Abstract
The concern regarding the toxicological effects of nanoparticles (NPs) on the terrestrial environment is increasing. To avoid risks of exposure to these NPs in the environment, it is essential to develop an understanding of their reactivity, toxicity, and persistency. Due to the increased usage of nano‐titanium dioxide (TiO2) in various industrial products, an exponential increase in exposure is expected, which would exacerbate concerns about its ecological risks. The present study is conducted to evaluate the size‐dependent effects of TiO2 NPs on the soil, especially on earthworm (Eudrilus euginiae). To date, many studies have been reported on the impact of TiO2 NPs on ecotoxicology. However, histotoxicology studies are sparse. This study serves to be the first report on the size‐dependent histotoxicological impact of nano‐TiO2 on earthworms particularly, E. euginiae. This report presents an intensive overall view of the longer time ecotoxicological impact of TiO2 nanomaterials on various biological parameters of earthworms at cellular levels. The results show that the survival and growth of adult earthworms are severely affected by the TiO2 NPs in the soil, which substantiates the adverse effects of TiO2 NPs on earthworms.
Inspec keywords: nanobiotechnology, nanoparticles, titanium compounds, semiconductor materials, toxicology, zoology, soil, cellular biophysics, particle size
Other keywords: toxicological impact, nanoparticles, Eudrilus euginiae, terrestrial environment, soil, earthworm, ecotoxicology, size‐dependent histotoxicological impact, nanomaterials, biological parameters, cellular levels, TiO2
1 Introduction
Nowadays, nanomaterials play an important role in the manufacture of numerous products that we use in our daily life. Among them, titanium dioxide (TiO2) is a well‐known photocatalytic semiconducting material having great potential as photoelectric converters with applications in solar cells, environmental purification and for the generation of hydrogen gas [1, 2]. The vast applications of TiO2 nanoparticles (NPs) also include various consumer goods and products of daily use, such as cosmetics, paints, dyes and varnishes, textiles, paper, plastics, and even in paving stones. So, the increased usage of these NPs in various fields has led to their presence in the soil and environment. Hence, the safety of NPs in relation to their toxicity, reactivity, and persistency, has to be analysed carefully before exposing these NPs to the terrestrial environment and ecosystem. Reports available on the effect of NPs on the natural environment could pose a threat to beneficial microbial communities such as those found in soil [1, 2, 3]. NPs can enter the soil through industrial spills, landfill sites or when sewage sludge is used as a fertiliser [4]. Due to the exceptional properties possessed by them, NPs are also able to pass through cell membranes in organisms. Reports are available on the toxic effects of TiO2 NPs stating the formation of reactive oxygen species (ROS) with water in the presence of ultraviolet irradiation and even in the absence of photo‐activation. ROS and free radicals cause oxidative stress and may play a major role in the NPs potential toxicity to organisms [5, 6, 7]. Also, the toxicity of NPs is related to their size [8]. However, the detailed interactions of these NPs with biological systems are relatively unknown [9]. Thus, leaching of NPs into the environment could be a serious problem.
In the present investigation, TiO2 NPs with different particle sizes have been synthesised by the sol–gel method and were characterised by X‐ray diffraction (XRD) and transmission electron microscopy (TEM). Our major concern is to evaluate the effect of these TiO2 NPs at the tissue level of biological organisation to improve the understanding of hazards and risks of NPs in the environment especially on earthworm (Eudrilus euginiae). The effect of commercially available nano‐TiO2 on selected earthworm species was also compared and analysed. Earthworms (from the family Eudrilidae) were selected as one of the key indicator organisms for eco‐toxicological testing of TiO2 NPs. Being a soil dwelling organism, it is involved in the process of soil formation and organic matter decomposition and is widely used for vermicomposting.
2 Materials and methods
2.1 Materials
Titanium (IV)‐n ‐butoxide (Ti(OBu)4) (98%, Alfa Aesar), Ce(NO3)3. 6H2 O (99.5%, Alfa Aesar), isopropyl alcohol (99%, Fisher Scientific) and HNO3 (99.9%, Merck) were used for the synthesis of TiO2. Distilled water was used in all the synthesis processes.
2.2 Synthesis of TiO2 NPs
The procedures for the synthesis of TiO2 NPs and Ce‐doped TiO2 NPs have been described elsewhere [10, 11]. Ce was doped to reduce particle size, and enhance visible light activity. The precursor solution was a mixture of 1:2 titanium (IV)‐n ‐butoxide (Ti(OC4 H9)4) and isopropyl alcohol ((CH3)2 CHOH). The required weighed % of Ce(NO3)3 ·6H2 O was dissolved in distilled water, and then mixed with another solution whose pH value was suitably adjusted to 2.0 by adding HNO3 under a continuous stirring process. The precursor solution was then added drop wise into this solution and stirred continuously for 3 h. After complete hydrolysis of Ti(OC4 H9)4, and performing gelling, ageing and drying for 13–20 h, the colloid was calcined at 400°C for 3 h to obtain Ce‐doped TiO2 NPs. Ce ion‐doped TiO2 at various molar concentrations 3, 2, and 1 mol % were denoted as T 1, T 2, and T 3, respectively. TiO2 NPs prepared by the same method without adding Ce(NO3)3 ·6H2 O, following the calcination at 400, 600 and 800°C for 3 h. TiO2 NPs calcined at 400, 600 and 800°C were denoted as T 4, T 5, and T 6, respectively. The order of increasing particle size is T 1 < T 2 < T 3 < T 4 < T 5 < T 6. Commercially available nano‐TiO2 (widely known as P25; purchased from Sigma‐Aldrich) with primary particle size of 21 nm was also used to test the effect on earthworms.
X‐ray powder diffraction (XRD) was carried out using a Bruker D8 Advance X‐ray diffractometer (k = 1.5406 Å, step size = 0.020°, dwell time 65.6 s, 40 kV, and 35 mA) with Cu‐Kα1 radiation in the 2θ range from 0° to 80°. TEM images of nanocrystalline TiO2 powder samples were obtained using a JEOL/JEM 2100 (Source: LaB6 and voltage: 200 kV).
2.3 Experimental set up
In the present study, exotic earthworm species Eudrilus eugeniae obtained from the local worm breeder associated with CARTZ, Thodupuzha, Kerala, India is used for the toxicological effect of TiO2 NPs. The stock of culture was maintained at room temperature in a culture medium made of cow dung, grass cutting and saw dust in a ratio of 3:1:0.5. The moisture was maintained by spraying water from time to time.
The experiments were carried out in triplicates including control. To assess the effect of TiO2 NPs on earthworms, 15 adult clitellate earthworms were introduced into the 2 kg of growth medium and admixed with 0.005% TiO2 NPs. After four weeks, the worms were counted by wet‐sieving and hand sorting. This counting has been done once in a month for six months to study the effect for a longer period.
Biological parameters, such as proliferation rate of earthworms, total heterotrophic bacterial (THB) count and fungal count, were estimated. Histology of E. euginiae has been carried out by denoting the pre‐clitellar region as anterior, the immediate post clitellar region as middle and the last body segments as posterior. Earthworms from each treatment were analysed according to the standard procedure [12].
3 Results and discussion
3.1 Morphology and particle size of synthesised TiO2 NPs
Fig. 1 shows the XRD patterns of TiO2 NP samples T 1–T 6. As shown in the figure, the peaks of samples T 1, T 2, T 3, T 4, T 5, and T 6 correspond to the tetragonal structure (space group: I 41 /amd) with lattice parameters a = 3.776 Å and c = 9.486 Å, which is in agreement with JCPDS file no. 73–1764 for TiO2. Some peaks of the rutile TiO2 sample can also be found in T 5 and T 6 which is indexed to a tetragonal structure (space group: P 42 /mnm) with lattice parameters a = 4.582 Å and c = 2.953 Å, which is in agreement with JCPDS file no.78–1510 for TiO2.
Fig. 1.
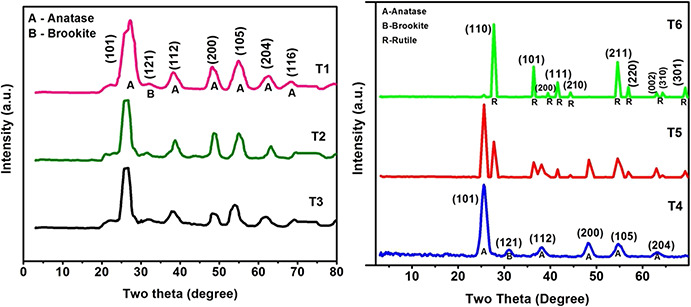
XRD patterns of T1, T2, T3, T4, T5, and T6
The average sizes of crystallites, calculated by using Sherrer's equation [13, 14, 15], were 4.56, 5.2, 5.87, 6, 24 and 33 nm, respectively, for T 1, T 2, T 3, T 4, T 5 and T 6 samples. Sizes of the doped samples (T 1–T 3) are found to be decreased when compared with pure TiO2 (T 4 sample), due to the effect of Ce doping. It confirms the significant effect of Ce doping on the crystallite size of TiO2 NPs. While for the calcined TiO2 samples (T 5 and T 6), particle size increases due to thermally promoted crystallite growth occurring during calcination.
TEM images of the synthesised samples in the order of increasing particle size (T 1–T 6) are shown in Fig. 2. Particle size distribution for each sample is shown in Table 1 and it shows that particle sizes obtained from TEM images are in agreement with that obtained using XRD analysis.
Fig. 2.
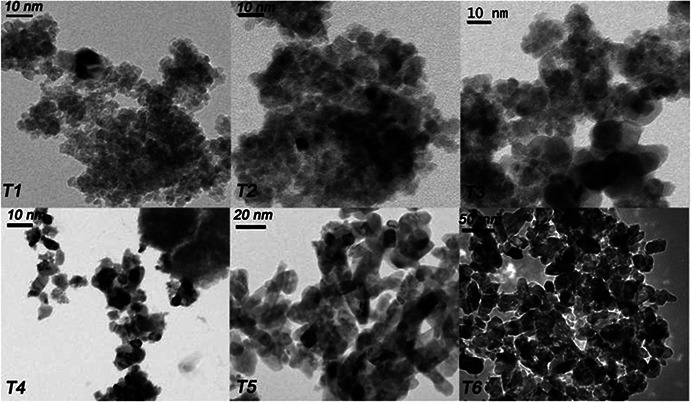
TEM images of T1, T2, T3, T4, T5, and T6 TiO2 samples
Table 1.
Particle size distribution of samples T 1–T 6
| Sample | Particle size distribution from TEM, nm | Particle size from XRD, nm |
|---|---|---|
| T 1 | 5–6 | 4.56 |
| T 2 | 5–6 | 5.2 |
| T 3 | 5–8 | 5.87 |
| T 4 | 5–10 | 6 |
| T 5 | 9–35 | 24 |
| T 6 | 25–40 | 33 |
3.2 Bio‐evaluation of earthworm and media
3.2.1 Histological effects of TiO2 NPs on earthworm
Histopathology analysis was done after 60 days exposure of NPs and studied the histological changes happening in the tested earthworms. The effect of TiO2 NPs on the cells of E. euginiae is shown in Figs. 3, 4, 5.
Fig. 3.
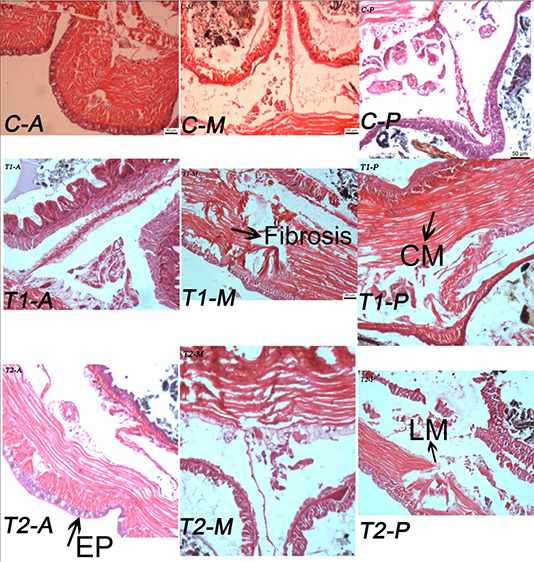
Effect of TiO2 NPs (T1 and T2) on histopathology studies of Eudrilus euginiae (scale: 50 µm; magnification 10 × 2048)
C: control tissue section of earth worms; T 1 and T 2: NP‐treated tissue section of earthworm; CM: circular muscle; LM: longitudinal muscle; EP: epithelium (A, anterior; M, middle; P, posterior)
Fig. 4.
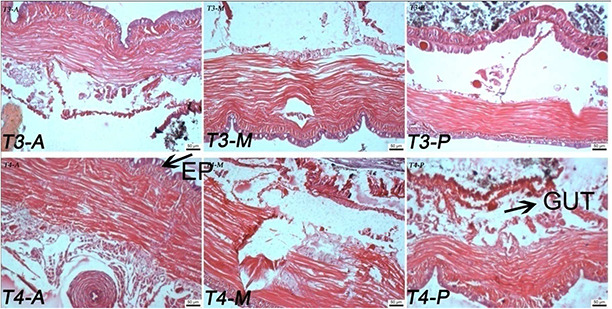
Effect of TiO2 NPs (T3 and T4) on histopathology studies of E. euginiae (scale: 50 µm; magnification 10 × 2048)
T 3 to T 4: NP‐treated tissue section of earthworm; EP: epithelium (A, anterior; M, middle; P, posterior)
Fig. 5.
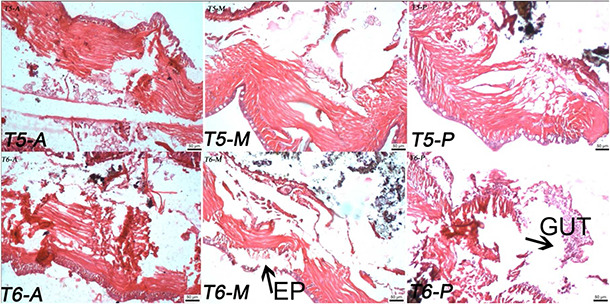
Effect of TiO2 NPs (T5 and T6) on histopathology studies of E. euginiae (scale: 50 µm; magnification 10 × 2048)
T 5 to T 6: NP treated tissue section of earthworm; EP: epithelium (A, anterior; M, middle; P, posterior)
In control, all the layers – epidermal, circular muscle and longitudinal cell layers – are found healthy without any change (Fig. 3). However, the earthworms treated with NPs show damage in the epidermal and circular muscle layer. For the samples T 1–T 3 (Figs. 3 and 4), the damage is more visible in the form of shrinking of cells inside the body walls of earthworms.
The worms exposed to T 4, T 5 and T 6 samples show more damages on the earthworm as cuticle damage, and pathological sign on epidermis, muscles, and intestinal barrier rather than T 1, T 2, T 3 and control (Figs. 4 and 5). Maximum cell damages in the form of breakage and shrinkage of the cells are observed in the T 6 sample (Fig. 5). The intensity of cell damage is in the order of T 6 > T 5 > T 4 > T 3 > T 2 > T 1.
In TiO2 ‐P25 (Fig. 6), the damage was intense with the entire epithelium being disintegrated and the epithelial cell layer was completely lost in certain regions. All these results suggest that NPs affect the histology of E. eugeniae leading to a negative effect on survival or reproduction depending on the type of NP exposed.
Fig. 6.
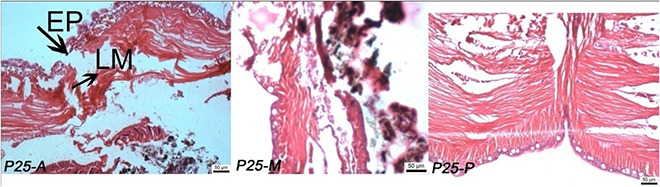
Effect of TiO2 NPs (P25) on histology studies of E. euginiae (scale: 50 µm; magnification 10 × 2048)
P25: NP‐treated tissue section of earthworm; EP: epithelium; LM: longitudinal muscle (A, anterior; M, middle; P, posterior)
The damage of the circular and longitudinal muscle cells leads to the disruptions in the peritoneum layer of an earthworm. The disruptions in the peritoneum may lead to making problems in the functioning of blood vessels, lymph vessels, and nerves. Thus, the exposure of the test organisms to different sized NPs resulted in various degrees of histopathological alterations in the internal cells. The study is in agreement with the results obtained by Van Der Ploeg et al. [14, 15].
3.2.2 Proliferation rate and survivability of earthworms
Table 2 shows the proliferation rate and survivability of earthworms under study. It is clear that the TiO2 NPs influence the proliferation rate of E. euginiae. It can be seen that samples T 1, T 2, and T 3 affected earthworm reproduction negatively, which may be due to their smaller particle sizes and presence of doped cerium ions compared with control. An increase in the proliferation rate up to 50% in samples T 4, T 5, and T 6 were observed in comparison with T 1, T 2, and T 3. P25 shows a slight effect on the proliferation rate. Similar results were obtained by Schlich et al. [16] and reported that uncoated TiO2 NPs did not alter the rate of reproduction of earthworm (Eisenia andrei) in winter.
Table 2.
Proliferation rate and survivability (mean ± SE, n = 3) of earthworms in samples
| Samples | Initial | Final |
|---|---|---|
| Control | 15 ± 0 | 59 ± 1.527 |
| T 1 | 15 ± 0 | 16 ± 2.645 |
| T 2 | 15 ± 0 | 17 ± 2.081 |
| T 3 | 15 ± 0 | 22 ± 1.0 |
| T 4 | 15 ± 0 | 54 ± 1.0 |
| T 5 | 15 ± 0 | 46 ± 0.5773 |
| T 6 | 15 ± 0 | 43 ± 1.0 |
| P25 | 15 ± 0 | 39 ± 0.5773 |
3.2.3 THB count and fungal count in media
Biological parameters like THB count and fungal count in the medium were analysed, and are presented in Fig. 7. It depicts that NP‐treated samples possess maximum THB count followed by control. An increase in the fungal counts in NP‐treated samples as compared with control is also observed, except for T 6. Samples T 1, T 2, and T 3 showed a decrease in proliferation rate due to the mortality of earthworms (Table 2). However, an increase in colony‐forming units (CFUs) and fungal count was observed in them too. This may be due to the organic substances provided by the mortal remains of earthworms for the growth of microbes. The decrease in the fungal count in samples T 4, T 5, T 6, and P25, where the mortality rate of earthworms is less, implies that the NP has bactericidal and fungicidal activity [16, 17, 18, 19]. This further concludes that TiO2 NPs are toxic enough to kill natural bacteria and fungi present in the soil, which is shown by the reduced number of CFUs.
Fig. 7.
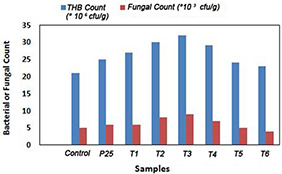
THB count and fungal count
4 Conclusion
TiO2 NPs are synthesised by the sol–gel method and characterised by XRD and TEM. Size‐dependent toxicological impact of TiO2 NPs on various biological parameters of E. euginiae has been evaluated. It is found that the TiO2 NPs exert negative effects on the earthworm proliferation rate and survivability. The percentage of mortality is found to be more with smaller TiO2 NPs when comparing with larger particles and control. An increase in CFUs and fungal count was observed for these NPs, attributed to the decrease in the proliferation rate and increase the mortality. Histological observations made on the earthworms exposed to various TiO2 NPs showed cuticle loss from the body wall and muscle damage in the form of breakage and shrinkage of the cells due to the formation of ROS and free radicals from TiO2 NPs, which play a major role in the NPs’ potential toxicity to organisms. These disruptions may cause problems in the functioning of blood vessels, lymph vessels and nerves of the organisms. The intensity of cell damage confirms the size dependence of TiO2 NPs on earthworms. Moreover, all these results indicate the deleterious effect of TiO2 NPs on the environment and eco‐system.
5 Acknowledgments
The authors acknowledge Nirmala College, Muvattupuzha for providing the opportunity to undertake this study. We are also thankful to SAIF, Cochin and Mangalore University, Mangalore, Karnataka.
6 References
- 1. Aruoja V. Dubourguier H.C. Kasemets K. et al.: ‘Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata ’, Sci. Total Environ., 2009, 407, pp. 1461 –1468 [DOI] [PubMed] [Google Scholar]
- 2. Blaise C. Gagne F. Ferard J.F. et al.: ‘Ecotoxicity of selected nanomaterials to aquatic organisms’, Environ. Toxicol., 2008, 23, pp. 591 –598 [DOI] [PubMed] [Google Scholar]
- 3. Bouche M.B.: ‘Earthworm species and ecotoxicological studies’, in Greig‐Smith P.W. Becker H. Edward P.J. et al. (Eds.): ‘Ecotoxicology of earthworms’ (Intercept Ltd, UK, 1992), pp. 20 –35 [Google Scholar]
- 4. Simonin M. Richaume A.: ‘Impact of engineered nanoparticles on the activity, abundance, and diversity of soil microbial communities: a review’, Environ. Sci. Pollut. Res., 2015, 22, pp. 13710 –13723. doi: 10.1007/s11356‐015‐ 4171‐x [DOI] [PubMed] [Google Scholar]
- 5. Gurr J.R. Wang A.S.S. Chen C.H. et al.: ‘Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells’, Toxicology, 2005, 213, pp. 66 –73 [DOI] [PubMed] [Google Scholar]
- 6. Reeves J.F. Davies S.J. Dodd N.J. et al.: ‘Hydroxyl radicals (·OH) are associated with titanium dioxide (TiO2) nanoparticle‐induced cytotoxicity and oxidative DNA damage in fish cells’, Mutation Res., 2008, 640, pp. 113 –122 [DOI] [PubMed] [Google Scholar]
- 7. Armelao L. Barreca D. Bottaro G. et al.: ‘Photocatalytic and antibacterial activity of TiO2 and Au/TiO2 nanosystems’, Nanotechnology, 2007, 18, p. 375709 [Google Scholar]
- 8. Chen Z. Meng H. Xing G. et al.: ‘Acute toxicological effects of copper nanoparticles in vivo’, Toxicol. Lett., 2006, 163, pp. 109 –120 [DOI] [PubMed] [Google Scholar]
- 9. Firdos A.K.: ‘Biotechnology fundamentals’ (Taylor & Francis, Boca Raton, USA, 2011, 2nd edn.) [Google Scholar]
- 10. Priyanka K.P. Anu Tresa S. Jaseentha O.P. et al.: ‘Cerium doped nanotitania‐extended spectral response for enhanced photocatalysis’, Mater. Res. Exp., 2014, 1, p. 015003 [Google Scholar]
- 11. Priyanka K.P. Sheena P.A. Aloysius Sabu N. et al.: ‘Characterization of nanophase TiO2 synthesized by sol–gel method’, Ind. J. Phys., 2014, 88, pp. 657 –663 [Google Scholar]
- 12. Vijaya T.M. Sushil K.M. Usha T. et al.: ‘Morphological and histological studies on the vermicomposting Indian earthworm Eudrilus eugeniae ’, World J. Zool., 2012, 7, pp. 165 –170 [Google Scholar]
- 13. Cullity B.D.: ‘Elements of X‐ray diffraction’ (Addison‐Wesley, MA, 1976) [Google Scholar]
- 14. Varghese T. Balakrishna K.M.: ‘Nanotechnology: an introduction to synthesis, characterization and applications of nanomaterials’ (Atlantic Publishers and Distributors, New Delhi, 2010) [Google Scholar]
- 15. Van Der Ploeg M.J.C. Baveco J.M. Van Der Hout A. et al.: ‘Effects of C60 nanoparticle exposure on earthworms (Lumbricus rubellus) and implications for population dynamics’, Environ. Pollut., 2011, 159, pp. 198 –203 [DOI] [PubMed] [Google Scholar]
- 16. Velhal S.G. Kulakrni S.D. Jaybhaye R.G.: ‘Titanium dioxide nanoparticles for control of microorganisms’, Int. J. Res. Chem. Environ., 2014, 4, pp. 192 –198 [Google Scholar]
- 17. Ameer A. Ahmed A.S. Mohammad O. et al.: ‘Antimicrobial activity of metal oxide nanoparticles against gram‐positive and gram‐negative bacteria: a comparative study’, Int. J. Nanomed., 2012, 7, pp. 6003 –6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maness P.C. Smolinski S. Blake D.M. et al.: ‘Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism’, Appl. Environ. Microbiol., 1999, 65, pp. 4094 –4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlich K. Terytze K. Hund‐Rinke K.: ‘Effect of TiO2 nanoparticles in the earthworm reproduction test’, Environ. Sci., 2012, 24, pp. 1 –10 [DOI] [PubMed] [Google Scholar]


