Abstract
The biosynthesis of silver nanoparticles (AgNPs) is substantial for its application in lots of fields. Tomato and grape fruit juices were used as a reducing and capping agents for the biosynthesis of AgNPs. Ultraviolet spectroscopic analysis offered peaks in the range of 396‒420 nm that indicate the production of AgNPs. Fourier transform infrared spectroscopy analysis revealed attachment of different functional groups with Ag ion in both tomato and grape fruit extracts NPs. The X‒ray diffraction analysis confirmed that the synthesised AgNPs have a face centred cubic confirmation. Scanning electron microscopy confirms the size of NPs that varies from 10 to 30 nm. The DPPH free radical scavenging assay, total antioxidant capacity, reducing power assay, total flavonoid contents and total phenolic contents determination confirmed that synthesised AgNPs are potent antioxidant agents; can be used as an effective scavenger of free radicals. Biosynthesised AgNPs also showed good antibacterial activity against Pseudomonas septica, Staphylococcus aureus, Micrococcus luteus, Enterobacter aerogenes, Bacillus subtilis and Salmonella typhi. Protein kinase inhibition activity showed a clear zone which indicates anticancerous potential of biosynthesised AgNPs. The efficacious bioactivities indicate that the tomato and grape derived AgNPs can be used efficiently in pharmaceutical and medical industries.
Inspec keywords: silver, nanoparticles, nanomedicine, biomedical materials, nanofabrication, Fourier transform infrared spectra, X‐ray diffraction, scanning electron microscopy, microorganisms, antibacterial activity, enzymes, cancer, ultraviolet spectra
Other keywords: silver nanoparticle green synthesis, grape juice, tomato juice, biological activity evaluation, ultraviolet spectroscopic analysis, silver nanoparticle production, Fourier transform infrared spectroscopy analysis, silver ion, X‐ray diffraction analysis, scanning electron microscopy, DPPH free radical scavenging assay, antioxidant capacity, flavonoid content, phenolic content determination, antioxidant agent, antibacterial activity, Pseudomonas septica, Staphylococcus aureus, Micrococcus luteus, Enterobacter aerogenes, Bacillus subtilis, Salmonella typhi, protein kinase, size 10 nm to 30 nm
1 Introduction
Nanotechnology is an inspiration and invention of modern fundamental science. Nanoparticles (NPs) are crystalline or amorphous with at least single dimension smaller than 1 µm. Many physical and chemical methods to produce NPs have been developed such as (i) co‐precipitation; (ii) sol‐gel processing; (iii) micro emulsions; (iv) hydrothermal/solvothermal; (iv) microwave synthesis; (v) sonochemical synthesis; (vi) inert gas condensation; (vii) pulsed laser ablation; (viii) spark discharge generation; (ix) chemical vapour synthesis; (x) spray pyrolysis; (xi) laser pyrolysis/photochemical synthesis; (xii) thermal plasma synthesis; and (xiii) flame spray pyrolysis. However, the problems with the chemical and physical methods are that the synthesis is expensive and can have toxic substances absorbed onto them [1]. There is a growing need to develop environmentally friendly processes of NPs synthesis that do not use toxic chemicals because these are or can be widely applied to human contacting areas [2]. For this reason, biological method provides a feasible alternative for the eco‐friendliness; cost effectiveness; easy to scale up process; and most important compatibility for pharmaceutical and biomedical applications as they do not use harsh, toxic and expensive chemicals/solvents, high pressure, energy and temperature in the synthesis protocols. All forms of life such as bacteria, fungi and plant can be employed for the biosynthesis of NPs [1]. Beside other characteristics of NP production protocols, phytochemicals present in biological system are also directly involved in the reduction of the ions and formation of NPs [3].
Among metallic NPs silver NPs (AgNPs) capped with biomolecules are new hope as multi factional nanoweapon can be used for the treatment and prevention of drug‐resistant microbes [4]. Electron spin resonance spectroscopy studies have revealed formation of free radicals by the AgNPs when in contact with bacteria, and these free radicals have the ability to damage the cell membrane and make it porous which can ultimately lead to cell death [5]. It has also been proposed that there can be release of Ag ions by the NPs [6] and these ions can interact with the thiol groups of many vital enzymes and inactivate them [7]. Other parameters of synthesised NPs such as antimycelial, antioxidant, anticancerous and so on have been reported and proven to be more effective during in vitro studies as compared with whole plant extract.
Tomato (Lycopersicon esculentum Mill.) ‘the most popular vegetable fruit’ contains vitamins, i.e. A, B and C; beta‐carotene; phytosterols, and so on. While, grape (Vitis vinifera), one of the most widely consumed fruit worldwide; contains many bioactive constituents including flavonoids, polyphenols, anthocyanins and stibene derivatives resveratrol. The components present in grape and tomato juices have been reported for anticarcinogenic, antimutagenic, antiangiogenics, antioxidant, antibacterial and antiviral properties and also have broad‐range of applications in pharmaceuticals, medical, cosmetic and food industry. Keeping in view the pharmacological properties of tomato and grapes; we investigated antibacterial; antioxidant and reducing power potential of AgNPs capped with biocomponents present in juices.
2 Materials and methods
2.1 Preparation of fruit extracts
Fresh grapes and tomato were purchased from the local market (Abpara, Islamabad, Pakistan). The fruits were washed thoroughly under running tap water followed by rinsing thrice with double distilled water. Fruits were separately squeezed and extracts were strained initially through a muslin cloth then passed through Wattmann filter paper No. 1. Finally the extracts were vacuum filtered through 0.45 μm filters (Millipore). The extracts were stored at 4°C till further experimental work.
2.2 Biosynthesis and characterisation of AgNPs
The AgNPs were synthesised by reacting aqueous solution of Ag nitrate (AgNO3) and fruit extract. The reaction mixture was kept in dark at room temperature to minimise photoactivation of AgNO3. The reduction of Ag ion to Ag was initially confirmed by the colour change. The reduced solution was centrifuged at 10,000 rpm for 5 min. The supernatant liquid was discarded and the pellet obtained was washed three times with distilled water to remove any undesired impurities. The purified dried AgNPs were used for the subsequent characterisation studies.
To determine the effect of Ag+ ion concentration on synthesis of NPs, the fruit juices were treated with different concentrations of AgNO3 (1, 5, 10, 20, 30, 40 and 50 mM) keeping the total volume constant in all the reaction vials. While to analyse the effect of temperature, initially the temperature of juices was raised upto 20, 40, 60, 80 and 100°C. Thereafter; 1 M AgNO3 was added to acquire AgNO3 concentration 20 and 30 mM. The tubes were observed till the colour change and the reaction were stopped by adding 1 ml acetone to each test tube and placed in ice cold water. To investigate pH effect, the juices were taken in small vials and its initial pH 4.2 was recorded for tomato juice and 4.0 for grape juice. pH of fruit juices was adjusted at 3, 5, 7 and 9 by using 0.1N HNO3 and 0.1N NaOH. The stock solution of AgNO3 (1 M) was used to make the concentration 20 mM and mixed with the fruit juices to start the reduction reaction.
The ultraviolet visible (UV–vis) spectrophotometric readings were recorded at a scanning speed of 200 to 800 nm with a resolution of 1 nm at a scan speed of 300 nm/min. The samples were diluted if required and dilution factor was noted. The recorded spectra were then replotted using Microsoft excel. Fourier transform infrared spectroscopy (FTIR) shows the presence of bio‐organic components which acted as a probable stabiliser for the synthesised AgNPs. After the synthesis of the AgNPs the biomolecules associated with it was identified by FTIR measurements. Dry powders of the AgNPs were obtained in the above mentioned manner. A carefully weighed quantity of the synthesised AgNP of dried powder was subjected to FTIR analysis (Schimadzu Fourier transform infrared spectrophotometer, model 270). Samples were exposed to an infrared source having the spectrum scanned in the range of 500–4000 cm−1 in order to obtain good signal/noise ratio. The various modes of vibrations were identified and assigned to determine the different functional groups present on the AgNPs.
The X‐ray diffraction patterns of AgNP were recorded according to the description of Wang [8]. The dried AgNPs were subjected for X‐ray diffraction patterns using X‐ray diffractometer (XRD) (X'pert PRO of PANalytical company). The XRD pattern measurements of drop‐coated film of AgNPs on glass substrate were recorded in a wide range of Bragg angles θ at a scanning rate of 2 θ angles/min. The generator was operated at a voltage of 40 kV and a current of 30 mA at room temperature. The scanning range was selected between 10° and 100° using nickel monochromatic Cu Kα radiation (θ = 1.5406 Å), NaI detector, variable slits and a 0.050 step size. The result of powder‐XRD was compared with standard JCPDS database values. The mean diameter of the AgNPs was calculated by the width of the XRD peaks, i.e. the data of full width at half‐maximum (FWHM) assuming that they are free from non‐uniform strains, using the Scherrer formula. D = k λ /β Cos θ where D is the average crystalline domain size perpendicular to the reflecting planes, K is the Scherrer coefficient (0.85), λ is the X‐ray wavelength (1.5406 Å), β is the angular FWHM in radians and θ is the diffraction angle or Bragg's angle.
2.3 Scanning electron microscopy (SEM) analysis of synthesised NPs
The size of synthesised grapes and tomato AgNPs were studied by JOEL‐JSM‐6490LA™ SEM operating at 20 kV with counting rate of 2838 cps.
2.4 Biological activities of biosynthesised AgNPs
The AgNPs synthesised by 20 and 30 mM AgNO3 of both tomato and grape fruit extracts were evaluated for biological activities. For tomato these are termed as T20 and T30 while for grapes these are termed as G20 and G30. To study the biological activities of biosynthesised NPs, 5 mg NPs were sampled in 1 ml dimethyl sulphoxide (DMSO) in an Eppendroff tube. The suspension was initially sonicated in water bath for about 30 min and this process was repeated each time when used. All the assays were performed in triplicate and results are presented as mean with standard deviation.
2.5 Antibacterial assay of AgNPs
The antibacterial activity of the synthesised AgNPs was determined against gram‐positive (Staphylococcus aureus, Micrococcus luteus and Bacillus subtilis) and gram‐negative (Enterobacter aerogenes, Pseudomonas septica and Salmonella typhi) bacterial strains. The in vitro sensitivity test was carried out by using the agar well diffusion assay method as described by Bibi et al. [9].
2.6 In vitro antioxidant assays of AgNPs
Total flavonoid contents were determined following the method of Rehman et al. [10] with some modifications. A volume of 20 μl of the sample was mixed with 10 μl of 1M potassium acetate buffer and 10 μl of 10% aluminium chloride in 160 μl of distilled water and incubated for 30 min at room temperature. After 30 min, absorbance was recorded at 405 nm wavelength on microplate reader. The total flavonoid content was quantified from a standard quercetin curve.
The total phenolic contents were determined by the Folin–Ciocalteu method [11] with little modifications. A 20 μl of each sample was mixed with 90 μl diluted stock solution of Folin–Ciocalteu reagent and incubate for 5 min at room temperature followed by the addition of 90 μl of 6% sodium carbonate (Na2 CO3) solution. Absorbance was recorded at 630 nm wavelength on using UV–vis spectrophotometer. The concentration of total phenolic content in the samples was determined as μg of gallic acid equivalent (GAE) by calibration curve.
The DPPH free radical scavenging assay was carried out by the method of Humaira et al. [12] with some alteration. A volume of 20 μl of each sample was mixed with 180 μl of DPPH reagent (3.2 mg of DPPH reagent/100 ml DMSO) followed by incubation for 1 h in the dark. The reduction of the DPPH radical was determined by recording the absorbance at 517 nm using UV–vis spectrophotometer. DPPH free radical‐scavenging activity was calculated by the following equation:
The antioxidant activity of the NPs was evaluated by phosphomolybdenum method according to the procedure of Rehman et al. [10] where
Ascorbic acid at 4 mg/ml was used as standard.
The reducing power assay was carried out by the method of Rehman et al. [10]. A 200 μl of sample was mixed with 500 μl of 0.2M phosphate buffer (pH 6.6) and 500 μl of 1% potassium ferricyanide and the mixture was incubated at 500°C for 20 min. Afterwards, 500 μl of 10% trichloroacetic acid was added and centrifuged at 3000 rpm for 10 min. Finally, 500 μl of the supernatant wax was mixed with 100 μl of 0.1% ferric chloride. The absorbance was measured at 630 nm wavelength using UV‐vis spectrophotometer. The intensity of reducing power is directly proportional to the absorbance of the reaction mixture. Ascorbic acid at 4 mg/ml was used as standard.
2.7 Protein kinase inhibition assay
Streptomyces (largest genus of Actinobacteria) was maintained on ISP4 medium (Difco Laboratories) for the preparation of spores and liquid tryptic soy broth (TSB) medium (Sigma‐Aldrich) was used for the propagation of mycelium. Streptomyces culture was refreshed on TSB medium in a shaker incubator at 28°C. An aliquot of 60 μl of refreshed culture was taken in an Eppendorf tube and mixed with 540 μl of sterile TSB media. The ISP‐4 medium was autoclaved and ∼ 20‒25 ml was poured into sterile petri plates and allowed to solidify. Sterile cotton swabs were used to culture inoculum homogeneously over the entire surface of the petri plates softly. The assay procedure was followed as described for antibacterial assay.
2.8 Statistical analysis
All the biological assays were performed in triplicate. The results are presented as mean with standard deviation. The mean values were further analysed for analysis of variance and least significant difference (LSD) at probability level p < 0.05 using MSTATC.
3 Results and discussion
3.1 Synthesis and characterisation of NPs
Synthesis, characterisation and application of AgNPs by reducing AgNO3 solution using fruit extract of grapes (V. vinifera) and tomato (L. esculentum) fruit extracts have been accomplished through green chemistry. The colour of the reaction mixture changed from yellowish brown to dark brown (Fig. 1) when the extract was exposed to an aqueous solution of Ag+ ions (AgNO3). The change in appearance seemed due to excitation of surface plasmon vibrations/resonance in the AgNPs. Depicted on the concentration of Ag ion revealed attachment of biomolecules forming the NPs and finally change in colour. It has been reported that AgNPs make the solution dark reddish to dark brown in colour [13, 14].
Fig. 1.

Colour changes of grape and tomato juices on treatment with different concentrations of AgNO3 , (a) Grapes, (b) Tomato.
From left to right AgNO3 concentration varies as 0, 1, 5, 10, 20, 30, 40 and 50 mM
The UV–vis spectral data of synthesised grape juice AgNPs shows that at low concentration of AgNO3 (1 to 20 mM) bands appeared in the range of 394–396 nm. While at 30, 40 and 50 mM the spectra were at 402, 413 and 419 nm, respectively (Fig. 2 A). The absorbance bands in the case of tomato fruit extracts the UV–vis spectra for 1, 5 and 10 mM AgNO3 appeared at 396 nm. While the absorbance peak for 20, 30, 40 and 50 mM AgNO3 concentration was observed at 402, 418, 419 and 420 nm, respectively (Fig. 2 B). Higher concentration of AgNO3 provided darker colour and synthesis of NPs was faster. In both cases, grapes and tomato; border peaks were observed when juices were treated with higher concentrations of AgNO3. It has been documented that the NPs formation increase while increasing the concentration of AgNO3 and metabolites present in the extracts play vital role in synthesis of NPs [15]. Roy et al. [14] also reported that the high OD of the solution suggests a high conversion of Ag+ to Ag0 as NP.
Fig. 2.
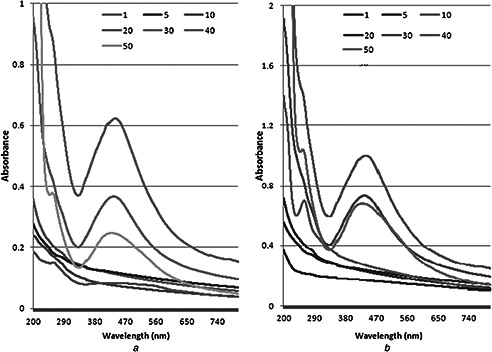
Effect of various AgNo3 concentrations on AgPNs synthesis by
(a) Grape fruit extract, (b) Tomato fruit extract
Increase in H+ ion concentration in the reaction mixture decreased the production of NPs. Colour formation was rapid at alkaline pH but agglomeration was observed. At neutral pH, the reaction started immediately as the AgNO3 was mixed with fruit extracts. The change in colour of reaction mixture was also observed at acidic pH, however, the intensity was low and visually the particles looked dispersed as compared with that produced at alkaline pH. Many reports describe that alkaline condition favour synthesis of AgNPs produced through green chemistry [16, 17]. However, Krishnaraj et al. [18] reported that there is no particle formation at acidic pH.
The change in colour varied depending upon heat provided. At 20°C no peak was observed in visual range. When the temperature rose up to 40°C, the band shifted to 396 nm which show the synthesis of NP. Further increase in temperature did not shift the band and the intensity remained constant at 396 nm conforming stable synthesis of NPs in both grape and tomato juices (Fig. 3). It was further observed that as the temperature of reaction mixture increase, the colour changes from lighter to denser. Such findings have previously been reported in [16, 17, 19]. The size might also vary due to the reduction in aggregation of the growing NPs and even the peak intensity enhances in response to time and temperature. It has been reported that on increase in temperature, the sharpness of peaks indicate synthesis of smaller NPs [17, 20]. Fayaz et al. [21] reported that band shift is due to the localisation of the surface plasmon resonance of the AgNPs and plasmon bands broaden with an absorption tail in the longer wavelength due to the size distribution of the particles [19].
Fig. 3.
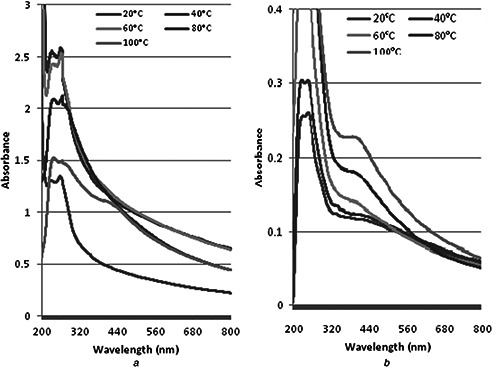
Effect of temperature on AgPNs synthesis of
(a) Grape fruit extract, (b) Tomato fruit extract
FTIR was used for determination of functional group associated with the synthesised AgNPs. The present study shows peak curves (Fig. 4) of the biosynthesised AgNPs from grape and tomato extract resulted a strong bands at 3255.1, 3270.3 and 3270.4 cm−1 (corresponds to O–H stretching of carboxylic acids); 2844.7, 2922.2, 2926, 2928.7 and 3119 cm−1 (corresponds to the C–H stretching of alkane, aldehyde and alkene or aromatic [22, 23]); 1733.6, 1736.0 and 1742.0 cm−1 (corresponds to the C=O stretching of ester or saturated aldehyde); 1539.5, 1540.3, 1558.4, 1575.8, 1584.2 and 1626.5 cm−1 (corresponds to C=C stretching of alkene), 1281.5, 1326.2, 1327.3, 1330, 1373.8, 1381.1, 1385.1 and 1397.3 cm−1 (corresponds to the C‒C stretching of alkanes), 1020, 1058, 1059.4 and 1063.8 cm−1 (corresponds to O–prim‒C stretching; [24, 25]). The biological molecules perform the dual functions of formation and stabilisation of AgNPs in the aqueous medium [26]. The synthesised NPs might be surrounded by amino acids, proteins and other essential metabolites present in the extract [3].
Fig. 4.
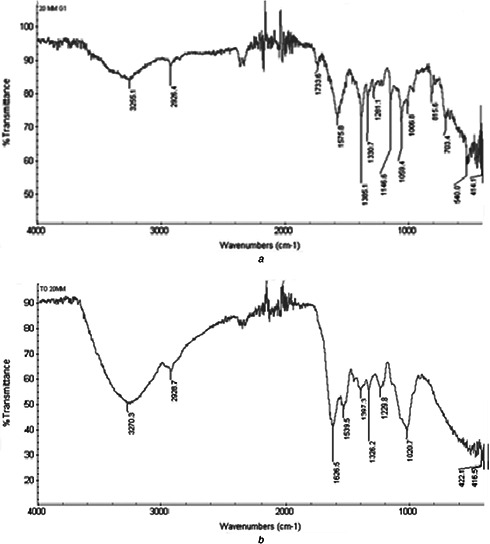
FTIR spectra overlay of NPs synthesised at 20 mM AgNO3 by
(a) Grape fruit extract, (b) Tomato fruit extract
Resultant analysis of AgNPs by XRD revealed four peaks of grapes NPs synthesised at 20 mM (27.59°, 32.16°, 35.21° and 46.19°) (Fig. 5 A) and four peaks of NPs synthesised at 30 mM. In case of tomato, NPs synthesised at 20 mM; three peaks (Fig. 5 B) were observed in the range of 27°–46°, i.e. at 27.94°, 32.48° and 46.44° and four peaks of NPs synthesised by 30 mM AgNO3. It was observed that NPs synthesised at both AgNO3 concentrations, the peak FWHM, area and length varied. Comparison with online JCPDS database indicated that the samples have a face centred cubic structure. Many reports describe that the NPs synthesised by reacting AgNO3 with biological solutions are face centred cubic with minor variations in peak values depending upon nature of extract; metabolites present and binding properties [27]. The average crystalline size of the AgNPs formed in the present process was calculated from the Debye–Scherrer's formula (D = k λ /β Cos θ) where k is Scherrer constant taken as 0.85, λ is the wavelength of the X‐rays, θ is the diffraction angle or Bragg's angle and β is the angular FWHM in radians, and found it in the range of 10–20 nm. The theoretically derived size of AgNPs synthesised at 20 mM AgNO3 were 20 and 9 nm and at 30 mM AgNO3 were 18 and 12 nm for grapes and tomato, respectively.
Fig. 5.
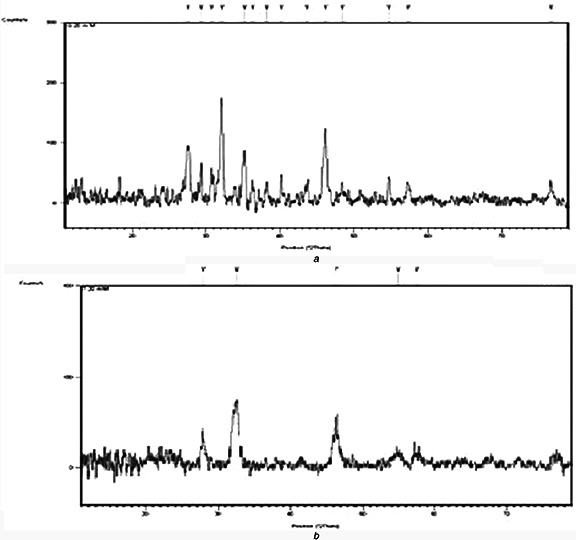
XRD spectra overlay of NPs synthesised at 20 mM AgNO3 by
(a) Grape fruit extract, (b) Tomato fruit extract
Scanning electron micrograph shows the variation in size of NPs by fruit extracts and concentration of Ag ions. The grape juice NP resulted in 10–18 nm when treated with 20 mM AgNO3. However, the size range increased 14–30 nm when treated with 30 mM AgNO3 (Fig. 6). Same behaviour was observed by tomato juice with variation in size 14–28 nm and 11–30 nm by 20 and 30 mM AgNO3 treatment, respectively (Fig. 6). The variation in size of AgNPs synthesised through green chemistry has been reported by many researchers [28].
Fig. 6.
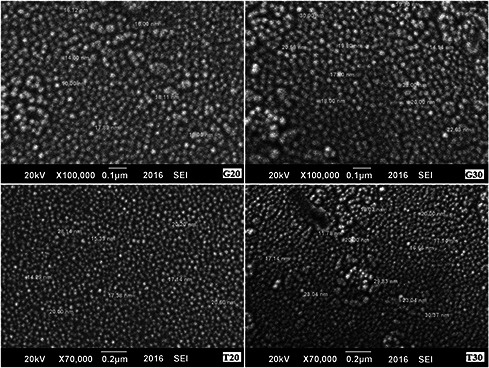
SEM of grape and tomato juices AgNPs synthesised by 20 and 30 mM AgNO3. T stands for tomato and G for grapes. 20 and 30 represents synthesis of NPs as 20 and 30 mM AgNO3, respectively
3.2 Biological activities of NPs
Tomato AgNPs synthesised at 20 and 30 mM AgNO3 showed 76 and 83% inhibition of free radicals, respectively (Table 1) and significant activity was also observed in case of grapes derived particles. Total antioxidant capacity was calculated based on the formation of the phosphomolybdenum complex. Total antioxidant capacity for tomato was 4.6 and 5.8 µg AAE/100 µg and grapes 6.4 and 8.3 µg AAE/100 µg, respectively. Fe (III) reduction is used as an indicator of electron‐donating activity, which is an important mechanism of phenolic antioxidant action. The reducing power of tomato and grape NPs derived at 20 and 30 mM are shown in Table 1. In the reducing power assay, the presence of antioxidants in the samples would result in the reducing of Fe3+ –Fe2+ by donating an electron. Increasing absorbance indicates an increase in the reductive ability. It is evident that the tomato and grape juice NPs showed reductive potential and could serve as electron donor, terminating the radical chain reaction.
Table 1.
Total flavonoids (µg QE/100 µg of sample), total phenolics (µg GAE/100 µg of sample), DPPH scavenging (%), total antioxidant (µg AAE/100 µg of sample) and reducing power (µg AAE/ 100 µg of sample) activities of grape and tomato fruit extracts AgNPs synthesised at 20 and 30 mM AgNO3 concentrations. T stands for tomato and G for grapes. 20 and 30 represents synthesis of NPs as 20 and 30 mM AgNO3, respectively. The small letters shows significant difference within the column determined by LSD at 0.05% probability
| Sample | Total flavonoids | Total phenolics | DPPH activity | Total antioxidant | Reducing power |
|---|---|---|---|---|---|
| T20 | 7.3 ± 1.4c | 0.35 ± 0.1b | 75 ± 3c | 4.6 ± 0.3d | 0.7 ± 0.2b |
| T30 | 14.2 ± 1.1a | 0.43 ± 0.1a | 83 ± 3.4a | 5.9 ± 0.8c | 1.9 ± 0.8a |
| G20 | 10.8 ± 0.7b | 0.28 ± 0.1d | 84 ± 2.9a | 6.4 ± 0.7b | 2 ± 0.4a |
| G30 | 5.7 ± 0.5d | 0.37 ± 0.2c | 80 ± 2b | 8.3 ± 0.9a | 2 ± 0.1a |
Flavonoids contain hydroxyl groups which are responsible for the radical scavenging effect. Tomato juice AgNPs derived by 20 and 30 mM AgNO3 total flavonoids contents were 7.3 and 14.1 µg QE/100 µg, respectively. While grape juice derived NPs at same concentrations the values were 10.8 µg QE/100 µg and 5.7 µg QE/100 µg, respectively. Phenolic compounds are a class of antioxidant agents acting as free radical terminators. In case of tomato, derived AgNPs at 30 mM AgNO3 show best result (0.34 µg GA/100 µg), as compared with 20 mM (0.42 µg GA/100 µg). The same was observed in case of grape juice. Total flavonoid and total phenolic contents determined for tomato and grape AgNPs demonstrated significant activities. The resulted values demonstrated formation of complex molecules by attachment of metabolites with Ag ion. The results also demonstrate that particles synthesised by 30 mM AgNO3, exhibited more activity as compared with 20 mM AgNPs (Table 1). Velavan et al. [29] reported that Cassia auriculata flower AgNPs exhibited strong antioxidant activity. Banerjee and Narendhirakannan [30] used an extract of Syzygium cumini (Jambul) seeds to produce AgNPs and found to have stronger antioxidant properties in vitro than the original extract, suggesting that it might be due to a preferential adsorption of the antioxidant material from the extract onto the surface of the NPs. These obtained AgNPs are advantageous in medical and pharmaceutical purposes. It also has potential applications in the biomedical field and can be produced commercially at large scale.
The tomato and grape extract derived AgNPs showed good antibacterial activity against different gram‐negative and gram‐positive strains. The zone of inhibition (Table 2) varied depending upon the concentration of Ag used to synthesise AgNPs (i.e. 20 and 30 mM), size of NPs and also the diffusion capability of NPs in the agar medium. Protein kinase inhibition assay for biosynthesised AgNP also showed good results against Streptomyces (Table 2) and the present study suggested that it can be used as protein kinase inhibitors. Roy et al. [14] studied the antibacterial activity of spherical shape biosynthesised AgNP from grape fruit extract with an average size of 18‒20 nm and reported that these NPs showed inhibition of growth and effective antibacterial activity. AgNPs synthesised from different plant extracts have been reported showing capability to kill different bacterial strains such as Dioscorea bulbifera; Anogeissus latifolia; Ocimum sanctum; banana peels; Mentha piperita; Calotropis gigantea; Ficus bengalensis (Marri) and Eucalyptus citriodora; Cinnamomum camphora; Ocimum tenuiflorum; Acalypha indica; and Citrus sinensis; and many others.
Table 2.
Antibacterial and protein kinase inhibition activity of AgNP synthesised from grape and tomato extracts. T stands for tomato and G for grapes. 20 and 30 represents synthesis of NPs as 20 and 30 mM AgNO3, respectively. The data is presented as mean with standard deviation. The small letters shows significant difference at p < 0.05 within the column. The capital letters shows significant difference within the row determined by LSD at 0.05% probability
| Zone of inhibition, mm | |||||||
|---|---|---|---|---|---|---|---|
| Sample | S. aureus | M. luteus | E. aerogenes | B. subtilis | P. septica | S. typhi | Protein kinase |
| T20 | 26 ± 1aA | 22 ± 1.1aBC | 17 ± 0.6cDE | 18 ± 0.7cD | 21 ± 1bC | 23 ± 1aB | 13 ± 0.5bE |
| T30 | 22 ± 0.5bA | 20 ± 1bB | 22 ± 0.9aA | 20 ± 1bB | 20 ± 0.8bB | 20 ± 0.7cB | 15 ± 0.4aC |
| G20 | 20 ± 0.7cC | 21 ± 1abBC | 22 ± 1.1aB | 22 ± 1aB | 27 ± 1.1aA | 19 ± 0.6dD | 10 ± 0.2dE |
| G30 | 25 ± 0.9aB | 19 ± 0.5bcD | 19 ± 1bD | 21 ± 0.8aC | 27 ± 1.2aA | 21 ± 1bC | 12 ± 0.2cE |
4 Conclusions
The biosynthesised AgNPs by grapes and tomato fruit extracts have potent antioxidant, antibacterial and protein kinase inhibitory activity. As free radicals are important contributors to various degenerative diseases, the observed antioxidant properties of biosynthesised AgNPs might be useful for the development of newer and more potent antioxidants and can be used as potential free radical scavengers against the various damages caused by free radicals making them biocompatible for its use in both biomedical and industrial applications. Though free radicals (oxidants) function as cellular messenger at micromoles level, however, excess of oxidants may deplete antioxidants in the wound that may also lead to tissue damage, extended inflammatory phase, less angiogenesis stage and decreased production of collagen and fibroblast. This antioxidant can also protect collagen and glycosaminoglycans from oxidation, which may speed the rate of wound closure. Antimicrobial and antioxidative potential of synthesised AgNPs depict that these can be used as protectant against microbes and as natural healer of wound.
5 References
- 1. Prabhu S. Poulose E.K.: ‘Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects’, Int. Nano Lett., 2012, 2, pp. 1 –10 [Google Scholar]
- 2. Schultz S. Smith D.R. Mock J.J. et al.: ‘Single‐target molecule detection with nonbleaching multicolor optical immunolabels’, PNAS, 2000, 97, pp. 996 –1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jae Y.S. Beom S.K.: ‘Rapid biological synthesis of silver nanoparticles using plant leaf extracts’, Bioprocess Biosyst. Eng., 2009, 32, (1), pp. 79 –84 [DOI] [PubMed] [Google Scholar]
- 4. Rai M.K. Deshmukh S.D. Ingle A.P. et al.: ‘Silver nanoparticles: the powerful nanoweapon against multidrug‐resistant bacteria’, J. Appl. Microbiol., 2012, 112, (5), pp. 841 –852 [DOI] [PubMed] [Google Scholar]
- 5. Kim J. Kim J. Kim J. et al.: ‘Characterization of as‐synthesized FeCo magnetic nanoparticles by coprecipitation method’, J. Appl. Phys., 2013, 113, (17), p. A313 [Google Scholar]
- 6. Feng Q.L. Wu J. Chen G.Q. et al.: ‘A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus ’, J. Biomed. Mater. Res., 2000, 52, (4), pp. 662 –668 [DOI] [PubMed] [Google Scholar]
- 7. Matsumura Y. Yoshikata K. Kunisaki S. et al.: ‘Mode of bacterial action of silver zeolite and its comparison with that of silver nitrate’, Appl. Environ. Microbiol., 2003, 69, (7), pp. 4278 –4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z.L.: ‘Characterization of nanophase materials’ (Wiley‐VCH, Weinheim, Germany, 2000, 1st edn.), pp. 37 –80 [Google Scholar]
- 9. Bibi Y. Nisa S. Chaudhary F.M. et al.: ‘Antibacterial activity of some selected medicinal plants of Pakistan’, BMC Complement. Altern. Med., 2011, 11, (1), p. 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rehman R. Chaudhary M.F. Khawar K. et al.: ‘In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential’, Biologia, 2014, 69, (3), pp. 341 –349 [Google Scholar]
- 11. Ali A. Phull A.R. Zia M. et al.: ‘Phytotoxicity of river Chenab sediments: in vitro morphological and biochemical response of Brassica napus L’, Nanotechnol. Monit. Manag., 2015, 4, pp. 74 –84 [Google Scholar]
- 12. Humaira F. Komal K. Muhammad Z. et al.: ‘Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: an in vitro biological and phytochemical investigation’, BMC Complement. Altern. Med., 2015, 15, p. 376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prakas R.T.U. Thiagarajan P.: ‘Syntheses and characterization of silver nanoparticles using Penicillium sp. isolated from soil’, Inter. J. Adv. Sci. Technol., 2012, 1, (2), pp. 137 –149 [Google Scholar]
- 14. Roy K. Biswas S. Banerjee P.C.: ‘Green synthesis of silver nanoparticles by using grape (Vitis vinifera) fruit extract: characterization of the particles and study of antibacterial activity’, Res. J. Pharm. Biol. Chem. Sci., 2013, 4, (1), pp. 1271 –1278 [Google Scholar]
- 15. Bar H. Bhui D.K. Sahoo P.G. et al.: ‘Green synthesis of silver nanoparticles using latex of Jatropha curcas ’, Colloids Surf. A Physicochem. Eng. Aspects, 2009, 339, pp. 134 –139 [Google Scholar]
- 16. Amin M. Anwar F. Janjua M.R. et al.: ‘Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum l. berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori ’, Int. J. Mol. Sci., 2012, 13, pp. 9923 –9941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vanaja M. Gnanajobitha G. Paulkumar K. et al.: ‘Phytosynthesis of silver nanoparticles by Cissus quadrangularis: influence of physicochemical factors’, J. Nano. Struct. Chem., 2013, 3, (17), pp. 1 –8 [Google Scholar]
- 18. Krishnaraj C. Ramachandran R. Mohan K. et al.: ‘Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi’, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2012, 93, pp. 95 –99 [DOI] [PubMed] [Google Scholar]
- 19. Jha A.K. Prasad K.: ‘Green fruit of chili (Capsicum annum L.) synthesizes nano silver’, Dig. Nanomater. Bios., 2011, 6, (4), pp. 1717 –1723 [Google Scholar]
- 20. Daizy P.: ‘Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis ’, Physica E, 2010, 42, (5), pp. 1417 –1424 [Google Scholar]
- 21. Fayaz A.M. Balaji K. Kalaichelvan P.T. et al.: ‘Fungal based synthesis of silver nanoparticles – an effect of temperature on the size of particles’, Colloids Surf. B Biointerfaces, 2009, 74, (1), pp. 123 –126 [DOI] [PubMed] [Google Scholar]
- 22. Socrates G.: ‘Infrared characteristic group frequencies’ (Wiley, New York: USA, 1980, 1st edn.), p. 153 [Google Scholar]
- 23. Renugadevi T.S. Gayathri S.: ‘FTIR and FT‐Raman spectral analysis of paclitaxel drugs’, Int. J. Pharm. Sci. Rev. Res., 2010, 2, (2), pp. 106 –110 [Google Scholar]
- 24. O'Coinceanainn M.O. Astill C. Schumm S.: ‘Potentiometric FTIR and NMR studies of the complexation of metals with the aflavin’, Dalton Trans., 2003, 3, (5), pp. 801 –807 [Google Scholar]
- 25. Susanto H. Feng Y. Ulbricht M.: ‘Fouling behavior during ultrafiltration of aqueous solutions of polyphenolic compounds’, J. Food Eng., 2009, 91, (2), pp. 333 –340 [Google Scholar]
- 26. Sathyavathi R. Krishna M.B. Rao S.V. et al.: ‘Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics’, Adv. Sci. Lett., 2010, 3, (2), pp. 138 –143 [Google Scholar]
- 27. Mitra B. Vishnudas D. Sant S.B. et al.: ‘Green‐synthesis and characterization of silver nanoparticles by aqueous leaf extracts of Cardiospermum helicacabum leaves’, Drug Invent. Today, 2012, 4, (2), pp. 340 –344 [Google Scholar]
- 28. Phull A.R. Abbas Q. Ali A. et al.: ‘Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata ’, Future J. Pharm. Sci., 2016, 2, (1), pp 31 –36 [Google Scholar]
- 29. Velavan S. Arivoli P. Mahadevan K.: ‘Biological reduction of silver nanoparticles using cassia auriculata flower extract and evaluation of their in vitro antioxidant activities’, Nanosci. Nanotechnol., 2012, 2, (1), pp. 30 –35 [Google Scholar]
- 30. Banerjee J. Narendhirakannan R.T.: ‘Biosynthesis of silver nanoparticles from Syzygium cumini (L.) seed extract and evaluation of their in vitro antioxidant activities’, Dig. J. Nanomater. Biostruct., 2011, 6, (3), pp. 961 –968 [Google Scholar]


