Abstract
Silver nanoparticles (AgNPs) have been biosynthesised through the extracts of Ribes khorassanicum fruits, which served as the reducing agents and capping agents. Biosynthesised AgNPs have been found to be ultraviolet–visible (UV–vis) absorption spectra since they have displayed one surface plasmon resonance peak at 438 nm, attesting the formation of spherical NPs. These particles have been characterised by UV–vis, field‐emission scanning electron microscopy, energy‐dispersive X‐ray spectroscopy, X‐ray diffraction, Fourier transform infrared spectroscopy, and transmission electron microscopy analysis. The formation of AgNPs at 1.0 mM concentration of AgNO3 has resulted in NPs that contained mean diameters in a range of 20–40 nm. The green‐synthesised AgNPs have demonstrated high antibacterial effect against pathogenic bacteria (i.e. Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa). Biosynthesising metal NPs through plant extracts can serve as the facile and eco‐friendly alternative for chemical and/or physical methods that are utilised for large‐scale nanometal fabrication in various medical and industrial applications.
Inspec keywords: X‐ray diffraction, X‐ray chemical analysis, nanofabrication, surface plasmon resonance, nanoparticles, antibacterial activity, microorganisms, scanning electron microscopy, silver, nanomedicine, visible spectra, ultraviolet spectra, transmission electron microscopy, Fourier transform infrared spectra, field emission scanning electron microscopy, biomedical materials
Other keywords: antibacterial properties, silver nanoparticles, reducing agents, capping agents, surface plasmon resonance peak, spherical NPs, field‐emission scanning electron microscopy, energy‐dispersive X‐ray spectroscopy, X‐ray diffraction, transmission electron microscopy analysis, plant extracts, ultraviolet‐visible absorption spectra, Fourier transform infrared spectroscopy, antibacterial effect, Ribes khorassanicum fruits, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, surface plasmon resonance, AgNO3 , Ag
1 Introduction
Silver nanoparticles (AgNPs) synthesis is an expanding research area due to their unique chemical, physical, and biological properties [1, 2, 3, 4] that can lead to a wide range of applications in spectroscopy, sensors, electronics, catalysis, and pharmaceutical sciences [5, 6, 7, 8]. Although several approaches (physical‐ and chemical‐based methods) have been reported in order to prepare AgNPs [9, 10, 11, 12], yet the biosynthesis of NPs can eliminate the requirement of using harmful chemicals while reducing the need for downstream procedures, and as a result make the biosynthesis protocol further low cost, fast, and less energy consuming [13, 14, 15]. For this purpose, utilising plant extracts are potentially advantageous over other biosynthesis methods, mainly microorganisms, due to the ease in improving and the lack of requiring an elaborate process of maintaining cell cultures [16].
Several plant extracts such as palm kernel [17], Prosopis fracta [18], Malva sylvestris [19], Coffea arabica [20], Moringa oleifera [21], Descurainia sophia [22], Clerodendrum phlomidis [23], Justicia adhatoda [24], Panax ginseng [25], Rheum turkestanicum [26], Diospyros paniculata [27], and other particular species have been used for the synthesis of AgNPs. Within this context, the present work describes the biosynthesis of AgNPs through employing the aqueous fruit extract of Ribes khorassanicum (R. khorassanicum) for the first time. R. khorassanicum is a unique medicinal plant with a large number of natural antioxidants, mainly anthocyanins – a group of natural compounds and secondary metabolites belonging to the flavonoid family – as suitable reducing agents in the reduction process of Ag+ to AgNPs [28]. Herein, AgNPs are easy to produce along with short preparation time and in moderate temperature. Different characterisation instruments including ultraviolet–visible (UV–vis) spectrophotometry, transmission electron microscopy (TEM), field‐emission scanning electron microscopy (FESEM), energy‐dispersive X‐ray spectroscopy (EDX), and Fourier transform infrared spectroscopy (FT‐IR) were performed to analyse the structure and formation of AgNPs. We have also examined the antibacterial effects of biosynthesised AgNPs by following disc diffusion Mueller–Hinton agar method on Gram‐positive (Staphylococcus aureus) and Gram‐negative bacteria (Escherichia coli and Pseudomonas aeruginosa). The difference in antibacterial effects between Gram‐positive and Gram‐negative bacteria was attributed to the structure of their perspective cell walls [29]. For example, the cell wall of Gram‐negative bacteria is included of a thin peptidoglycan layer and a lipopolysaccharide layer [30]. In the end, observations have strongly recommended the use of ‘green’ AgNPs to prevent bacterial biofilms in medical applications.
2 Materials and methods
2.1 Materials
Fresh R. khorassanicum fruits were collected from an eastern–southern area in Iran, and Ag nitrate (AgNO3, pre‐analysis) was procured from Carlo Erba (France). The R. khorassanicum fruits were cleansed and dried for 3 days at room temperature. The laboratory experiments were applied by double distilled water (ddw).
2.2 Preparation of R. khorassanicum fruits extracts
R. khorassanicum fruits extract (using 3.0 g of dried shoots) was prepared in 250 ml of ddw. Then, it was placed in a shaker for 24 h and was sieved using a Whatman #1 filter. The obtained extract (red colour) was reserved in a refrigerator (4°C).
2.3 Biosynthesis of AgNPs
The fruit extract (10.0 ml) was added to 90 ml of AgNO3 (1.0 mM) and was vigorously stirred at 70°C for 1 h. The obtained AgNPs solution (dark brown colour) was lyophilised for 24 h and it was scrapped out for forthcoming studies.
2.4 Characterisation of AgNPs
The bio‐prepared AgNPs was analysed using many tools, e.g. UV–vis (double beam spectrophotometer, CECIL, CE 9500, UK), FESEM + EDX (TESCAN, MIRA 3), TEM (Leo 912 AB, 120 kV, Zeiss, Germany), X‐ray diffraction (XRD) (D8, ADVANCE‐BRUKER, Germany), and FT‐IR (Shimadzu 8400, Japan).
2.5 Antibacterial activity
Method of disc diffusion was applied to the antibacterial evaluation of AgNPs. The bacterial culture was modified to the colony (equivalent to 0.5 McFarland) for an overnight. Then, discs that were soaked by AgNPs (15 µl) were placed on the immunised agars [31]. Besides the discs soaked by extract (0.1% v/v), the rest was labelled as the control (–). All of the plates were incubated (37°C) for 24 h. Gentamicin (GM) and streptomycin were used as standards in this work.
3 Results and discussion
The schematic plan of AgNPs biosynthesis is shown in Fig. 1. The fruit extract of R. khorassanicum was used as a natural reducing and capping agent. As shown in Fig. 1, mixing the extract with Ag+ solution has resulted in the change of solution colour from light red to dark brown due to the bio‐preparation of AgNPs [32].
Fig. 1.
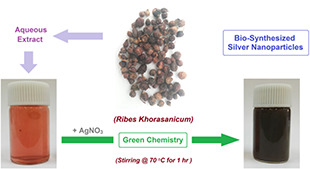
Schematic plan for biosynthesis of AgNPs using R. khorasanicum seeds extracts
3.1 UV–vis spectral analysis
UV–vis spectrophotometric analysis has provided sufficient evidence for NPs preparations. AgNPs that have been biosynthesised by R. khorassanicum fruits extract have illustrated a sharp characteristic surface plasmon resonance (SPR) peak at 438 nm, which confirms their biosynthesis (Fig. 2). Previous works have reported that AgNPs give an absorption peak between 410 and 450 nm due to the SPR phenomenon [33, 34, 35, 36, 37].
Fig. 2.
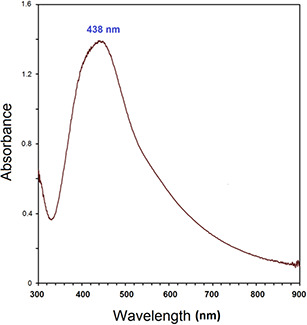
UV–vis spectrum of biosynthesised AgNPs using R. khorasanicum seeds extracts
3.2 TEM micrograph analysis
The morphology and size of biosynthesised AgNPs were observed through a high‐magnification TEM image (Fig. 3). AgNPs seemed to be mainly spherical and nano‐sized particles with diameters in the range of 20–40 nm. It is noted from this image that the NPs are very fine, while covered by little organic material as residual biomolecules of the fruit extracts.
Fig. 3.
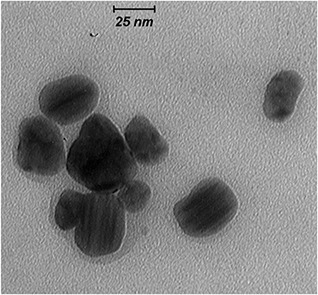
High‐magnification ( × 80,000) TEM image of biosynthesised AgNPs using R. khorasanicum seeds extracts
3.3 FESEM and EDX analyses
The FESEM image of AgNPs demonstrated in Fig. 4 displays the spherical AgNPs with a mean diameter in a range of 15–45 nm, which stands in agreement with the particle size illustrated in the TEM image. As represented in Fig. 4 (inset), EDX result reveals a sharp signal in the Ag region and confirms the formation of AgNPs. Owing to SPR, AgNPs generally show a characteristic absorption peak at ∼3 keV [38]. EDX spectrum has displayed a strong absorption peak for Ag along with a weak oxygen peak that may have originated from the extract (biomolecules) bound to the surface of AgNPs, indicating on the reduction of Ag+ to elemental Ag. The other peak corresponding to gold (Au) in the EDX would be an artefact of Au coating on the sample. There were no extra peaks observed for Ag compounds due to the complete reduction of Ag ions and/or compounds to AgNPs, as shown in the spectrum.
Fig. 4.
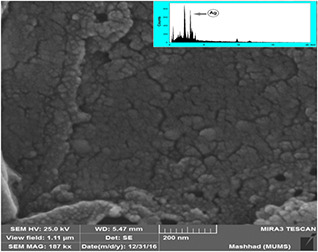
FESEM and EDX spectrum of biosynthesised AgNPs
3.4 XRD pattern
Fig. 5 illustrated the XRD result of the biosynthesised AgNPs. The characteristic Miller indices at 38.1°, 44.2°, 64.2°, 77.4°, and 81.8° are corresponding to the (111), (200), (220), (311), and (222), respectively. Consistently, it approves that the obtained AgNPs have face‐centred cubic structure (JCPDS # 01‐087‐0597).
Fig. 5.
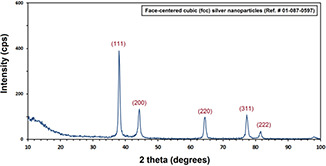
XRD pattern of biosynthesised AgNPs
3.5 FT‐IR spectral analysis
The spectral analysis of FT‐IR result was employed in recognising the existence of various organic functional groups due to their reducing, capping, and stabilising functions. FT‐IR spectrum (Fig. 6 b) has demonstrated bands at 3417, 2918, 1719, 1607, 1384, and 1072 cm−1, representing the presence of biomolecules along with AgNPs. The strong band at 3417 cm−1 corresponds to O–H (νst.) due to existing phenolic compounds in ddw [39]. The characteristic bands in 2918 cm−1 are accredited to the C–H (νst.) of aromatic compounds [40]. The band in 1719 cm−1 seem to be assigned for C–C (νst.; non‐conjugated), while a band in 1607 cm−1 correlates to C = O (νst.; amide) [41]. The peak of C = C group (at 1398 cm−1) absent for AgNPs is possible, due to the reduction of Ag+ to Ag° [42]. The peak at 1070 cm−1, which is absent for the AgNPs FT‐IR spectrum, is the bending vibration of the C–O stretch and could be due to the stabilisation of AgNPs through this group [43]. Similarly, the same phytochemical compounds and biomolecules that are present in plant extracts have been reported to be capable of acting as the important roles of reducing and capping agents in the synthesis of AgNPs [37].
Fig. 6.
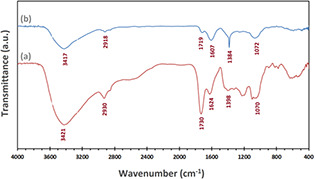
FT‐IR spectra of
(a) Biosynthesised AgNPs, (b) R. khorasanicum fruits extracts
3.6 Assessment of antibacterial effects
The antibacterial effects of biosynthesised AgNPs have been assessed by disc diffusion technique [44]. As represented in Fig. 7, indicating the high antibacterial properties of these biosynthesised particles. AgNPs have demonstrated high antibacterial effects in opposition to Gram ( + ), i.e. S. aureus and Gram (–), i.e. E. coli and P. aeruginosa bacteria, which has been reported previously as well [45, 46]. The inhibition zones (IZs) have been recognised in Table 1, while each experiment has been performed three times. The precise mechanism of antibacterial effects in AgNPs is not clearly known and yet remains as a debated topic. However, there are many theories on this matter such as the ability of AgNPs to get attached to the bacterial cell wall, and finally penetrate (through changing the structure of cell membrane) it, leading to the cells annihilation [47]. In this manner, the formation of free radicals by AgNPs and releasing Ag+ from the surface of AgNPs could be considered as another procedure that destroys the cells [48, 49]. In addition, AgNPs are talented to inhibit bacterial growth due to their small mean diameter (large surface area) that provides adequate contact to bacteria [50].
Fig. 7.

Antibacterial activity of biosynthesised AgNPs against human pathogenic bacteria
Table 1.
Diameter of IZs by biosynthesised AgNPs
| Bacteria | IZ, mm | |||
|---|---|---|---|---|
| Test | Control (–) | Control ( + ) | ||
| AgNPs | Water | S | GM | |
| E. coli | 8.9 | NA | 11.3 | 15.0 |
| P. aeruginosa | 9.1 | NA | 9.8 | 15.1 |
| S. aureus | 12.6 | NA | 16.3 | 26.0 |
NA = not appearing, GM = gentamicin, and S = streptomycin.
4 Conclusion
We have proposed a novel approach in this paper regarding the biosynthesis of AgNPs from fruits extract of R. khorassanicum. The biosynthesised particles contained a spherical shape and the size that ranged around 20–40 nm as observed in TEM/FESEM and UV–vis/EDX analyses. These particular NPs have shown antibacterial activity against Gram‐negative and Gram‐positive human pathogenic bacteria. Thus, it is concluded that the biosynthesis of AgNPs in plant extracts and through the use of R. khorassanicum fruits can stand as a ‘green’, cost‐effective, facile, and eco‐friendly method that excludes the hazards that may arise out of utilising harmful reducing and/or capping agents. Furthermore, this process has the potential of scaling up for the industrial applications to increase the yield of NPs significantly, which doubtlessly would begin its commercial viability in medicine.
5 Acknowledgment
M. Darroudi acknowledges the technical/instrumental supports for this project provided by the Urmia University and Mashhad University of Medical Sciences in the Ph.D. thesis of Dr. M.E.T. Yazdi.
6 References
- 1. Iravani S.: ‘Green synthesis of metal nanoparticles using plants’, Green Chem., 2011, 13, (10), pp. 2638 –2650 [Google Scholar]
- 2. Zamiri R. Azmi B.Z. Naseri M.G. et al.: ‘Laser based fabrication of chitosan mediated silver nanoparticles’, Appl. Phys. A, Mater. Sci. Process., 2011, 105, (1), pp. 255 –259 [Google Scholar]
- 3. Pahlavan Noghabi M. Parizadeh M.R. Ghayour‐Mobarhan M. et al.: ‘Green synthesis of silver nanoparticles and investigation of their colorimetric sensing and cytotoxicity effects’, J. Mol. Struct., 2017, 1146, (Supplement C), pp. 499 –503 [Google Scholar]
- 4. Kumari A. Guliani A. Singla R. et al.: ‘Silver nanoparticles synthesised using plant extracts show strong antibacterial activity’, IET Nanobiotechnol., 2014, 9, (3), pp. 142 –152 [DOI] [PubMed] [Google Scholar]
- 5. Abbasi E. Milani M. Fekri Aval S. et al.: ‘Silver nanoparticles: synthesis methods, bio‐applications and properties’, Crit. Rev. Microbiol., 2016, 42, (2), pp. 173 –180 [DOI] [PubMed] [Google Scholar]
- 6. Shenashen M.A. El‐Safty S.A. Elshehy E.A.: ‘Synthesis, morphological control, and properties of silver nanoparticles in potential applications’, Part. Part. Syst. Charact., 2014, 31, (3), pp. 293 –316 [Google Scholar]
- 7. Chen A. Chatterjee S.: ‘Nanomaterials based electrochemical sensors for biomedical applications’, Chem. Soc. Rev., 2013, 42, (12), pp. 5425 –5438 [DOI] [PubMed] [Google Scholar]
- 8. Elemike E.E. Onwudiwe D.C. Ekennia A.C. et al.: ‘Synthesis and characterisation of silver nanoparticles using leaf extract of Artemisia afra and their in vitro antimicrobial and antioxidant activities’, IET Nanobiotechnol., 2018, 12, (6), pp. 722 –726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zamiri R. Azmi Z. Abbastabar H. et al.: ‘Laser‐fabricated castor oil‐capped silver nanoparticles’, Int. J. Nanomed., 2011, 6, pp. 565 –568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Darroudi M. Ahmad M.B. Zamiri R. et al.: ‘Time‐dependent preparation of gelatin‐stabilized silver nanoparticles by pulsed Nd:YAG laser’, Solid State Sci., 2011, 13, (3), pp. 520 –524 [Google Scholar]
- 11. Shameli K. Ahmad M.B. Yunus W.M.Z.W. et al.: ‘Synthesis and characterization of silver/polylactide nanocomposites’, World. Acad. Sci. Eng. Technol., 2010, 64, pp. 28 –32 [Google Scholar]
- 12. Khatami M. Pourseyedi S.: ‘ Phoenix dactylifera (date palm) pit aqueous extract mediated novel route for synthesis high stable silver nanoparticles with high antifungal and antibacterial activity’, IET Nanobiotechnol., 2015, 9, (4), pp. 184 –190 [DOI] [PubMed] [Google Scholar]
- 13. Tamuly C. Hazarika M. Bordoli M. et al.: ‘Photocatalytic activity of Ag nanoparticles synthesized by using Piper pedicellatum C. DC fruits’, Mater. Lett., 2013, 102, pp. 1 –4 [Google Scholar]
- 14. Lee H.J. Song J.Y. Kim B.S.: ‘Biological synthesis of copper nanoparticles using Magnolia kobus leaf extract and their antibacterial activity’, J. Chem. Technol. Biotechnol., 2013, 88, (11), pp. 1971 –1977 [Google Scholar]
- 15. Sharma P. Pant S. Rai S. et al.: ‘Green synthesis and characterization of silver nanoparticles by Allium cepa L. to produce silver nano‐coated fabric and their antimicrobial evaluation’, Appl. Organomet. Chem., 2018, 32, (3) [Google Scholar]
- 16. Njagi E.C. Huang H. Stafford L. et al.: ‘Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts’, Langmuir, 2010, 27, (1), pp. 264 –271 [DOI] [PubMed] [Google Scholar]
- 17. Khatami M. Kharazi S. Kishani Farahani Z. et al.: ‘The anti‐cancer effect of octagon and spherical silver nanoparticles on MCF‐7 breast cancer cell line’, Tehran Univ. Med. J., 2017, 75, (1), pp. 72 –76 [Google Scholar]
- 18. Miri A. Sarani M. Rezazade Bazaz M. et al.: ‘Plant‐mediated biosynthesis of silver nanoparticles using Prosopis farcta extract and its antibacterial properties’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2015, 141, pp. 287 –291 [DOI] [PubMed] [Google Scholar]
- 19. Esfanddarani H.M. Kajani A.A. Bordbar A.‐K.: ‘Green synthesis of silver nanoparticles using flower extract of Malva sylvestris and investigation of their antibacterial activity’, IET Nanobiotechnol., 2017, 12, (4), pp. 412 –416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dhand V. Soumya L. Bharadwaj S. et al.: ‘Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity’, Mater. Sci. Eng. C, 2016, 58, pp. 36 –43 [DOI] [PubMed] [Google Scholar]
- 21. Hussain M. Raja N.I. Mashwni Z. et al.: ‘Green synthesis and characterisation of silver nanoparticles and their effects on antimicrobial efficacy and biochemical profiling in Citrus reticulata ’, IET Nanobiotechnol., 2018, 12, (4), pp. 514 –519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khatami M. Mehnipor R. Sobhani Poor M.H. et al.: ‘Facile biosynthesis of silver nanoparticles using Descurainia sophia and evaluation of their antibacterial and antifungal properties’, J. Cluster Sci., 2016, 27, (5), pp. 1601 –1612 [Google Scholar]
- 23. Sriranjani R. Srinithya B. Vellingiri V. et al.: ‘Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities’, J. Mol. Liq., 2016, 220, pp. 926 –930 [Google Scholar]
- 24. Latha D. Arulvasu C. Prabu P. et al.: ‘Photocatalytic activity of biosynthesized silver nanoparticle from leaf extract of Justicia adhatoda ’, Mech., Mater. Sci. Eng. J., 2017, 9, (1) [Google Scholar]
- 25. Singh P. Kim Y. Zhang D. et al.: ‘Biological synthesis of nanoparticles from plants and microorganisms’, Trends Biotechnol., 2016, 34, (7), pp. 588 –599 [DOI] [PubMed] [Google Scholar]
- 26. Taghavizadeh Yazdi M.E. Khara J. Sadeghnia H.R. et al.: ‘Biosynthesis, characterization, and antibacterial activity of silver nanoparticles using Rheum turkestanicum shoots extract’, Res. Chem. Intermed., 2018, 44, (2), pp. 1325 –1334 [Google Scholar]
- 27. Rao N.H. Lakshmidevi N. Pammi S.V.N. et al.: ‘Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities’, Mater. Sci. Eng. C, 2016, 62, pp. 553 –557 [DOI] [PubMed] [Google Scholar]
- 28. Adibi F. Ejtehadi H. Abrishamchi P.: ‘Phytochemicals and antibacterial effects of Ribes khorassanicum Saghafi & Assadi, an endemic plant species to North–East of Khorasan’, J. Med. Plants, 2007, 4, (24), pp. 64 –73 [Google Scholar]
- 29. Siddhartha S. Tanamy B. Arnab R. et al.: ‘Characterization of enhanced antibacterial effects of novel silver nanoparticles’, Nanotechnology, 2007, 18, (22), p. 225103 [DOI] [PubMed] [Google Scholar]
- 30. Tamboli D.P. Lee D.S.: ‘Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against Gram‐positive and Gram‐negative bacteria’, J. Hazard. Mater., 2013, 260, pp. 878 –884 [DOI] [PubMed] [Google Scholar]
- 31. Dovi K. Yoel K. Eugene R. et al.: ‘Antimicrobial activity of the reef sponge Amphimedon viridis from the Red Sea: evidence for selective toxicity’, Aquat. Microb. Ecol., 2001, 24, (1), pp. 9 –16 [Google Scholar]
- 32. Muthu K. Priya S.: ‘Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction’, Spectrochim. Acta, Part A, 2017, 179, pp. 66 –72 [DOI] [PubMed] [Google Scholar]
- 33. Darroudi M. Ahmad M.B. Abdullah A.H. et al.: ‘Green synthesis and characterization of gelatin‐based and sugar‐reduced silver nanoparticles’, Int. J. Nanomed., 2011, 6, (1), pp. 569 –574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Darroudi M. Ahmad M.B. Abdullah A.H. et al.: ‘Effect of accelerator in green synthesis of silver nanoparticles’, Int. J. Mol. Sci., 2010, 11, (10), pp. 3898 –3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Darroudi M. Ahmad M.B. Hakimi M. et al.: ‘Preparation, characterization, and antibacterial activity of γ ‐irradiated silver nanoparticles in aqueous gelatin’, Int. J. Miner., Metall. Mater., 2013, 20, (4), pp. 403 –409 [Google Scholar]
- 36. Darroudi M. Ahmad M.B. Mashreghi M.: ‘Gelatinous silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity’, J. Optoelectron. Adv. Mater., 2014, 16, (1–2), pp. 182 –187 [Google Scholar]
- 37. Kathiravan V. Ravi S. Ashok Kumar S.: ‘Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity’, Spectrochim. Acta A, 2014, 130, pp. 116 –121 [DOI] [PubMed] [Google Scholar]
- 38. Jyoti K. Baunthiyal M. Singh A.: ‘Characterization of silver nanoparticles synthesized using Urtica dioica Linn. Leaves and their synergistic effects with antibiotics’, J. Radiat. Res. Appl. Sci., 2016, 9, (3), pp. 217 –227 [Google Scholar]
- 39. Prathna T.C. Chandrasekaran N. Raichur A.M. et al.: ‘Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size’, Colloids Surf. B, Biointerfaces, 2011, 82, (1), pp. 152 –159 [DOI] [PubMed] [Google Scholar]
- 40. Atarod M. Nasrollahzadeh M. Mohammad Sajadi S.: ‘Green synthesis of Pd/RGO/Fe3 O4 nanocomposite using Withania coagulans leaf extract and its application as magnetically separable and reusable catalyst for the reduction of 4‐nitrophenol’, J. Colloid Interface Sci., 2016, 465, pp. 249 –258 [DOI] [PubMed] [Google Scholar]
- 41. Prakash P. Gnanaprasakam P. Emmanuel R. et al.: ‘Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi linn. for enhanced antibacterial activity against multi drug resistant clinical isolates’, Colloids Surf. B, Biointerfaces, 2013, 108, pp. 255 –259 [DOI] [PubMed] [Google Scholar]
- 42. Balavijayalakshmi J. Ramalakshmi V.: ‘ Carica papaya peel mediated synthesis of silver nanoparticles and its antibacterial activity against human pathogens’, J. Appl. Res. Technol. JART, 2017, 15, (6), pp. 413 –422 [Google Scholar]
- 43. He Y. Du Z. Lv H. et al.: ‘Green synthesis of silver nanoparticles by Chrysanthemum morifolium Ramat. Extract and their application in clinical ultrasound gel’, Int. J. Nanomed., 2013, 8, p. 1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ashok Kumar S. Ravi S. Kathiravan V. et al.: ‘Synthesis of silver nanoparticles using A. indicum leaf extract and their antibacterial activity’, Spectrochim. Acta, Part A, 2015, 134, pp. 34 –39 [DOI] [PubMed] [Google Scholar]
- 45. Verma V.C. Kharwar R.N. Gange A.C.: ‘Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus ’, Nanomedicine, 2009, 5, (1), pp. 33 –40 [DOI] [PubMed] [Google Scholar]
- 46. Wang W. Xiao K. He T. et al.: ‘Synthesis and characterization of Ag nanoparticles decorated mesoporous sintered activated carbon with antibacterial and adsorptive properties’, J. Alloys Compd., 2015, 647, pp. 1007 –1012 [Google Scholar]
- 47. Sondi I. Salopek‐Sondi B.: ‘Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram‐negative bacteria’, J. Colloid Interface Sci., 2004, 275, (1), pp. 177 –182 [DOI] [PubMed] [Google Scholar]
- 48. Feng Q. Wu J. Chen G. et al.: ‘A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus ’, J. Biomed. Mater. Res., 2000, 52, (4), pp. 662 –668 [DOI] [PubMed] [Google Scholar]
- 49. Matsumura Y. Yoshikata K. Kunisaki S. et al.: ‘Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate’, Appl. Environ. Microbiol., 2003, 69, (7), pp. 4278 –4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kanmani P. Rhim J.‐W.: ‘Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films’, Food Chem., 2014, 148, pp. 162 –169 [DOI] [PubMed] [Google Scholar]


