a significant effect on EE. With the increase of polyelectrolyte quantity, there was an increase of EE. Maximum EE was obtained from the combination of 3 mg polyelectrolyte (PSS/PAH) with 25 mg GNPs (Fig. 4 A). The trend was same for GNPs prepared from gelatin A (TPP‐GNP‐A) and gelatin B (TPP‐GNP‐B). Number of polyelectrolyte layers (0, 2, 4 and 6) over GNPs as well as incubation time (0.5, 1, 1.5, 2 and 4 h) significantly influenced EE (Fig. 4 B). Highest EE (∼80%) was obtained in GNPs with six polyelectrolyte layers incubated for 4 h. Polyelectrolyte layers probably provided more binding sites for TPP. The highest EE was found at pH 4 and a further increase of pH resulted in decrease of EE (Fig. 4 C). This trend was similar for TPP‐GNP‐A and TPP‐GNP‐B although the former showed comparatively better EE than the latter (Fig. 4 B). Higher pH showed significantly lower EE. Among different factors influencing the stability of TPP, pH is the most crucial, the rate of oxidation of TPP increases with pH [24]. Therefore, decreased EE at higher pH may be due to oxidation or degradation of TPP. A recent experiment with remarkable protein fouling resistant and better antibacterial activity was achieved via LbL assembly on graphane oxide nanosheets [25]. Romanelli et al. [26] prepared bio‐composite scaffolds by utilising LbL assembly technique which has potential clinical applications in bone tissue regeneration.
Fig. 4.
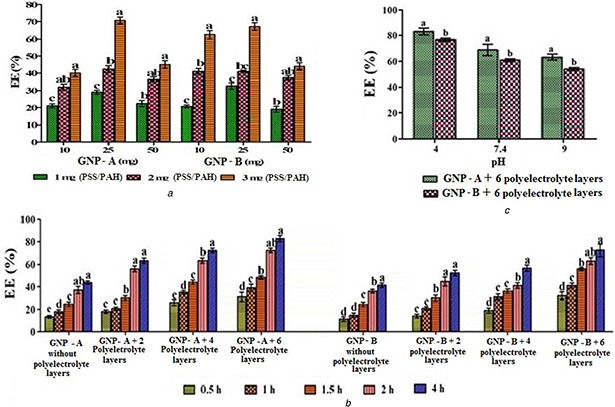
Influence of different factors on EE of GNPs. Different quantities of polyelectrolyte (PSS/PAH) used for GNP preparation and different quantities of GNPs (with two polyelectrolyte layers) incubated at pH 4 for four h (A), GNPs (25 mg) with different number of layers and incubation time at pH 4 (B) and GNPs (25 mg) with six polyelectrolyte layers incubated for four h at different pH (C). Means followed by the same latter in each GNP type are not significantly different at P ≤ 0. 05 according to DMRT
3.3 In vitro release of TPP from TPP‐GNPs
In a preliminary experiment, a slow and sustained release of TPP from TPP‐GNPs was obtained at pH 7.4. Therefore, further experiments on the release of TPP were conducted at pH 7.4. The number of polyelectrolyte layers over the GNPs showed a significant effect on the release of TPP at pH 7.4. The fastest rate of TPP release was recorded in TPP‐GNPs without polyelectrolyte layer; the rate of release was gradually and significantly slowed down with the increase in number of polyelectrolyte layers (Figs. 5 A and B). Burst release in the first hour (>20% release) was observed only in TPP‐GNPs without polyelectrolyte layer. This might be due to the release of TPP present over or just beneath the surface of nanoparticles. The absence of initial burst release in nanoparticles with polyelectrolyte layers might be due to the protection given by polyelectrolyte layers and thereby slowed the rate of release. Hydrophilicity of polymer influences the burst release. Gelatin is a hydrophilic polymer, therefore, TPP‐GNPs without polyelectrolyte layer facilitated water uptake from the release medium resulting in burst release. TPP‐GNPs with polyelectrolyte layers might have inhibited water uptake and thereby burst release. Polyelectrolyte layers have significantly influenced the rate of release of TPP from TPP‐GNPs. To achieve 99–100% release of TPP, TPP‐GNPs without polyelectrolyte layer took 24 h whereas TPP‐GNPs with six polyelectrolyte layers took 120–150 h which indicated the possibility of slow and sustained release by manipulating polyelectrolyte layers. Although the trend was same in TPP‐GNP‐A and TPP‐GNP‐B, the release was always faster in TPP‐GNPs prepared from gelatine A than that from gelatine B. Shutava et al. [12] have also reported a similar effect of polyelectrolyte layers but they compared between GNPs without and with two polyelectrolyte layers only.
Fig. 5.
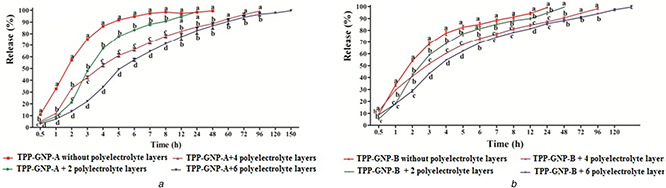
Influence of polyelectrolyte layers on release of TPP from TPP‐GNPs prepared from gelatin A (A) and B (B) with different polyelectrolyte layers and incubated in pH 7. 4. Data are mean ± S.E. Means followed by the same latter at each time interval are not significantly different at P ≤ 0.05 according to DMRT
3.4 Mathematical modelling, release kinetics and mechanism
TPP release data was fitted to mathematical models (Table 1). Among different types of TPP‐GNPs, first order kinetics was observed in the release of TPP from TPP‐GNPs without polyelectrolyte layers, which represented the concentration dependent release of TPP. The release of TPP from TPP‐GNP‐6L prepared from gelatin A was fitted into Korsmeyer–Peppas model whereas that from gelatin B was fitted into Higuchi model.
Table 1.
Influence of polyelectrolyte layers on TPP release kinetics and release mechanism
| Type of TPP‐GNP | Zero order | First order | Hixson–Crowel | Higuchi | Korsmayer–Peppas | Drug release mechanism | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R 2 | k 0 | R 2 | k 1 | R 2 | k HC | R 2 | k H | R 2 | N | ||
| TPP‐GNP‐A without polyelectrolyte layer | 0.904 | 1.05 | 0.991 | 2.86 | 0.904 | 3.88 | 0.957 | 27.58 | 0.948 | 0.52 | Anomalous |
| TPP‐GNP‐A with six polyelectrolyte layers | 0.987 | 3.5 | 0.98 | 2.51 | 0.989 | 3.58 | 0.971 | 29.36 | 0.991 | 1.17 | super case ii |
| TPP‐GNP‐B without polyelectrolyte layer | 0.856 | 0.97 | 0.984 | 3.12 | 0.935 | 2.05 | 0.937 | 28.98 | 0.951 | 0.46 | anomalous |
| TPP‐GNP‐B with six polyelectrolyte layers | 0.960 | 2.7 | 0.977 | 2.98 | 0.983 | 3.31 | 0.991 | 30.58 | 0.989 | 0.74 | anomalous |
TPP‐GNP‐A: TPP‐GNP prepared from gelatin A; TPP‐GNP‐B: TPP‐GNP prepared from gelatin B.
Mechanism of TPP release was determined based on ‘n’ value obtained from Korysmeyer–Peppas model (Table 1). The value of ‘n’ is limited based on its release model, where it is 0.43 for release from spheres, 0.45 for slabs and 0.5 for cylinders. In our study, 0.43 was considered as a limiting factor for ‘n’, as the release of TPP is from spherical particles. Generally n values <0.43 is considered as diffusion (Fickian release); n values ranging between 0.43 and 0.89 are considered as anomalous transport (diffusion with erosion) and n values >0.89 is regarded as super case II transport (erosion). Mechanism of TPP release observed for TPP‐GNPs without polyelectrolyte layers was likely to be anomalous transport, with a coupling of diffusion and erosion mechanism (n values of 0.46 and 0.85). For TPP‐GNP‐A with six polyelectrolyte layers, release mechanism was super case II transport (‘n’ values of 1.17.) whereas that from TPP‐GNP‐B was anomalous (‘n’ value between 0.46 and 0.74). Mathematical modelling indicated that polyelectrolyte layers have an influence on release kinetics. In our study, the predominant mode of TPP release was an anomalous type (diffusion was coupled with erosion) whereas, others have reported diffusion mode of release of drug from GNP [27, 28, 29, 30, 31]. This difference indicates that drug release mechanism not only depends on the type of polymer but also the type of drug.
3.5 In vitro TPP release in simulated biological fluids from TPP‐GNPs with six polyelectrolyte layers
Release of TPP from TPP‐GNPs with six polyelectrolyte layers was evaluated up to three minutes in SSF (pH 6.8) where release was negligible (<1% for fasted and <1.5% for fed state in both TPP‐GNP‐A and TPP‐GNP‐B) which was followed by 2 h incubation in SGF (pH 1.2) where the release of TPP in fasted and fed state were 9.14 and 15.12% from TPP‐GNP‐A and 17.38 and 28.38% from TPP‐GNP‐B, respectively, (Fig. 6). Shpigelman et al. [32] also reported practically no release of EGCG from β‐lactoglobulin nanoparticles for the first 90 min while the significant release was noted after 90 min in simulated gastric fluid. Contrary to our observation, burst release of TPP (over 50% in first 5–10 min) from chitosan‐pectin coated alginate microparticles in simulated gastric fluid (pH 1.2) was recently reported [33] which may be due to different materials used for formation of the particles affecting interaction with TPP. The release of TPP from TPP‐GNPs‐A and TPP‐GNP‐B in fasted and fed state was evaluated up to 8 h of incubation in SIF where a maximum release of TPP in this condition was 83% (fed state from TPP‐GNP‐A). When the TPP‐GNPs were transferred to SCF (pH 7) a maximum of 90% release of TPP (fed state from TPP‐GNP‐A) was noted. These data indicate retention of about 50% TPP in the TPP‐GNP up to 5 h in SIF and TPP‐GNP‐B performed better than TPP‐GNP‐A. Slow release of TPP in the acidic medium means more retention of TPP in TPP‐GNPs and thereby protection of TPP from degradation in the acidic environment. Khan et al. [34] also reported a slow release of EGCG from chitosan nanoparticles in the acidic medium even at 24 h while the release was much faster in intestinal fluid.
Fig. 6.
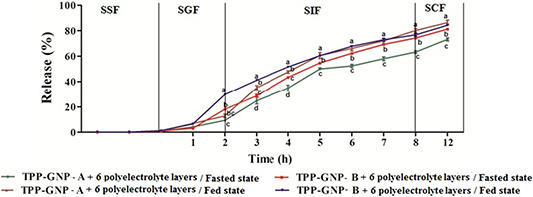
In vitro drug release profile of TPP‐GNP prepared from gelatin A (TPP‐GNP‐A) and TPP‐GNP prepared from gelatin B (TPP‐GNP‐B) in simulated biological fluids under fasted and fed conditions. Data are mean ± S.E. Means followed by the same latter at each time interval are not significantly different at P ≤ 0.05 according to DMRT
3.5 Pharmacokinetics of TPP‐GNPs with six polyelectrolyte layers
The oral administration showed positive influence on pharmacokinetic parameters due to encapsulation of TPP into GNPs (Table 2). The maximum TPP concentration (27.42 µg.ml−1) was obtained at 5.9 h by TPP‐GNP‐A whereas it took 4.6 h to reach maximum TPP concentration (24.07 µg.ml−1) by TPP‐GNP‐B. In the case of free TPP, maximum concentration (15.73 µg.ml−1) was obtained at 3.32 h. This almost two‐fold increase in maximum TPP concentration due to encapsulation of TPP into GNPs indicated protection of TPP from degradation while passing through GI tract. Moreover, the sustained release of TPP from TPP‐GNPs is evident as it took nearly ∼6 h to reach at maximum concentration compared with ∼3 h in case of free TPP. Slow elimination rate (K el) significantly prolonged the T 1/2a. TPP exposure over time (AUC0‐∞) was significantly higher in TPP‐GNP‐A (176.7 ml−1) and TPP‐GNP‐B (158.2 ml−1) than free TPP (108.2 ml−1). There was a substantial increase in MRT for TPP‐GNPs compared with free TPP. This higher MRT along with higher AUC0‐∞ indicates higher bioavailability of TPP when encapsulated into GNP. The rate of total clearance of TPP‐GNP‐A and TPP‐GNP‐B (0.4 and 0.59 ml.h−1 respectively) were slower than that of free TPP (1.12 ml.h−1). All these pharmacokinetics data indicate the efficacy of TPP‐GNP as a sustained release system for TPP. Encapsulation of TPP into GNPs has favoured slow and sustained release with improved pharmacokinetics and bioavailability. This will prolong the action of TPP.
Table 2.
In vivo pharmacokinetic parameters of free TPP and TPP‐GNPs after administration of a single oral dose of 50 mg/kg. Data represented at mean ± S.E. (n = 5)
| Parameters | Free TPP | TPP‐GNP‐A with 6 polyelectrolyte layers | Fold increase/decrease | TPP‐GNP‐B with 6 polyelectrolyte layers | Fold increase/decrease |
|---|---|---|---|---|---|
| C max, µg.ml−1 | 15.73 ± 1.2, c | 27.42 ± 0.8, a | +1.74 | 24.07 ± 1.25, b | +1.53 |
| T max, h | 3.32 ± 0.21, c | 5.9 ± 0.87, a | +1.77 | 4.6 ± 0.42, b | +1.38 |
| K a, h−1 | 0.44 ± 0.01, a | 0.40 ± 0.05, c | −0.90 | 0.421 ± 0.03, b | −0.95 |
| K el, h−1 | 0.17 ± 0.03, a | 0.05 ± 0.05, c | −0.29 | 0.07 ± 0.09, b | −0.41 |
| T 1/2 a | 4.17 ± 2.1, c | 13.8 ± 1.8, a | +3.3 | 8.7 ± 1.9, b | +2.08 |
| T 1/2 el | 1.3 ± 0.85, c | 1.7 ± 0.74, a | +1.3 | 1.5 ± 0.81, b | +1.15 |
| AUC0‐∞, µg.h.ml−1 | 108.2 ± 6.4, c | 176.7 ± 8.2, a | +1.63 | 158.2 ± 6.9, b | +1.46 |
| MRT, h | 8.35 ± 3.1, c | 22.5 ± 2.8, a | +2.69 | 16.36 ± 6.7, b | +1.95 |
| Vd, L | 7.44 ± 0.2, b | 8.06 ± 0.71, a | +1.08 | 6.66 ± 0.54, c | −0.89 |
| Cl, ml.h−1 | 1.12 ± 0.48, a | 0.4 ± 0.07, c | −0.35 | 0.59 ± 0.04, b | −0.53 |
Means followed by different letters in a row indicate a significant difference between the means at p ≤ 0.05 according to DMRT. Increase: ‘+’, decrease: ‘−’.
A significant difference in the pharmacokinetics of free TPP and TPP‐GNPs were observed. The area under the curve (AUC) for TPP‐GNPs was significantly increased compared with free TPP. Previous results by Saraogi et al. [35] showed a higher level of rifampicin in plasma of mice when loaded in gelatin nanocarriers. Vichasilp et al. [36] reported, the AUC of rats with mulberry 1‐deoxynojirimycin (DNJ) loaded in gelatin microsphere was significantly increased, compared with animal dosed with free DNJ. In another study, the pharmacokinetics of cryptolepine hydrochloride (CHN) loaded GNP showed significantly longer plasma residence time than the plain drug [37]. Narayanan et al. [38] reported the bioavailability of ibuprofen‐sodium (IbS) as around 4 fold higher for PEGylated GNP than the free IbS. Recently, the half‐life (T 1/2) and AUC of the EGFR2R‐lytic hybrid peptide were enhanced by gelatin hydrogel nanoparticles [39].
4 Conclusion
We have synthesised spherical GNPs with high loading and sustained release of TPP. Our data have established the role of polyelectrolyte layers on EE, release and release kinetics of TPP. Control of loading/release was possible by changing the number of polyelectrolyte layers. Although the predominant mode of drug release was anomalous type, TPP‐GNP‐A with six polyelectrolyte layers has showed super case II type. Comparative pharmacokinetics analysis of free TPP and TPP‐GNPs in rabbit model has proved TPP‐GNPs as a better system providing sustained release and higher plasma concentration of TPP. Thus, encapsulation of TPP into GNP has resulted in a controlled drug release, with better drug concentration in plasma, thereby improving the bioavailability. Hence, TPP‐GNPs with six polyelectrolyte layers may be utilised as a potential tool for the delivery of TPP with improved therapeutic efficacy. This result will pave the way for further development of TPP as a therapeutic agent.
5 Acknowledgments
The authors are thankful to the management of VIT for providing facilities. Financial support from NTRF, Tea Board, Kolkata is also gratefully acknowledged.
6 References
- 1. Zaveri N.T.: ‘Green tea and its polyphenolic catechins: medicinal uses in cancer and non‐cancer applications’, Life Sci., 2006, 78, pp. 2073 –2080 [DOI] [PubMed] [Google Scholar]
- 2. Zhu Q.Y. Zhang A. Tsang D. et al.: ‘Stability of green tea catechins’, J. Agric. Food Chem., 1997, 45, pp. 4624 –4628 [Google Scholar]
- 3. Mochizuki M. Yamazaki S. Kano K. et al.: ‘Kinetic analysis and mechanistic aspects of autoxidation of catechins’, Biochim. Biophys. Acta, 2002, 1569, pp. 35 –44 [DOI] [PubMed] [Google Scholar]
- 4. Dube A. Ng K. Nicolazzo J.A. et al.: ‘Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution’, Food Chem., 2010, 122, pp. 662 –667 [Google Scholar]
- 5. Zhang L. Zheng Y. Chow M.S. et al.: ‘Investigation of intestinal absorption and disposition of green tea catechins by caco‐2 monolayer model’, Int. J. Pharm., 2004, 287, pp. 1 –12 [DOI] [PubMed] [Google Scholar]
- 6. Chow H.H.S. Cai Y. Alberts D.S. et al.: ‘Phase I pharmacokinetic study of tea polyphenols following single‐dose administration of epigallocatechin gallate and polyphenon E’, Cancer Epidem. Biomar., 2001, 10, pp. 53 –58 [PubMed] [Google Scholar]
- 7. Jung Y.D. Kim M.S. Shin B.A. et al.: ‘EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells’, Brit. J. Cancer, 2001, 84, pp. 844 –850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chow H.H.S. Hakim I.A. Vining D.R. et al.: ‘Effects of dosing condition on the oral bioavailability of Green Tea catechins after single‐dose administration of polyphenon E in healthy individuals’, Clin. Cancer Res., 2005, 11, pp. 4627 –4633 [DOI] [PubMed] [Google Scholar]
- 9. Kommareddy S. Amiji M.: ‘Biodistribution and pharmacokinetic analysis of long‐circulating thiolated gelatin nanoparticles following systemic administration in breast cancer‐bearing mice’, J. Pharma. Sci‐US, 2007, 96, pp. 397 –407 [DOI] [PubMed] [Google Scholar]
- 10. Busch S. Schwarz U. Kniep R.: ‘Chemical and structural investigations of biomimetically grown fluorapatite gelatin composite aggregates’, Adv. Funct. Mater., 2003, 13, pp. 189 –198 [Google Scholar]
- 11. Khade S.M. Behera B. Sagiri S.S. et al.: ‘Gelatin‐PEG based metronidazole‐loaded vaginal delivery systems: preparation, characterization and in vitro antimicrobial efficiency’, Iran. Polym. J., 2014, 23, pp. 171 –184 [Google Scholar]
- 12. Shutava T.G. Shantanu S. Balkundi Vangala P. et al.: ‘Layer‐by‐Layer‐coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols’, ACS Nano, 2009, 3, pp. 1877 –1885 [DOI] [PubMed] [Google Scholar]
- 13. Elzoghby A.O.: ‘Gelatin‐based nanoparticles as drug and gene delivery systems: Reviewing three decades of research’, J. Control Release, 2013, 172, pp. 1075 –1091 [DOI] [PubMed] [Google Scholar]
- 14. Mazumder M.A.J.: ‘Polyelectrolyte complexation between cationic and anionic polyelectrolytes with complementary polymer‐bound reactive groups of amine and acetoacetate: effect of mono‐and divalent salts’, Iran. Polym. J., 2014, 23, pp. 445 –455 [Google Scholar]
- 15. Coester C.J. Langer K. Van Briesen H.: ‘Gelatin nanoparticles by two‐step desolvation‐a new preparation method, surface modifications and cell uptake’, J. Microencapsul., 2000, 17, pp. 187 –193 [DOI] [PubMed] [Google Scholar]
- 16. Swain T. Hillis W.E.: ‘The phenolic constituents of Prunus domestica ‘, J. Sci. Food. Agric., 1959, 10, pp. 63 –68 [Google Scholar]
- 17. Marques M.R.C. Loebenberg R. Almukainzi M.: ‘Simulated biological fluids with possible application in dissolution testing’, Dissolut. Technol., 2011, 18, pp. 15 –28 [Google Scholar]
- 18. Korsmeyer R.W. Gurny R. Doelker E. et al.: ‘Mechanism of solute release from porous hydrophilic polymers’, Int. J. Pharmaceut., 1983, 15, pp. 23 –25 [Google Scholar]
- 19. Attia I.A. El‐Gizawy S.A. Fouda M.A. et al.: ‘Influence of a liposomal formulation on the oral bioavailability of acyclovir in rabbits’, AAPS Pharm. Sci. Tech., 2007, 8, pp. 206 –212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nahar M. Mishra D. Dubey V. et al.: ‘Development, characterization, and toxicity evaluation of amphotericin B‐loaded gelatin nanoparticles’, Nanomed. Nanotechnol., 2008, 4, pp. 252 –261 [DOI] [PubMed] [Google Scholar]
- 21. Shutava T.G. Shantanu S. Balkundi V.P. et al.: ‘Layer‐by‐layer‐coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols’, ACS Nano., 2009, 3, pp. 1877 –1885 [DOI] [PubMed] [Google Scholar]
- 22. Jain S.K. Gupta Y. Jain A. et al.: ‘Mannosylated gelatin nanoparticles bearing an anti‐HIV drug didanosine for site specific delivery’, Nanomed: Nanotech. Biol. Med., 2008, 4, pp. 41 –48 [DOI] [PubMed] [Google Scholar]
- 23. Ofokansi K. Winter G. Fricker G. et al.: ‘Matrix loaded biodegradable gelatin nanoparticles as new approach to improve drug loading and delivery’, Euro. J. Pharm. Biopharm., 2010, 76, pp. 1 –9 [DOI] [PubMed] [Google Scholar]
- 24. Su Y.L. Leung L.K. Huang Y. et al.: ‘Stability of tea theaflavins and catechins’, Food Chem., 2003, 83, pp. 189 –195 [Google Scholar]
- 25. Zhang Z.B. Wu J.J. Su Y. et al.: ‘Layer‐by‐layer assembly of grapheme oxide on polypropylene macroporous membranes via click chemistry to improve antibacterial and antifouling performance’, Appl. Surf. Sci., 2015, 332, pp. 300 –307 [Google Scholar]
- 26. Romanelli S.M. Fath K.R. Phekoo A.P. et al.: ‘Layer‐by‐layer assembly of peptide based bioorganic‐inorganic hybrid scaffolds and their interactions with osteoblastic MC3T3‐E1 cells’, Mater. Sci. Eng., C., 2015, 51, pp. 316 –328 [DOI] [PubMed] [Google Scholar]
- 27. Saravanan M. Bhaskar K. Maharajan G.G. et al.: ‘Ultrasonically controlled release and targeted delivery of diclofenac sodium via gelatin magnetic microspheres’, Int. J. Pharm. Pharm. Sci., 2004, 283, pp. 71 –82 [DOI] [PubMed] [Google Scholar]
- 28. Bajpai A.K. Choubey J.: ‘Design of gelatin nanoparticles as swelling controlled delivery system for chloroquine phosphate’, J. Mater. Sci. Mater. M., 2006, 17, pp. 345 –358 [DOI] [PubMed] [Google Scholar]
- 29. Chen Y.C. Yu S.H. Tsai G.J. et al.: ‘Novel technology for the preparation of self assembled catechins/gelatin nanoparticles and their characterization’, J. Agric. Food Chem., 2010, 58, pp. 6728 –6734 [DOI] [PubMed] [Google Scholar]
- 30. Thakur G. Mitra A. Basak A. et al.: ‘Characterization and scanning electron microscopic investigation of cross linked freeze dried gelatin matrices for study of drug diffusivity and release kinetics’, Micron., 2012, 43, pp. 311 –320 [DOI] [PubMed] [Google Scholar]
- 31. Zhou H. Sun X. Zhang L. et al.: ‘Fabrication of biopolymeric complex coacervation core micelles for efficient tea polyphenols delivery via green tea process’, Langmuir, 2012, 28, pp. 14553 –14561 [DOI] [PubMed] [Google Scholar]
- 32. Shpigelman A. Cohen Y. Livney Y.D.: ‘Thermally‐induced β‐lactoglobuline EGCG nanovehicles: loading, stability, sensory and digestive‐release study’, Food Hydrocolloids, 2012, 29, pp. 57 –67 [Google Scholar]
- 33. Belscak‐Cvitanovic A. Dordevic V. Karlovic S. et al.: ‘Protein‐reinforced and chitosan‐pectin coated alginate microparticles for delivery of flavan‐3‐ol antioxidants and caffeine from green tea extract’, Food Hydrocolloids, 2015, 51, pp. 361 –374 [Google Scholar]
- 34. Khan N. Bharali D.J. Adhami V.M. et al.: ‘Oral administration of naturally occurring chitosan‐based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model’, Carcinogenesis, 2014, 35, pp. 415 –423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saraogi G.K. Gupta P. Gupta U.D. et al.: ‘Gelatin nanocarriers as potential vectors for effective management of tuberculosis’, Int. J. Pharm. Pharm. Sci., 2010, 385, pp. 143 –149 [DOI] [PubMed] [Google Scholar]
- 36. Vichasilp C. Nakagawa K. Sookwong P. et al.: ‘A novel gelatin crosslinking method retards release of mulberry 1‐deoxynojirimycin providing a prolonged hypoglycaemic effect’, Food Chem., 2012, 134, pp. 1823 –1830 [DOI] [PubMed] [Google Scholar]
- 37. Kuntworbe N. Ofori M. Addo P. et al.: ‘Pharmacokinetics and in vivo chemosuppressive activity studies on cryptolepine hydrochloride and cryptolepine hydrochloride‐loaded gelatin nanoformulation designed for parental administration for the treatment of malaria’, Acta Trop., 2013, 127, pp. 165 –173 [DOI] [PubMed] [Google Scholar]
- 38. Narayanan D. Geena M.G. Lakshmi H. et al.: ‘Poly‐(ethylene glycol) modified gelatin nanoparticles for sustained delivery of the anti‐inflammatory drug Ibuprofen—Sodium: an in vitro and in vivo analysis’, Nanomed. Nanotechnol., 2013, 9, pp. 818 –828 [DOI] [PubMed] [Google Scholar]
- 39. Gaowa A. Horibe T. Kohno M. et al.: ‘Combination of hybrid peptide with biodegradable gelatin hydrogel for controlled release and enhancement of anti‐tumor activity in vivo ‘, J. Control Release, 2014, 176, pp. 1 –7 [DOI] [PubMed] [Google Scholar]


